Dietary uptake in pike (Esox lucius) of some polychlorinated biphenyls, polychlorinated naphthalenes and polybrominated diphenyl ethers administered in natural diet
Abstract
The dietary uptake of 12 halogenated diaromatic compounds was studied using northern pike (Esox lucius L.) fed with rainbow trout (Oncorhynchus mykiss (Walbaum)). Before the trout were fed to the pike, they had been injected with a cocktail of five polychlorinated biphenyls, four polychlorinated naphthalenes, and three polybrominated diphenyl ethers, dissolved in rainbow trout lipid. The reported uptake efficiencies (E) were in the range 35 to 90% and differ in some respect from earlier studies. The E-values for those substances with effective cross sections (ECS) >9.5 Å were considerably higher than expected if the membrane permeation at dietary uptake was restricted as proposed previously in the literature. There was no hydrophobicity dependency of the total dietary uptake efficiency as suggested by an earlier proposed empirical model. The difference between the results presented here and earlier studies is likely to depend on cotransport with lipids and/or proteins through a mediated, possibly active uptake of hydrophobic organic compounds (HOC) in the gastrointestinal tract enabled by the actual exposure method. For the proposed mediated/active uptake of HOCs, the uptake efficiency varied with molecular weight and was greatest for a molecular weight of approximately 450.
INTRODUCTION
The high concentrations of some hydrophobic organic compounds (HOCs), e.g., the polychlorinated biphenyls (PCBs), present in predatory fish can be explained by effective dietary uptake and slow diffusive clearance rates [1]. The effectiveness of dietary uptake is an important factor when estimating the biomagnification potential of various HOCs. Dietary uptake of HOCs has been investigated in several laboratory studies but mostly with fish that have been fed with dried food items, i.e., food pellets. Opperhuizen and coworkers [2, 3] reported that the molecular size of some extensively halogenated HOCs restricted the bioconcentration of these compounds because permeation through the membranes of the gill cells was prevented. The studies reported that molecules with an effective cross section (ECS) >9.5Å were not taken up through the gills. Opperhuizen and Sijm in a literature study [4] also reported that dietary uptake of some large molecule HOCs was highly inefficient and concluded that the 9.5-Å limit largely restricted dietary uptake too. This would mean that dietary HOC uptake is diffusive and that the membranes of the cells in the gills and the gastrointestinal tract (GI tract) have similarities with respect to membrane permeation. The results from these bioaccumulation studies seem to be in conflict with the reported presence of anthropogenic HOCs with ECS >9.5 Å in studies of biological samples from the environment [5-7]. The difference in uptake efficiency pattern could be due to the difference in the food matrix under natural, compared to experimental conditions. Gobas et al. [8] reviewed dietary uptake of HOCs and concluded that dietary HOC uptake was diffusive. Later, the diffusive model of dietary uptake of HOCs received further support in a study [9] in which the authors exposed fish to HOCs via food pellets of different lipid concentration. The reported lack of dependency of uptake efficiency on the lipid concentration of the food fit the authors' model of diffusive dietary uptake of HOCs.
Possible differences in HOC uptake efficiency in fish due to different food qualities has been relatively little studied. Sijm et al. [10] reported that differences in dietary uptake efficiencies of some HOCs in fish were dependent on the food matrixes used and stressed the importance of using food that resembles the food of the fish under natural conditions when investigating dietary uptake of HOCs in the laboratory. In several vertebrates, other dietary uptake mechanisms than diffusion are known to be involved in the uptake of substances that constitute parts of the natural diet [11-15]. It is possible that these mechanisms could also be involved in dietary uptake of HOCs under natural conditions.
The objective of this study was therefore to evaluate possible differences in dietary uptake efficiency of HOCs using a natural fish food matrix (living rainbow trout) compared to earlier exposure methods. It was intended to determine if this dietary exposure method could explain the presence of different anthropogenic, highly halogenated hydrocarbons in biological environmental samples.
