Exposure of captive feral pigeons to fonofos-treated seed in a semifield experiment
Abstract
This study aimed to investigate factors affecting risks to birds from toxic seed treatments under typical field use. Feral pigeons (Columba livia) were exposed for 2 d to fonofos-treated wheat seeds by holding them in pens in plots sown at two depths following standard husbandry in winter sowings in English fenland. In the shallow plots, seeds were readily available to pigeons on the soil surface (8.20 seeds/0.25 m2) while in the deep plots little seed was left uncovered (0.20 seeds/0.25 m2). Serum butyrylcholinesterase analysis revealed negligible exposure to fonofos of birds from deep plots but significant exposure of those in shallow plots. Data on body weight and the availability of exposed seed showed that pigeons in the shallow plots stopped eating treated seeds without depleting those exposed on the soil even though the seed consumed was less than 26% of birds' daily food requirements. Neither mortality nor signs of poisoning were observed. Though seeds were treated at the approved rate (1,080 mg a.i./kg seed), fonofos concentration in seeds from the hopper (586.5 mg/kg) and from plots during testing (260.2 and 201.7 mg/kg for days +1 and +2 after sowing, respectively) was substantially lower. In this experiment, the avoidance of fonofos-treated seeds together with the low concentration of fonofos in seeds prevented pigeons from ingesting a lethal dose. In the wild, however, pigeons are poisoned occasionally by fonofos, and it has yet to be determined under what conditions this occurs.
INTRODUCTION
The organophosphorous insecticide fonofos (O-ethyl S-phenyl [RS]-ethylphosphonodithioate) is approved in the United Kingdom as a seed treatment for winter cereals [1]. Acute oral toxicity values obtained from laboratory tests have shown that fonofos is highly toxic to birds (median 50% lethal dose [LD50] values for eight bird species range from 5.2 to 42.1 mg/kg [2-5]). Several cases of bird poisoning involving fonofos-treated seeds [6-8] have shown that this use of fonofos does pose a significant risk to birds. However, the frequency of poisoning incidents reported in the UK is lower than might expected for such a toxic compound taking into account that (1) several species feed on newly sown fields, (2) birds feeding on seeds treated at the recommended application rate would ingest more than a lethal dose per day if they fed exclusively on treated seeds, and (3) though not widely used, fonofos seed treatments are common in some areas, e.g., parts of eastern England. We are therefore using fonofos as a model to investigate the factors affecting the risks to birds from seed treatments, with the aim of providing an improved basis for risk assessment [9].
In this paper, we present results from a semifield experiment to investigate birds' responses to fonofos-treated seeds on newly sown fields. This approach allows more control than is possible with free-ranging birds and is more realistic than presenting seed in pots or hoppers as is normal in aviary studies [10]. Feral pigeons were used because they are one of the species that has been involved in fonofos poisoning incidents and they acclimate well to captivity. The birds were kept in cages on experimental plots that were cultivated and drilled following standard agricultural practice in fenland. Ingestion of treated seeds was monitored by measuring changes in body weight and cholinesterase activities, and observations were made to record any mortality or sublethal effects.
MATERIALS AND METHODS
Birds
Adult feral pigeons (Columba livia) were kept in outdoor communal aviaries at Worplesdon (Surrey, UK) for at least 5 weeks prior to the initiation of the experiment. Birds were fed with maintenance food ad libitum. Birds were given free access to grit and water throughout the study. The experiment was carried out in three (aviary, cage, and pen) phases during October-November 1994.
