Comparison of three marine screening tests and four Oslo and Paris commission procedures to evaluate toxicity of offshore chemicals
Abstract
The results from the screening toxicity tests Artemia salina, Microtox®, and Mitochondria RET test were compared with those obtained from OSPAR (Oslo and Paris Commissions)-authorized procedures for testing of offshore chemicals (Skeletonema costatum, Acartia tonsa, Abra alba, and Corophium volutator). In this study 82 test substances (26 non-water soluble) were included. The Microtox test was found to be the most sensitive of the three screening tests. Microtox and Mitochondria RET test results showed good correlation with results from Acartia and Skeletonema testing, and it was concluded that the Microtox test was a suitable screening test as a base for assessment of further testing, especially regarding water-soluble chemicals. Sensitivity of Artemia salina to the tested chemicals was too low for it to be an appropriate bioassay organism for screening testing. A very good correlation was found between the results obtained with the Skeletonema and Acartia tests. The results indicated no need for more than one of the Skeletonema or Acartia tests if the Skeletonema median effective concentration or Acartia median lethal concentration was greater than 200 mg/L. The sediment-reworker tests (A. alba or C. volutator) for chemicals that are likely to end up in the sediments (non-water soluble or surfactants) should be performed, independent of results from screening tests and other OSPAR species.
INTRODUCTION
North Sea operators have been faced with more stringent regulations concerning the use of chemicals since 1989. The Oslo and Paris Commissions (OSPAR) have proposed a test system for all chemical products used offshore. Round-robin tests of various marine toxicity tests in 1990 through 1993 were initiated by the Paris Commisson (PARCOM) [1, 2] for development of standardized toxicity test systems for offshore chemicals to be used in the North Sea. The PARCOM has concluded that all offshore chemicals/products must be tested with one alga, one herbivore, and one sediment-reworking species or one fish species. Products that are likely to end up in the sediment (non-water soluble or surface active) must be tested with a sediment reworker, and products that are ideally water soluble must be tested with a fish test (Scophtalmus). In the round-robin test a large number of species were used, and the species Skeletonema costatum, Acartia tonsa, and Corophium volutator were choosen as standard test organisms for alga, herbivore, and sediment reworker, respectively.
The uses of resources related to OSPAR requirements (whether some of the tests could be omitted) should be more closely evaluated. An initiative was taken in 1991 by the Norwegian Oil Industry Association (OLF) to evaluate screening toxicity tests in comparison with OSPAR tests for determination of acute and chronic marine toxicity of offshore production chemicals. This project was carried out by Aquateam. Different screening toxicity tests were compared with OSPAR's authorized procedures for testing of offshore chemicals. The screening tests are simple and will give the test result after a short time, whereas the OSPAR tests are more time consuming and expensive. The OLF project [3] included three screening-test candidates (Artemia salina, Microtox®, and Mitochondria RET test), and two OSPAR tests (S. costatum and Acartia tonsa 24 h). In addition, from 1991 to 1994, Aquateam's laboratory performed toxicity tests on a large number of chemicals and evaluated test results from a large number of OSPAR tests, including two other test species (the sediment reworkers Abra alba and C. volutator) as well as screening tests for different oil companies and chemical suppliers. In an internal Aquateam project, experiences from all these tests have been evaluated.
The purpose of this project was to evaluate the suitability of the screening toxicity tests and compare the screening-test results with results from the OSPAR authorized test, and to compare the results obtained from the different OSPAR authorized tests. Because only the 24-h Acartia test was performed in the period when most of the screening tests were performed, it was also decided to compare results from the Acartia 24- and 48-h test.
MATERIALS AND METHODS
The study included 82 test substances of which 26 were non-water soluble. All substances were tested in the period 1991 to 1994. The following categories of chemicals were tested: scale inhibitors, corrosion inhibitors, biocides, emulsion breakers, and flocculants. The test methods used follow.
Microtox®
The Microtox test is a rapid screening test that gives results within few hours. The Microtox system [4] is a simple standardized toxicity test system that utilizes a suspension of marine luminescent bacteria (Photobacterium phosphoreum) as the bioassay organism. Two replicates for each test concentration were used. The water-accommodated fraction of the non-water-soluble chemicals was tested.