MATERIALS AND METHODS
Experimental organisms
The experiments were performed at the Department of Zoology at the Stockholm University, Stockholm, Sweden, between September 1994 and February 1995. Fifteen pike (Esox lucius L.) (10–30 g each) from Kjuloträsk, Finland, were kept in separate 50-L aquaria with continuously stirred tap water at 16°C. The water was aerated before placing the pike in the aquaria. A 16-h day and 8-h night light cycle was used. One hundred rainbow trout (Oncorhynchus mykiss (Walbaum)), obtained from Åkersberga Fiskodling, Stockholm, Sweden were kept in a flow-through system with aerated tap water in a 200-L aquarium and fed with pellets (ET 91, EWOS AQUA A/S, Södertälje, Sweden). The weight of the trout was between 2 and 10 g. The pike were held in the aquaria 2 months before the exposure feeding. During that time they were fed two to five rainbow trout each. The production of feces was observed in order to examine the time needed for digestion. Trout and pike were weighed at each feeding occasion. The lipid content of the trout and the pike was approximately 3% wet weight and 1.5% wet weight, respectively (whole body).
| Systematic name | Abbreviation | Log Kow | ECS (Å) | Molecular weight |
|---|---|---|---|---|
| PCBs | ||||
| 2,4′,5-TriCB | PCB 31a | 5.67e | 8.7 | 258j |
| 2,2′,5,5′-TetraCB | PCB 52a | 5.84e | 8.7h | 292j |
| 3,3′,4,4′-TetraCB | PCB 77a | 6.36e | 8.7 | 292j |
| 2,3′,4,4′,5-PentaCB | PCB 118a | 6.74e | 8.7 | 326j |
| 2,2′,4,4′,5,5′-HexaCB | PCB 153a | 6.92e | 8.7h | 360j |
| PCNs | ||||
| (1,2,3,4,6,7-) + (l,2,3,5,6,7-)HexaCN**b | HxCN-A | 6.98f | No value | 335j |
| 1,2,4,5,6,8-HexaCN | PCN 71c | 6.98f | 9.7 | 335j |
| 1,2,3,4,5,6,7-HeptaCN | PCN 73c | 7.69f | 9.7h | 369j |
| 1,2,3,4,5,6,7,8-OctaCN | PCN 75c | 8.4i | 9.8i | 404j |
| PBDEs | ||||
| 2,2′,4,4′-TetraBDE | PBDE 47d | 5.87–6.16g | 8.1 | 485j |
| 2,2′,4,4′,5-PentaBDE | PBDE 99d | 6.64–6.97g | 9.6 | 563j |
| 2,2′,4,4′,5,5′-HexaBDE | PBDE 153d | 6.86–7.92g | 9.6 | 646j |
- a IUPAC number.
- b Co-eluting on GC.
- c Numbered according to the IUPAC rules as proposed in Wiedmann and Ballschmiter [32].
- d Numbered according to the IUPAC numbering system of the PCBs.
- c Hawker and Conell [33].
- f Calculated using the method described in Hansch and Leo [34], using the log Kow value 8.4 for PCN 75 reported in Opperhuizen et al. [2].
- g World Health Organization [35].
- h Opperhuizen [3].
- i Opperhuizen et al. [2].
- j Longman [23].
Exposure
A cocktail (Table 1) of five PCBs, three polychlorinated naphthalenes (PCNs), and three polybrominated diphenylethers (PBDEs) were dissolved in lipid from rainbow trout muscle tissue extracted with 1:1 acetone-hexane. The PCBs, PCNs, and PBDEs had been synthesized according to methods in the literature [16-21]. The concentration was approximately 90 ng/μl lipid for each substance. Spiked fish lipid extract (10 μl) was injected in the dorsal muscle tissue of the rainbow trout, after which they were directly given to the pike. The trout were taken by the pike after a few seconds. A minimum of 9 d after feeding with the spiked trout, the pike were sacrificed by hitting them on the head. The period of 9 d had been shown to be just sufficient for the pike to digest the trout.
Extraction and clean-up
After sacrificing the pike, they were stored at −20°C until analysis. Prior to analyses, the gastrointestinal (GI) tract was removed to exclude possible residues of the substances that had not been absorbed. The pike were weighed before and after removal of the GI tract.
The pike were homogenized and extracted in 5:2 acetone-hexane (both Merck, pa) and 9:1 hexane-diethylether (Merck, pa; Prolabo, rectapur, respectively) essentially by the method of Jenssen et al. [22]. Prior to extraction, 150 ng PCB 189 was added as internal standard. After extraction, the samples were cleaned up using a 1-cm-diameter silica gel column (Kieselgel 60, 0.063–0.200 mm, Merck) consisting of the following four layers: 1 cm SiO2 with 10% w/w H2O, 3 cm SiO2 with 56% w/w KOH, 3 cm SiO2 with 40% w/w H2SO4, and 3 cm SiO2 on top which was added 0.5 cm anhydrous NaSO4. The volume of the eluate was reduced to about 1.5 ml.
Three untreated rainbow trout and two untreated pike were extracted and cleaned up in the same way. Three 10-μl replicates of the cocktail spiked rainbow trout lipid extract were also cleaned up and analyzed.