Aviary phase. Our experiment required all the birds to be tested in the field to have experience foraging for buried seeds because in the newly sown experimental plots all or most of the seeds would be covered by soil. Twenty-four naive birds were trained to dig for buried seeds by social learning [11], using two birds experienced in digging as tutors. One tutor and four naive birds were placed together in a large outdoor aviary for a 2-d training period. In the training period, two trays (445 × 330 × 160 mm), each containing 125 g of wheat seeds covered by a 3-cm layer of soil and 30 seeds exposed on the surface, were offered to the birds during a 5-h session (0930–1430) on 2 consecutive days. From 1430 to 1630, seeds were provided ad libitum in other trays without soil, and birds were then deprived of food overnight. At 1630 on the second day the trained birds were transferred to screened outdoor aviaries where they were kept individually. During the following 7 d, each bird was tested individually for digging behavior using the previous schedule with minor modification; food was provided for 2 d but only one tray per aviary was used. Each bird was given 55 g of buried seeds and 10 (∼0.5 g) exposed seeds from 0930 to 1430. Food consumption (g dry weight) during the 5-h session on the second day was measured for each bird and used in the assignment of birds to experimental groups (see below).
Before taking the birds to the field study area, they were acclimated for 3 d to the portable pens that were used in the field experimental plots. The only food presented in the pens was untreated dyed wheat seeds scattered on the soil. Between the aviary and cage phases there was a period of 6 d in which birds were kept in communal aviaries and fed with maintenance food.
Cage phase. Birds were taken to the field study site (ADAS Arthur Rickwood Experimental Farm, Mepal, Cambridgeshire, UK) (52°24′N, 0°06′E) 1 week before the day on which the experimental plots were sown (sowing day = day 0). Birds were housed individually in metal cages (0.90 × 0.45 × 0.35 m) placed in a large barn with a translucent roof permitting a natural light-dark (∼7.5:16.5-h) cycle. Maintenance food was provided in a bowl for 24 h/d until day −5, when birds were starved overnight starting at 1500. On days −4 to 0, the only food given was 50 g/bird/d of untreated dyed seeds offered for 6 h (0900–1500). Daily food consumption (g fresh weight) was measured on days −4, −3, and −2 as the difference between the weight of the sample offered to each bird at 0900 and the weight of the food remaining in the bowl plus spillage at 1500.
Pen phase. On days +1 and +2 pigeons were placed for 6 h (0830–1430) in the pens (two birds/pen) situated in the experimental plots. Pens were portable enclosures (3 × 2 × 1 m) made of a plastic frame and black plastic netting. Birds were removed from the pens and held in metal cages in the barn overnight. Pens remained in the same place throughout the 6-h period each day but their locations were changed each day. All pens were provided with two bowls containing water and grit, respectively. In the control pens, 115 g of untreated dyed wheat seeds were scattered on the soil each day. The areas of the sown plots where the pens were situated were left undisturbed after sowing, thus only fonofos-treated wheat seeds were available. Birds were checked for signs of poisoning and abnormal bird behavior immediately before retrieving them from pens at 1430 on days +1 and +2.
Biochemical analyses were carried out to determine whether birds were exposed to fonofos, a cholinesterase inhibitor [12]. Serum butyrylcholinesterase (BChE) activity was measured spectrophotometrically. Assays and blood sampling procedures employed are fully described in Thompson and Walker [13]. Blood samples were taken on days 0 (pretreatment) and +2 (posttreatment) between 1600 and 1800. Preliminary analysis had shown that feral pigeon serum BChE inhibited by fonofos does not reactivate when preserved at −20°C (H.M. Thompson, personal communication), thus serum samples were kept at −20°C until they could be analyzed. Brain acetylcholinesterase (AChE) activity was assayed also, as detailed in Thompson et al. [14]. Cholinesterase activity was measured as μmol of substrate hydrolyzed per min per ml of plasma (serum BChE) or g of tissue (brain AChE). Birds were killed humanely by cervical dislocation followed by decapitation on day +2 between 1600 and 1800 and carcasses stored at −20°C until brain analyses were carried out. Pigeons were weighed to the nearest ±1 g on days −5, −2, 0, +1, and +2 between 1500 and 1530.