Mitochondria RET test
The Mitochondria RET test is a rapid screening test. The test is described by Blondin et al. [5]. An organelle, mitochondria from bovine heart, is used. The bioassay is based on the phenomenon of energy-coupled reverse electron transfer (RET). The RET responses permit rapid, simple, and sensitive detection of acute toxicity by spectrophotometric recording of the rate of NAD+ reduction. The production of NADH is then measured at 340 nm, so that inhibition of NAD+ reduction is defined as the toxic response. At least two replicates for each test dosage were used. The non-water-soluble chemicals were dissolved in ethanol prior to the test.
Artemia salina 24-h lethality test
The test procedure is described by Solbakken [6]. The bioassay organism is the brine shrimp. The test is easy, as the dry eggs can be obtained from pet shops at any season, and the eggs are hatched after 2 d. Dead and living animals can easily be separated. Three replicates of 50 animals each were used for each test dosage. The test was performed in room temperature and with a light intensity of 1,000 lux. The water-accommodated fraction of the non-water-soluble chemicals was tested.
Skeletonema costatum 72-h growth inhibition test
The test procedure is described in a standard from the International Organisation for Standardisation [7]. The test estimates the growth inhibition of the algae relative to a control. The assay time was 72 h, and the algal growth was measured with fluorescense. The strain NIVA (BAC-1) and algal medium described by Guillard and Ryther [8] was used. The tests were performed in a constant-temperature room with a temperature of 20.0 ± 2.4°C and light intensity of 7,000 to 10,000 lux. Three replicates for each test dosage were used. The water-accommodated fraction of the non-water-soluble chemicals was tested.
Acartia tonsa 24- and 48-h lethality test
The test procedure is described in a draft for a standard from the International Organisation for Standardisation [9]. The bioassay organism is a marine calanoid copepod. Eggs can be stored in the refrigerator. The animals are large enough to be used in the test 14 to 21 d after hatching. The tests were performed with synchronous cultured animals in a constant-temperature room with a temperature of 20.0 ± 2.4°C. Four replicates of five animals each were used for each test concentration. Dead and living animals were counted after 24 and 48 h. The water-accommodated fraction of the non-water-soluble chemicals was tested.
Abra alba test
The test procedure is described by Strømgren [10]. The bioassay organism is the deposit feeder mollusc A. alba. The test parameter is defecation rate during 96 to 120 h of exposure, measured as dry weight of fecal pellets. Twenty-five to 30 animals were used for each test dosage. The test was performed at a temperature of 11°C. The water-soluble substances are added directly to the medium (water + sediment). The non-water-soluble substances were microencapsulated together with sediment particles (the test substance was mixed with concentrated sediment suspension containing gelatine and Acacia gum solution). The A. alba tests were conducted by Prof. Tor Strømgren, Bioconsult, Trondheim.
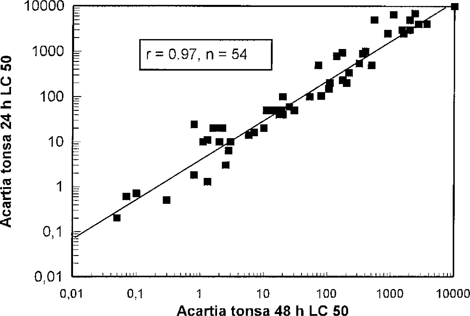
Acartia tonsa 24-h LC50 in relation to A. tonsa 48-h LC50. Each point represents testing of one chemical.
Corophium volutator 10-d lethality test
The test procedure is described in a harmonized protocol from Ministry of Agriculture, Fisheries and Food, UK, and Environment & Resources Technology, UK [11]. The bioassay organism is the isopod C. volutator, which is a deposit feeder/ sediment reworker. Three replicates of 20 animals or two replicates of 10 animals were used. The animals were exposed to sediment spiked with the test substance for a period of 10 d. The test was performed at a temperature of 15°C.
The test results were compared in correlation plots. A spreadsheet program, LOTUS 1-2-3 version 5.1, was used. The correlation coefficient was calculated for both logarithmic and linear function. A simplified test set-up for testing during product development is proposed.
RESULTS AND DISCUSSION
The test results obtained from the screening tests were compared with test results from the OSPAR A. tonsa test. Test results for Acartia are given after 24-h, because only a 24-h test was performed in the period when the screening tests were performed. However, a significant correlation (99.9%) between median lethal concentration (LC50) values after 24 and 48 h was found (Fig. 1). The lethal doses (dosage [LC50] multiplied by exposure time) obtained in the 48-h test were slightly lower than for the 24-h test (Fig. 2). This was an expected result, because the prolonged exposure in tests with no feeding was expected to contribute to the lethality. No differences were observed between water-soluble and non-water-soluble chemicals.