Chemical analysis
Gas chromatography-mass spectrometry (GC-MS) analyses were performed (1 μl samples, column-injected) using a Fisons GC 8000/MD 800 equipped with a Crompack CP-Sil 8 CB column with He as carrier gas. Response factors for quantification were calculated from the response on GC-MS to a standard solution of the studied substances, treated in the same way as the samples. The extraction efficiency was investigated by calculating the recovery of the internal standard using 13C-labeled PCB 153 as an injection spike in two samples. The recovery was 70.0%.
| Unspiked trout (n = 3) | 10 μl spiked fat (n = 3) | Exposed pike (n = 15) | Unexposed pike (n = 2) | |||||
|---|---|---|---|---|---|---|---|---|
| Substance | Mean (ng) | SE | Mean (ng) | SE | Mean (ng) | SE | Mean (ng) | SE |
| PCB 31 | 10 | 2 | 802 | 102 | 376 | 30 | 1 | 0.3 |
| PCB 52 | 32 | 4 | 928 | 116 | 525 | 46 | 1 | 0 |
| PCB 77 | 8 | 0.6 | 905 | 72 | 412 | 36 | nd | 0 |
| PCB 118 | 29 | 3 | 967 | 107 | 623 | 52 | 1 | 0.1 |
| PCB 153 | 98 | 6 | 948 | 55 | 922 | 89 | 5 | 0.7 |
| HxCN-A | nda | 0 | 1,249 | 76 | 813 | 68 | nd | 0 |
| PCN 71 | nd | 0 | 1,206 | 143 | 937 | 78 | nd | 0 |
| PCN 73 | nd | 0 | 630 | 35 | 428 | 28 | nd | 0 |
| PCN 75 | nd | 0 | 812 | 68 | 283 | 18 | nd | 0 |
| PBDE 47 | 52 | 8 | 845 | 97 | 950 | 96 | 3 | 0 |
| PBDE 99 | 5 | 0.4 | 717 | 40 | 454 | 39 | nd | 0 |
| PBDE 153 | nd | 0 | 731 | 42 | 294 | 3 | nd | 0 |
- a Not detected.
RESULTS AND DISCUSSION
HOC amounts and uptake efficiencies
The octanol-water partition coefficient (log Kow) values, the molecular weights, and the ECS values of the studied substances are listed in Table 1. The ECSs for the PBDEs were calculated by adding bond lengths and van der Waals radii [23] for the atoms in the molecules, taking into account the shape of the molecules. The ECS values of the PBDEs depends on the twisting of the molecule. Here, the lowest possible ECS values are chosen. For those of the PCBs and PCNs that lack reported literature values, the ECS have been approximated to the same values as those reported for similarly chlorinated congeners.
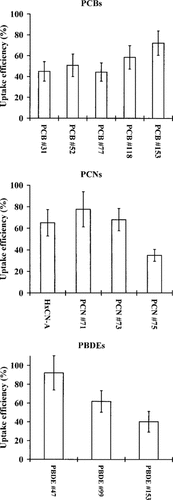
Uptake efficiencies of the different substances. Bars represent 95% confidence intervals.
No correlation was found between E for all compounds and log Kow, ECS, or molecular weights in the present study. Therefore, the conclusion must be that none of these parameters alone can explain the efficiency of dietary uptake. However, within the different groups of compounds there are two different trends. For the PCBs, E is positively correlated with degree of halogenation, while for the PCNs and PBDEs E is negatively correlated with degree of halogenation. The reason for these correlations is unknown and the degree of halogenation of these substances is correlated with several different parameters, such as size, molecular weight, and lipophilicity, of which all could have importance for uptake by organisms.
Membrane permeation
Opperhuizen et al. [2] reported that molecules with ECS >9.5 A, for example, PCNs 73 and 75, were not taken up by gills because permeation in the cell membranes of the gill cells of different fish species was prevented. Opperhuizen and Sijm [4] in a literature study of dietary uptake of polychlorinated dibenzo-p-dioxin/polychlorinated dibenzofurans (PCDD/Fs) stated that dietary uptake of HOCs was essentially restricted by the 9.5-Å limit even if the membranes of the cells in the GI tract might have a higher fluidity than the membranes of the cells in the gills due to a difference in lipid composition. The proposed hindered dietary uptake of highly halogenated HOCs fit the observations of several earlier studies of bioaccumulation of HOCs from dried fish food [24-27]. Also the authors of these studies suggested that uptake through biological membranes was blocked for the large molecule substances by steric hindrance. The results in the present study differ from those in the earlier studies. PCNs 73 and 75 with ECS of 9.7 and 9.8 have dietary uptake efficiencies of 68% and 35%, respectively. PCN 71, with an ECS of 9.7, had a dietary uptake efficiency of 78%. PBDE 99 and 153 that have ECS of 9.6 Å had dietary uptake efficiencies of 62% and 40%, respectively. Consequently, the way of administrating the HOCs in the present study seems to have enabled the uptake of HOCs that would not have been assimilated from dried fish food.