Plots layout, agricultural operations, and sowing depth
A field with a loamy peat soil was used in the experiment. Eight drilled plots were arranged in two parallel rows separated by an 18-m strip. Distance between plots in the same row was 6.52 m. Plot size was 18 × 5.78 m (104 m2) but the actual drilled area measured 91 m2 as there were two 0.37-m-wide tramlines in each, defining three subplots per plot. The four pens with the control birds were placed randomly on the strip between the two rows. In this strip, presowing cultivations were the same as in the plots but it was left undrilled.
Conventional tillage and sowing techniques for fenland were followed. The field was ploughed to 20 cm depth 12 d before sowing. Plots were seeded with an Øyjord drill [15] on 28 November (day 0). Seeding density was 450 seeds/m2 and row spacing 12 cm. Two target sowing depths were used, 25 mm for the shallow plots and 55 mm for the deep plots. Actual sowing depth was measured at the end of January 1995, after plant emergence. Four drill lengths (including two rows each) of approximately 50 cm each were taken at random in every plot, and 25 seedlings per length measured (n = 100 plants per plot). Sowing depth was measured as the length (to the nearest 1 mm) of the white part of the stem, from the top of the seed to the point where the green part appears above the soil.
Exposed seeds
The density of exposed seeds per plot was estimated from the number of seeds found on the soil surface in five 0.5 × 0.5-m (0.25-m2) quadrats per plot. The position of the quadrats was determined randomly and marked with sticks allowing repetition of the sampling in exactly the same position over several days. Seed counts were carried out on days 0 (between 1130 and 1230), +1, and +2 (between 1430 and 1530). Because the density of exposed seeds did not differ between days either in the shallow (one-way ANOVA, F = 0.04, d.f. = 2,9, p = 0.96) or in the deep (F = 0.12, d.f. = 2,9, p = 0.89) plots, only data from the day of the highest readings (day +1) have been used.
The availability of exposed seeds inside the pens at the start of each test day was not measured to avoid disturbing the soil surface and so altering the outcome of the sowing. The availability of exposed seeds in each pen was estimated from the density of exposed seeds in the plot taking into account the actual drilled surface in each pen and assuming an average weight per seed of 0.05055 g based on the thousand grain weight of the seeds used in the sowing. After taking the birds away from the pens it was not possible to do a reliable count inside the pens as the soil surface was disturbed while catching the birds.
Treatment of seeds and chemical analysis
Wheat seeds (cv. Brigadier), dressed with fonofos 3 d before sowing, were obtained from a commercial supplier (Dunns Long Sutton, Spalding, Lincolnshire, UK). Four samples of seeds were taken from the hopper immediately before sowing. Four samples of exposed seeds (one per shallow plot) and eight samples of buried seeds (one per plot) were taken on days +1 and +2. Samples were kept at −20°C until analysis. Seeds were homogenized in acetone using a Ultra-Turrax homogenizer for 2.5 min and the volume adjusted to 100 ml with acetone. The extracts were analyzed by gas chromatography using a Hewlett Packard 5890A gas chromatograph equipped with a nitrogen-phosphorous detector and a 15-m × 0.53-mm internal diameter DB1 column. All samples had residues well above the minimum detection limit for fonofos, which was 1 mg a.i./kg seed.
Fonofos-treated seeds are pink in color due to the use of rhodamine in the seed treatment formulation. To ensure that birds did not avoid pink-colored seeds [16], birds were fed for several days in the aviary and cage phases with rhodaminedyed seed.
Experimental design and statistical analysis
The experiment had a completely randomized design including one factor (sowing depth) with three levels for birds (shallow, deep, and control), two levels for the density of exposed seeds (shallow and deep plots) and four replicates. The number of elements per replicate was two for bird body weight and cholinesterase analysis, five for the density of exposed seeds, and four for fonofos residues in exposed seeds and from the hopper and eight for buried seeds. A mean value was calculated for each replicate. Mean values were used in all statistical analyses.