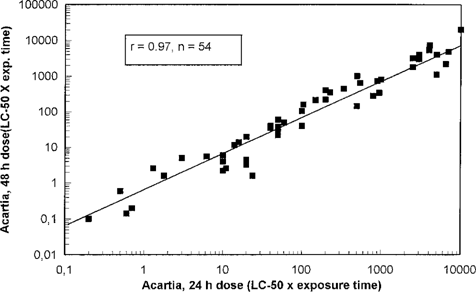
Acartia tonsa 48-h dose (LC50 × exposure time) in relation to A. tonsa 24-h dose. Each point represents testing of one chemical.
| Correlation coefficient (r) | |||
|---|---|---|---|
| Test | Number | Logarithmic scale | Linear scale |
| Artemia vs. Acartia (all chemicals) | 14 | 0.71 | 0.42 |
| Artemia vs. Acartia (water-soluble chemicals) | 10 | 0.63 | 0.34 |
| Microtox® vs. Acartia (all chemicals) | 14 | 0.85 | 0.54 |
| Microtox vs. Acartia (water soluble chemicals) | 10 | 0.89 | 0.49 |
| Mitochondria vs. Acartia (all chemicals) | 11 | 0.89 | 0.91 |
| Mitochondria vs. Acartia (water-soluble chemicals) | 7 | 0.96 | 0.90 |
- a EC50 = median effective concentration, LC50 = median lethal concentration.
Table 1 and Figures Fig. 3.-Fig. 5. show the results from the screening tests compared to the Acartia 24-h test. No good correlation was found between A. salina and Acartia (Fig. 3). Artemia generally showed a lower sensitivity to the toxic agents than did the other toxicity tests. The LC50 values were high (LC50 > 100 mg/L) for all tested chemicals except for two.
A significant correlation (99.9%) was found between Microtox and Acartia (Fig. 4), and an even better correlation occurred between Mitochondria and Acartia (Fig. 5). The correlation was slightly better if only test results from water-soluble chemicals were used. However, the number of chemicals tested was too low for drawing any final conclusions. The non-water-soluble chemicals were found to be more toxic relative to Microtox and Mitochondria than to Acartia. This could be explained by the different sample preparation methods used in the different tests. In the Mitochondria test, the non-water-soluble test substances were dissolved in ethanol prior to testing, and the toxic agents were more available than in the Acartia test where only the water-soluble fraction of the chemicals was used. The low median effective concentration (EC50) values obtained in some of the Microtox tests compared to the Acartia tests are difficult to explain.
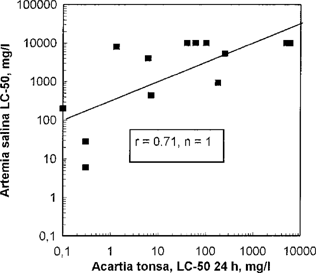
Artemia salina LC50 in relation to Acartia tonsa 24-h LC50. Each point represents testing of one chemical.
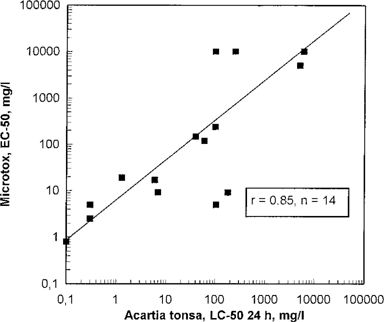
Microtox EC50 in relation to Acartia tonsa 24-h LC50. Each point represents testing of one chemical.
The EC50 values from the Microtox and Mitochondria tests were similiar with low-toxicity chemicals; however, Microtox was found to be the most sensitive of the two screening tests. These two screening tests seem to be a good indicator of toxicity levels, especially for water-soluble test substances. If the EC50 value of Mitochondria or Microtox was > 10,000 mg/L, the Acartia 24-h LC50 was found to be ≪2,000 mg/L and the Acartia 48-h LC50 was found to be ≪1,000 mg/L (Figs. Fig. 4., Fig. 5.). In such cases it should not be necessary to test for alga and herbivore to categorize chemicals. If the EC50 value of Microtox was >2,000 mg/L, the Acartia 24-h LC50 value was found to be >400 mg/L and the Acartia 48-h LC50 value was found to be >200 mg/L. For more toxic chemicals, OSPAR tests with organisms on different trophic levels will be necessary. Microtox was found to have a higher sensitivity than Mitochondria to toxic agents, especially for lower EC50 values. Microtox EC50 was approximately one-tenth of Mitochondria EC50.