In one of the studies included in the literature study [4] by Opperhuizen and Sijm, octachloradipenz-p-dioxin (OCDD) was reported to have been taken up from food despite ECS >9.5Å. Opperhuizen and Sijm [4] suggested that this unexpected uptake might have occurred due to mediated uptake, enabled by the oil carrier that had been used when exposing the fish in that particular study. The high lipid concentration of the food was proposed to have caused this mediated uptake. In the present study, the HOCs were dissolved in fish lipids. The lipid content of the spiked prey fish was however only approximately 3% w/w (fresh weight) i.e., far from that of pure oil. Accordingly, it does not seem likely that the lipid concentration per se enabled mediated uptake that would not have occurred during feeding with dried fish food. If there is a mediated uptake mechanism that was enabled in the present experimental set-up and that is not working in experimental set-ups using pellet feeding, it seems more likely that it is the dissolving of the HOCs in the rainbow trout lipid extract that has enabled it. When spiking pellets, the studied HOCs are often dissolved in an organic solvent that is added to the pellets; the solvent is then evaporated. There is a possibility that the studied substances by this means are adsorbed to the pellets rather than absorbed into lipids. There might therefore be a difference in how closely associated the HOCs are to the food matrix, leading to differences in uptake efficiency during food assimilation. The proposed mediated uptake may, due to this difference, work more efficiently when exposing fish to HOCs in fish lipid extract than in dried fish food.
The way of exposing fish in the present study can be considered to mimic the situation under natural conditions better than pellet feeding because the HOCs present under natural conditions can be expected to be dissolved in the lipid pools of the fish. This conclusion is strengthened by the observed presence of some large molecule (ECS > 9.5 Å) anthropogenic HOCs in samples of biological material from the environment. For example, uptake in biota has been reported for OCDD [5], PCNs 71 and 73 [7] and PBDE 99 [6]. The injected lipids in the trout of the present study might be somewhat more bioavailable than lipids of the natural lipid pools of the trout, leading to quantitative overestimates of the calculated E values. However, because the digestibility of fish lipids in fish is generally high (85–96%) [28], this effect should not severely change the interpretations.
In fish samples from the environment, Jansson et al. [6] reported a lower relative abundance of the highly brominated congeners compared with the commercial products believed to be responsible for the PBDE contamination. In fish consumers such as osprey (Pandion haliaetus), ringed seal (Pusa hispida), and grey seal (Halicherus grypus) this difference in relative abundance was even bigger. This pattern is also reflected in the results of the present study. Consequently, the mechanisms of dietary uptake of PBDEs in fish, mammals, and birds seem to share common properties with respect to selectiveness.
In teleost fishes, dietary absorption of lipids has been shown to be diffusive, driven forward by the concentration gradient that is maintained by the resynthesis of lipids from absorbed lipid constituents within the enterocytes [29, 30]. In several vertebrates, other dietary uptake mechanisms than diffusion from the food into the organism are known to occur. Part of the uptake of fatty acids, monoglycerides, and phospholipids, for example, have been shown to be mediated by proteins in the cell membranes of the epithelial cell in the intestine [11, 15]. The uptake of cholesterol was shown by Thurnhofer and Hauser [12] to be protein mediated. The membrane protein that was reported by Stremmel [11] to be involved in lipid uptake was apparently also active, using ATP as an energy source. Stremmel [11] also suggested that other substrates such as hydrophobic vitamins could be cotransported with lipids from the food into the cells of the GI tract. The same mechanism might also result in dietary uptake of HOCs that would not penetrate the cell membranes through diffusion because of their large molecular size. Because HOCs can be expected to be associated with lipids and fatty acids, part of the HOC uptake might also be mediated. Support for this can be found in a study by Vetter et al. [31] in which it is shown that benzo(α)pyrene is coassimilated with lipids in the killifish (Fundulus heteroclitus).