Plots were assigned to experimental groups (shallow or deep sowing) at random. Birds were assigned to groups and plots in a two-stage randomization process. In the first step, birds were ranked by food consumption in the 5-h period of individual testing for digging behavior in the second day of the aviary phase to obtain eight clusters of three individuals each. Within clusters, each bird was assigned at random to one of the three groups. Food intake did not differ between groups. Birds were assigned to plots randomly.
Statistical analyses were performed using Statgraphics® 4.0 [17] and SPSS® 6.0 [18]. The level of significance was set at p ≤ 0.05. Values shown are means ± SE. For two sample comparisons, t-tests (two-tailed) were used unless conditions of normality and homogeneity of variances were not met, in which case Mann-Whitney U-tests were employed. Sowing depth data were square-root transformed ([y]1/2) [19] before t-test analysis. Two-way ANOVAs compared fonofos residues in buried seeds between shallow and deep plots and between exposed and buried seeds (data from shallow and deep plots pooled) on days +1 and +2. One-way ANOVAs were used to test differences between the three experimental groups for birds' food consumption in the aviary and cage phases, absolute body weight, and serum and brain cholinesterase activities. A multiple comparison procedure (Tukey's test) was used to compare mean serum BChE activity between groups when the overall ANOVA gave significant differences. Body weight changes by percentage were analyzed using Kruskal-Wallis and Mann-Whitney U-tests. Relationships between bird body weight variation and density of exposed seeds in the pens were tested using Spearman rank correlation coefficient analyses.
RESULTS
Aviary phase
The 24 birds selected for the experiment were all successful in foraging for buried seeds at a depth of 30 mm after the sessions of social learning. During the 5-h period of individual testing for digging behavior, food consumption varied between 4.41 and 26.67 g (dry weight). Assignment of birds to experimental groups was based on this variable, so mean food consumption was similar in the three groups (control, 19.42 ± 2.56; shallow, 19.21 ± 1.36; deep, 18.64 ± 2.17) (one-way ANOVA, p = 0.96).
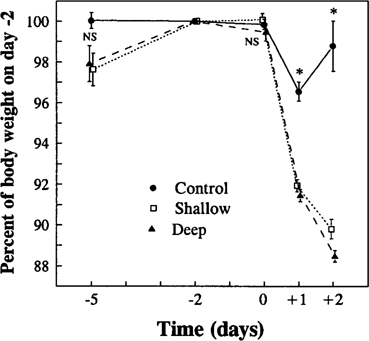
Change in pigeon body weight at the end of the cage phase (days —5 to 0) and during the pen phase (days +1 and +2). Data show means ± SE, n = 4. Percentages were calculated for each bird based on its body weight on day −2. Differences between experimental groups within days were tested using Kruskal-Wallis tests (NS, not significant; *p < 0.05).
Cage phase
As expected, mean food consumption (g fresh weight) of dyed untreated wheat seeds on days −4, −3, and −2 did not differ between control (22.41 ± 2.10), shallow (26.31 ± 1.05), and deep (25.25 ± 0.87) groups (one-way ANOVA,/> = 0.20).
Body weight variation also did not differ between groups during the cage phase, although body weight of birds from shallow and deep groups did not stabilize until day −2 (Fig. 1). Because body weight on day −2 was stable and there were no differences between groups (control, 498.75 ± 12.89 g; shallow, 482.75 ± 12.52; deep, 493.25 ± 7.81) (one-way ANOVA, p = 0.71), these individual body weights were used to calculate percentages of change in the cage and pen phases.
Pen phase
Availability of seeds. Wheat seeds were the only food resource available to the birds during the pen phase. Seed availability differed between shallow and deep plots in two ways. First, the density of exposed seeds (number/0.25 m2) was higher in the shallow (8.20 ± 1.83) than in the deep (0.20 ± 0.20) plots (Mann-Whitney U-test, p = 0.03). Fonofos-treated seeds were readily available on the soil surface in all shallow plots, while in most deep plots no seed was left unburied (Table 1). Second, buried seeds were placed significantly deeper in the deep (47.65 ± 3.69 mm) than in the shallow (19.78 ± 1.01 mm) plots (t-test, p = 0.0002). Deep sowing placed most seeds greater than 30 mm below the surface (Fig. 2), which was the depth used in the aviary phase during the training of birds for digging. By contrast, shallow sowing left most buried seeds less than 30 mm below the surface (Fig. 2).