The round-robin chemicals were tested with Microtox and the results were compared to results from OSPAR tests by Whale et al. [12]. It was concluded that the Microtox test compared favorably with all the other test procedures in respect to its sensitivity.
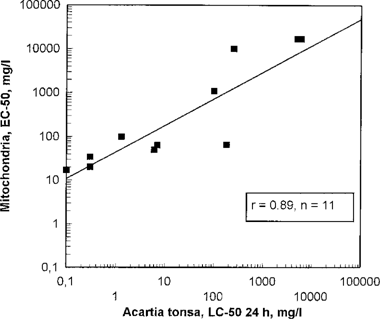
Mitochondria EC50 in relation to Acartia tonsa 24-h LC50. Each point represents testing of one chemical.
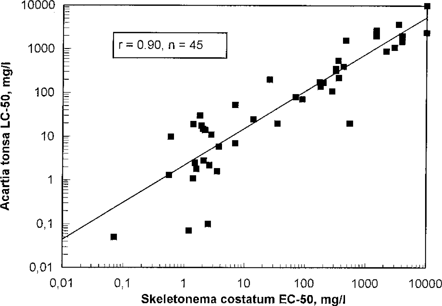
Acartia tonsa 48-h LC50 in relation to Skeletonema costatum EC50. Water-soluble and non-water-soluble chemicals. Each point represents testing of one chemical.
Figure 6 and Table 2 show relations between Acartia 48-h LC50 and Skeletonema 72-h EC50 values. There is a significant correlation (99.9%) between these results. The correlation was especially good for water-soluble chemicals. The Skeletonema EC50 72-h and the Acartia LC50 48-h results obtained were fairly equal, especially for chemicals with low toxicity. Table 2 shows correlation coefficients for chemicals with high and low toxicity, and for all chemicals within these categories, and for only water-soluble chemicals. These results indicate that it should not be necessary to test chemicals with more than one of these organisms if the first test gives a LC50 or EC50 value >200 mg/L. For chemicals with higher toxicity, the test results showed higher variability, and because the chemicals will be classified as toxic if the EC50 or LC50 are lower than 100 mg/L, testing with both an alga and a herbivore are needed.
In Figures Fig. 7., Fig. 8. test results from the sediment reworkers A. alba (5-d EC50) and C. volutator (10-d LC50) were compared with Acartia (48-h LC50). No correlations were found. Both sediment reworkers were found to have a lower sensitivity to the toxic agents compared to the herbivore Acartia. Interpretation of the test results from the sediment reworker tests are not easy, and the test results cannot be directly compared. Most of the tested chemicals showed an A. alba EC50 value > 100 mg/kg sediment or a C. volutator LC50 value >1,000 mg/kg sediment. Unfortunately, none of the chemicals in this study was tested with both Abra and Corophium. Results from the round-robin test with sediment-reworking species in 1993 [2] indicated that C. volutator is more sensitive than A. alba to these chemicals, but no good correlations were found. The toxicity was found to be generally higher for non-water-soluble chemicals than for water-soluble ones, as expected, because the non-water-soluble chemicals are more likely to end up in the sediment. The sediment in a Corophium test was spiked with the test substance, and the water-soluble chemicals/components probably dissolved into the water phase, and were therefore less available as food for the test organism. However, at higher test dosages the water-soluble chemicals could affect Corophium through ingestion with the sediment or by mechanisms other than by feeding. In the Abra test, the non-water-soluble substances were microencapsulated together with sediment particles, and should therefore be more easily available as food for the organisms. Sediment reworker tests must be performed for all chemicals that are likely to end up in the sediment, independent of results from screening tests or other OSPAR tests.
| Correlation coefficient (r) | |||
|---|---|---|---|
| Tests | Number | Logarithmic scale | Linear scale |
| All chemicals | 45 | 0.90 | 0.82 |
| All soluble chemicals | 29 | 0.92 | 0.79 |
| Chemicals with Skeletonema EC50 < 100 mg/L | 24 | 0.66 | 0.83 |
| Water-soluble chemicals with Skeletonema EC50 < 100 mg/L | 17 | 0.70 | 0.79 |
| Chemicals with Skeletonema EC50 > 100 mg/L | 16 | 0.71 | 0.92 |
| Water-soluble chemicals with Skeletonema EC50 > 100 mg/L | 12 | 0.81 | 0.60 |
- a LC50 = median lethal concentration, EC50 = median effective concentration.