There is also the possibility that HOC molecules can be associated with proteins and cotransported across cell membranes. In many teleosts, whole proteins are taken up from the food by different mechanisms, for example pinocytosis [13, 14]. Such proteins can have a molecular weight more than two orders of magnitude higher than large molecule HOCs that have been reported not to be taken up in the GI tract because of blocked membrane permeation. If mediated uptake of HOCs occurs, no blocked permeation for substances with ECS >9.5 Å can be expected, whereas HOCs with ECS <9.5 Å might show both diffusive and mediated uptake, resulting in higher total uptake.
Comparison with empirical model
The empirical model for dietary uptake efficiency proposed by Gobas et al. [8] cannot explain the pattern of uptake efficiencies in this study (Fig. 2). In this empirical model, dietary uptake of HOCs is assumed to be diffusive and controlled by a series of diffusion resistances in aqueous and lipid phases.
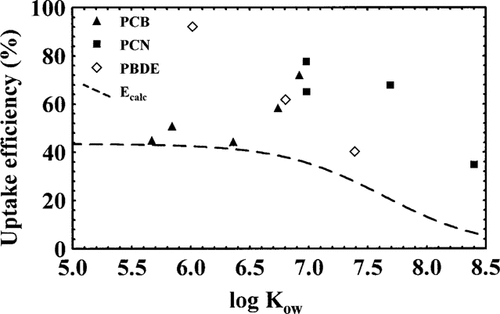
Calculated (as described in Gobas et al. [8], Ecalc) and observed uptake efficiencies of the different substances. The observed values are from the present study.
The relatively inefficient uptake of highly halogenated hydrocarbons was explained by Gobas et al. [8] by the slow diffusion through aqueous phases for highly hydrophobic substances. In Figure 2, the line represents the values of HOC uptake predicted from the empirical model. Apparently, all substances studied in the present study are taken up at higher efficiency than predicted from the empirical model. Some of them, e.g., PBDE 47, PCN 71, and PCB 153, are taken up twice as efficiently as predicted.
The reason for the difference between the predicted E values and the E values reported here might be in the mechanisms of dietary HOC uptake and how they depend on the quality of the food as discussed above. The uptake of HOCs was assumed by Gobas et al. [8] to be exclusively diffusive. The authors do not give further details about the types of food used in the experiments from which the uptake efficiencies were calculated, but it is assumed that the fish were fed with dried fish food pellets as is most often the case. In a later study [9], Gobas et al. showed that the HOC uptake from dried fish food was altogether diffusive.
If a mediated, possibly active uptake of HOCs occurred in the present study, the total uptake should be higher than predicted by the empirical model described above because the diffusive HOC uptake should be working together with the assumed mediated uptake. The results are in accordance with this. All the observed uptake efficiencies in this study are higher than predicted from the empirical model. In fact, most of them are higher than the proposed maximum uptake efficiency of about 43%. Even for the substance with the lowest E in this study, PCN 75, the uptake is substantial. PCN 75 has an E value of 35% in the present study that should be compared with the predicted value of <10%. It does not seem likely that the different forms of food would significantly change the extent of diffusive uptake, under the assumption that the digestibility of the food does not differ significantly.
The lack of lipophilicity dependency of the E values reported here is another difference compared to the study of Gobas et al. [8]. This makes the assumption of active uptake in the GI tract even more plausible than diffusive uptake through cell membranes only. Because lipophilicity controls diffusive uptake in the gill, it can also be expected to control diffusive uptake in the GI tract, which was also assumed in the study by Gobas et al. [8]. To confirm the hypothetical mediated uptake (mechanisms unknown), subcellular studies of the uptake in the epithelial cells of the GI tract are needed.
Residual uptake

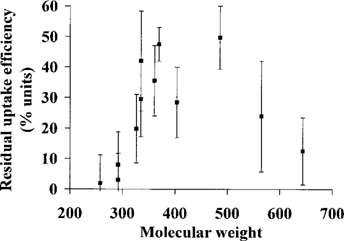
Residual uptake efficiencies of the different substances. Bars represent 95% confidence intervals.
It should be noted that the extent of the suggested mediated HOC uptake as calculated here is probably an underestimate. If such an uptake occurs in the same or a more proximal part of the GI tract than the diffusive HOC uptake, the HOC concentration in the GI tract is subsequently reduced and accordingly so is the diffusive uptake. It is implied in the calculation of Eres that the extent of the diffusive uptake is not reduced by the suggested mediated uptake.
The results of the present study imply that the composition of the diet may have a greater importance on the absorption of HOCs in fish than supposed previously. This issue is important and needs further scientific attention.
Acknowledgements
The authors are grateful to Barrie Webster for reviewing the manuscript. The Swedish National Research Council provided financial support for this study.