At the end of the 6-h period in which birds were in the plots on days +1 and +2, there were a few exposed seeds in the pens of all shallow plots while no seed was found on the soil surface inside the pens of the deep plots. In the control pens, a large quantity of seed remained on the soil on both days.
Residues of fonofos. Residues in buried seeds did not differ between shallow and deep plots on days +1 and +2 (two-way ANOVA, p = 0.39, plots; p = 0.13, days; p = 0.52, interaction), thus all buried seed samples were considered together for further analysis. Neither were any differences between exposed and buried seeds within days (two-way ANOVA, p = 0.76, seed placement). The levels of fonofos in exposed and buried seeds decreased rapidly within the 2 d after sowing (Fig. 3). Residues fell by 56% on day +1 with respect to hopper levels at the time of sowing (586.5 ± 47.24 mg/kg). Residues were higher on day +1 (260.2 ± 22.92) than on day +2 (201.7 ± 17.11), but differences were not significant (t-test, p = 0.053).
| Mean fonofos residues (mg/seed) | Estimated fonofos (mg) per penc | ||||||
|---|---|---|---|---|---|---|---|
| Plot no. | Group | No. per pena | Weight (g) per penb | Day +1 | Day +2 | Day +1 | Day +2 |
| 5 | Shallow | 73 | 3.69 | 0.0169 | 0.0100 | 1.234 | 0.730 |
| 7 | Shallow | 238 | 12.03 | 0.0242 | 0.0179 | 5.760 | 4.260 |
| 12 | Shallow | 141 | 7.13 | 0.0167 | 0.0147 | 2.355 | 2.073 |
| 14 | Shallow | 210 | 10.62 | 0.0095 | 0.0125 | 1.995 | 2.625 |
| 1 | Deep | 0 | 0 | — | — | — | — |
| 3 | Deep | 0 | 0 | — | — | — | — |
| 10 | Deep | 0 | 0 | — | — | — | — |
| 16 | Deep | 16 | 0.81 | —d | —d | 0.269e | 0.221e |
| c1 | Control | 2,275 | 115 | — | — | — | — |
| c2 | Control | 2,275 | 115 | — | — | — | — |
| c3 | Control | 2,275 | 115 | — | — | — | — |
| c4 | Control | 2,275 | 115 | — | — | — | — |
- a Estimate based on the mean number of exposed seeds per plot and on the actual drilled surface in the pen (5.04 m2) for shallow and deep plots. Based on the 115 g of seeds scattered inside each pen and the estimated average weight of a seed (0.05055 g) for the control pens.
- b Estimated fresh weight calculated from the values of the previous column and the average weight of a seed (0.05055 g) for shallow and deep plots. Actual amount for control pens.
- c Calculated from the estimated number of exposed seeds per pen and the mean fonofos residues in exposed seeds.
- d No samples for residue analysis were taken because there were too few exposed seeds.
- e Estimate based on mean residues in exposed seeds from shallow plots.
Bird mortality. There was no bird mortality at any stage of the experiment and no signs of pesticide poisoning were observed in the birds during the pen phase.
Body weight. Comparisons of body weight between day 0 and days +1 and +2 showed that control birds lost body weight significantly on day +1 with respect to day 0 (Mann-Whitney U-test, p = 0.03) but they recovered weight on day +2 (p = 0.47). Birds in shallow and deep plots lost much more weight than birds in the control plots (Fig. 1). With respect to the control birds, the average weight loss of birds in the shallow and deep groups was 4.6% and 5.1% on day +1 and 9.0% and 10.3% on day +2, respectively. Body weight loss did not differ significantly between shallow and deep groups within days either on day +1 (Mann-Whitney U-test, p = 0.31) or on day +2 (p = 0.11).