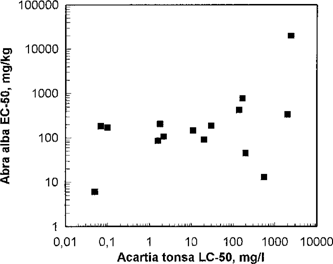
Abra alba EC50 in relation to Acartia tonsa 48-h LC50. Each point represents testing of one chemical.
CONCLUSION
The Microtox test was shown to be one of the most sensitive of the three screening tests. Microtox results also showed a good correlation with results obtained from Acartia and Skeletonema testing of the same products.
The Mitochondria RET test was found to have comparable sensitivity to that of the Microtox test and showed a better correlation with results from Acartia tests. In the Mitochondria test, the non-water-soluble test substances, however, were dissolved in ethanol prior to testing, and the sample preparation method was not comparable with the one used for the Skeletonema, Acartia, and Microtox tests.
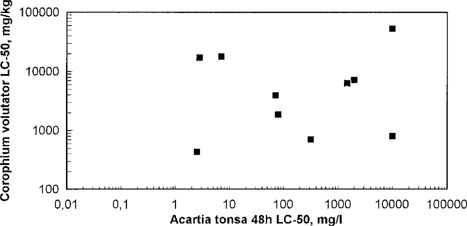
Corophium volutator LC50 in relation to Acartia tonsa 48-h LC50. Each point represents testing of one chemical.
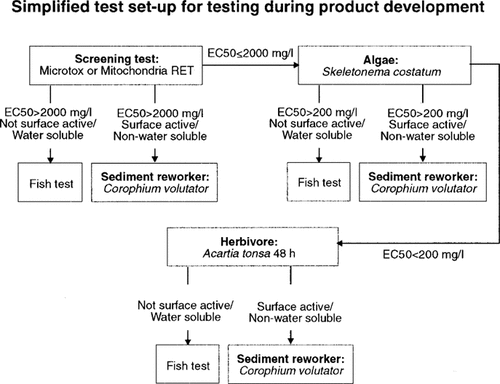
Proposed test setup for testing during product development.
Artemia salina was not found to be an appropriate bioassay organism for screening testing of offshore chemicals because of the low sensitivity to chemicals that were toxic to the OSPAR species.
It was concluded that the Microtox and Mitochondria tests were the most suitable screening tests as a base for assessment for further testing, especially regarding water-soluble chemicals. Results from testing of a limited number of chemicals (n = 14) showed that water-soluble chemicals with very low toxicity, Microtox and Mitochondria EC50 > 10,000, gave Acartia 24-h LC50 ≪2,000 mg/L and Acartia 48-h LC50 ≪1,000 mg/L, and Skeletonema 72-h EC50 > 1,000 mg/L. If a Microtox EC50 value was >2,000 mg/L, the Acartia 48-h LC50 was found to be >200 mg/L. Thus, alga or herbivore testing of water-soluble chemicals should not be necessary before the final classification of the chemical.
A very good correlation also exists between the results obtained with the Skeletonema and Acartia tests, especially for chemicals with low toxicity. For chemicals with higher toxicity (EC50 or LC50 <200 mg/L), the correlation was not as good; the test results showed larger variability, and it would probably be necessary to test for both an alga and a herbivore. Test results for a limited number of water-soluble chemicals indicated that if a chemical is tested with either Skeletonema or Acartia and the EC50 or LC50 are >200 mg/L, there is no need for testing with the other. A closer evaluation of the toxicity of different groups of chemicals should, however, be performed to evaluate the uses of resources related to OSPAR requirements for Skeletonema and Acartia testing of all chemicals.
The sediment reworker tests are specially suited for chemicals that are likely to end up in the sediments (non-water-soluble chemicals or surfactants). This test should always be performed, independent of any results obtained from screening tests or other OSPAR species.
For the Acartia test, the lethal doses (LC50 multiplied by exposure time) were approximately equal for 24-h and 48-h tests.
Based on this study a simplified test setup for testing during product development is proposed (Fig. 9).
Acknowledgements
We would like to thank the Norwegian Research Council for financial support.