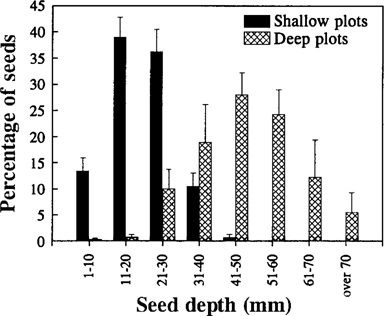
Seed depth distribution of buried seeds by 10-mm intervals in the shallow and deep plots. Data are means ± SE, n = 4.
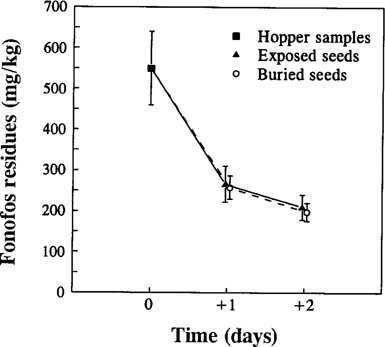
Concentration of fonofos (mg a.i./kg seed) in seeds taken from the hopper (day 0), and in exposed and buried seeds collected from the plots on days +1 and +2. Values are means ± SE, n = 4 for hopper samples and exposed seeds and n = 8 for buried seeds.
Body weight variation was highly correlated with availability of exposed seed in the pens on both days +1 (Spearman, rs = 0.81, p < 0.01) and +2 (rs = 0.90, p < 0.01) when plots from the three experimental groups were included in the analyses. When only data from shallow and deep plots were considered, the relationship approached significance on day +2 (rs = 0.71, p = 0.06) but not on day +1 (rs = 041, p = 0.27), suggesting that birds in the shallow plots ate more of the available seed on day +2 than on day +1 and that intake was related to density of exposed seeds.
Cholinesterase analysis. Serum BChE activity before the pen phase did not differ between the control (1.74 ± 0.07 μmol/min/ml), shallow (1.57 ± 0.13), and deep (1.57 ±0.13) groups (one-way ANOVA, p = 0.52). Posttreatment BChE activity was significantly lower in the shallow (0.62 ± 0.11) than in the control (1.80 ± 0.07) groups (Tukey's test, p < 0.01), but there were no differences between the deep (1.57 ± 0.22) and the control groups (Tukey's test, p < 0.01). This suggests that all birds in the shallow group were exposed to fonofos because they showed substantial BChE inhibition (59.68 ± 5.40%, n = 8, range = 40.58–86.93) with respect to pretreatment levels. BChE was inhibited by 55.70% in one bird of the deep group indicating that it was exposed to fonofos. The other seven birds from the deep group were not exposed probably because the maximum BChE inhibition was 12%, lower than the maximum change found in the control group (23%).
Brain AChE was not depressed in any bird. AChE activity was similar in control (25.30 ± 0.72 μmol/min/g), shallow (25.34 ± 0.65), and deep (25.88 ± 0.29) groups (one-way ANOVA, p = 0.74).
DISCUSSION
Pigeons ingested some treated seed, as evidenced by the significant decrease in serum BChE activity, a reliable indicator of avian exposure to fonofos [12] and other OP pesticides [14]. However, no mortality or signs of poisoning were observed at any stage of this study, nor was there any significant inhibition of brain cholinesterase (50% inhibition of brain AChE is considered indicative of a life-threatening exposure to OP compounds [20]). The two main factors that may explain the lack of acute effects are the avoidance of treated seeds by pigeons and the low concentration of fonofos in the seeds. Results suggest that these and other factors that limited exposure in this experiment could be expected to moderate exposure in the wild.
The concentration of fonofos in the seeds was lower than the recommended application rate, which reduced the risk of acute effects on birds (see calculations below). Fonofos residues were low because the experiment accurately simulated field conditions of the use of fonofos as a seed treatment. Two factors can affect the actual concentration of pesticide in seeds after sowing: the amount of active ingredient retained by seeds during the dressing process and the degradation of fonofos. The mean concentration of 54% of the target dose found in seeds taken from the hopper is within the range of 46–87% found in other field trials where microencapsulated formulations of fonofos were used also [21]. Similar loading efficiency has been frequently found in commercially dressed seeds, indicating that low loading efficiency is a trait affecting seed treatments in general [22].
Fonofos residues declined further after sowing, to reach 65% of presowing levels after 2 d. A similar pattern of fonofos residue decay in unburied wheat seeds has been found in commercial fenland fields near the study area (unpublished results). Therefore, it can be concluded that the low concentration of fonofos in seed available to birds is consistent with conditions of current use of fonofos as a seed treatment in winter cereals in fenland. However, birds may sometimes have access to concentrations much nearer to the approved application rate, for example if seed is spilt when loading the drill, and it may be under these conditions that mortality occurs.
Avoidance of fonofos-treated seeds by pigeons was another factor limiting exposure in this experiment. The clearest evidence for this comes from the results for the shallow group. The estimated availability of exposed seeds in the pens in the shallow plots ranged from 1.8 to 6.0 g/bird/d (calculated from data in Table 1). Because the daily mean food intake of the birds in the shallow group during the cage phase was 26 g (range 23–31 g), exposed seeds comprised only 6–26% of daily food requirements. Despite this, the birds did not consume all the exposed seeds available; the birds chose to go hungry rather than consume fonofos-treated seeds. A similar behavior has been observed in other bird species avoiding pesticide-treated food in no-choice laboratory studies, in which severe body weight losses were associated with reductions in daily food consumption up to over 90% of controls [23, 24]. It is interesting to note that Bennett [25] found that bobwhites offered a choice of untreated food and chlorpyrifos- or methyl parathion-treated foods developed conditioned aversions only after 12–24 h of exposure. By contrast, fonofos-treated seeds in our experiment presumably induced a faster avoidance response, soon after exposure, because pigeons ingested only a few of the available seeds. However, as they ingested a significant amount before stopping, it seems unlikely that they were responding to odor or taste (primary repellency, sensu Rogers [26]).
Birds in this experiment had access to seed in excess of their daily requirement until the day before testing and were then deprived of food overnight. Birds in the wild have limited and variable food supplies and might often be significantly hungrier than those in this trial. Increased hunger could interfere with the avoidance response either by increasing motivation to feed to the point where it overcomes any nauseating effects of intoxication, or by increasing the rate of feeding such that a lethal dose is consumed before the onset of intoxication [27]. Some poisoning incidents have involved migrating birds that probably were in poor body condition [28]. The differences found between days +1 and +2 in the relationship between body weight change and seed availability suggest that most of the exposure to birds in the shallow group occurred on day +2, which would be consistent with a response to increasing hunger. However, pigeons did not deplete exposed seeds, indicating that hunger was not high enough to overcome the avoidance response in this experiment.
Because pigeons in the wild do sometimes consume lethal doses, there must be some conditions under which the avoidance response fails to protect them. In our experiment, pigeons stopped eating treated seeds long before ingesting a lethal dose, as the following data show. Calculations using the mean concentration of fonofos in the seeds in day +2 (202 mg/kg), the mean body weight of pigeons of the shallow group on day +1 (444 g), and assuming that they ate 5.3 g of treated seeds on day +2 (20% of their daily mean food intake in the cage phase) show that birds would have ingested 2.4 mg/kg of fonofos, which is less than 1/5 of an LD50 dose (13.3 mg/kg [2]). If fonofos residues in the seeds had been the recommended rate for winter cereals (1,080 mg/kg) and assuming a similar intake, birds would have ingested a 12.8-mg/kg dose, practically a lethal dose.
Other factors that could alter the extent of exposure and the avoidance response in the wild include the availability of alternative food [29], social feeding [30, 31], and group size [32]. In order to improve the reliability of pesticide risk assessment it is necessary to improve our understanding of the factors affecting risk and the way in which they interact.
Risk management
Sowing treated seed more deeply than current practice might reduce risk to birds by making it less available and hence reducing exposure. Label instructions for fonofos and other seed treatments specify relatively shallow sowing depths (typically ∼25 mm) designed to maximize the efficacy of the treatment, so it would be necessary to seek a balance between efficacy and risk reduction [9]. Sowing deeper did reduce exposure in this experiment, as shown by the difference in serum cholinesterase inhibition between deep and shallow groups. Exposure in deep sown plots was reduced primarily because few or no treated seeds were left on the soil surface, while many were available on the shallow plots. The lack of exposure to fonofos of most birds from the deep group shows that they obtained very little or no buried seeds. However, the experiment did not provide conclusive results on whether placing seed deeper than recommended reduces the risk of lethal poisoning because no mortality occurred in either treatment. Results from deep plots would suggest so, but the fact that the density of exposed seed differed between shallow and deep plots and that birds in the shallow group did not deplete the exposed seeds preclude comparisons between groups on the likelihood of birds obtaining seeds buried at different depths.
Under the conditions of the experiment, the reduction in exposure was not critical as no mortality occurred even at the shallower depth of about 20 mm. With hungrier wild birds and higher levels of fonofos on the seed, sowing deeper may be important in avoiding mortality. Better information is required on the conditions under which poisoning occurs in the wild in order to optimize recommendations for risk management.
Utility of semifield experiments
Semifield experiments (also termed pen trials, pen studies, or cage trials) typically involve birds being held captive in temporary pens or cages placed in the habitat of interest. The possibility of using them in the assessment of pesticide risks to birds has been recognized for many years [10] and they are identified as an option in current European guidance [33]. Their usefulness in refining the interpretation of laboratory toxicity studies rests primarily on their ability to simulate field conditions. In particular, being able to reproduce actual farm practice in terms of crop, husbandry, machinery used, and application of pesticide. Semifield experiments also have advantages over field studies with free-ranging animals, providing better control of conditions and ensuring that the subjects can be observed and captured repeatedly to monitor the time course of exposure and effects. All of these benefits were experienced in this study, and our results identified several factors that restricted exposure in the experiment and that might also moderate exposure in the wild. However, the lack of acute effects in our study implies that the experimental conditions did not correspond to worst-case conditions in the wild, as mortalities of both feral pigeons and woodpigeons occur at least occasionally. A similar example has been documented for diazinon, which has been involved in a number of poisoning incidents in North America but caused no mortality in a semifield experiment with Canada Geese [27]. Semifield experiments should therefore be used and interpreted with caution: in particular, it should not be assumed that a lack of mortality implies none will occur in the wild. Paradoxically, to be sure of imposing worst-case conditions, the experimenter must already have detailed knowledge of the factors affecting risk in the wild, in which case an experiment would be unnecessary. Semifield experiments are therefore likely to be most useful for exploring the factors affecting risk and extrapolating to other conditions, rather than as a definitive test of effects. There is a need for a wider review of semifield approaches to identify those that are most useful for pesticide risk assessment, as no recent guidance is available.
Acknowledgements
We thank Rachel Chivers, Justin Hart, Helen McKay, Phil Prosser, and Mark Taylor for assistance during field work; Colin McCoy and Ainsley Jones for fonofos analysis; Helen Thompson for cholinesterase assays; John Kilpatrick for agronomic advice; Jason Mottran for conducting the agricultural operations and sowing depth measurements; Steve Langton for statistical advice; and Helen McKay and Helen Thompson for comments on the manuscript. This research was supported by funding from the UK Pesticides Safety Directorate and a European Union (AIR Programme) Postdoctoral Fellowship to J.A. Pascual.




