Interactive effects on the erod-inducing potency of polyhalogenated aromatic hydrocarbons in the chicken embryo hepatocyte assay
Abstract
The chicken embryo hepatocyte-7-ethoxyresorufin O-deethylase (EROD) assay is used as a method to measure the amount of 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) equivalents (TEQs) in environmental samples. A common feature of EROD-induction assays in vertebrates is that they generate biphasic dose-response relationships that show dose-related increases of the EROD induction to a maximum activity, followed by a dose-related decrease at higher concentrations. In general, the maximum achievable enzyme activity decreases with increasing median effective concentration (EC50). This suggests that aryl hydrocarbon (Ah)-receptor binding affinity is not the only factor determining the enzyme activity. An additional factor can obscure the maximum EROD activity (Ymax) and EC50 of the enzyme activity. Cytotoxicity and competitive inhibition are ruled out as possible influencing factors. Coadministration of 2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl (PCB 153) and 2, 3, 7, 8-TCDD significantly reduces the EC50 value compared to administration of TCDD alone. The dose-related decrease at higher concentrations has been suggested to be induced by mechanisms other than the Ah-receptor-related mechanism responsible for the observed increases at low concentrations. These interactive effects have serious consequences for risk assessment based on bioassay-derived TEQs.
INTRODUCTION
Polyhalogenated aromatic hydrocarbons (PHAHs) include polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs). Polychlorinated biphenyls are products of industry and are used as dielectric fluids, flame retardants, plasticizers, and lubricants. Polychlorinated dibenzo-p-dioxins and PCDFs are by-products in PCB and herbicide production and are formed during combustion processes. Because of their lipophylic nature and resistance to metabolic breakdown, these compounds are retained in body lipids and accumulate in foodchains. Polychlorinated dibenzo-p-dioxins, PCDFs, and PCBs are found in all compartments of the environment and are a potential risk to humans and wildlife [1-5]. Toxic potencies of environmental mixtures can be expressed compared to the most toxic compound, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), in TCDD equivalents (TEQs). Total TEQ concentrations can be determined using a bioassay in which a mixture is compared with TCDD with respect to the induction of an aryl hydrocarbon (Ah)-receptor-mediated effect. An example of such a bioassay is the measurement of Ah-receptor-mediated cytochrome P4501A1 (CYP1A1) induction by dioxinlike compounds in vivo or in vitro, using 7-ethoxyresorufin O-deethylase (EROD) activity as an indicator. Ethoxyresorufin is specifically metabolized by CYP1A1 [6]. A good correlation has been found between CYP1A1 induction in cultured hepatocytes and other toxic endpoints in in vitro as well as in in vivo studies [7, 8]. It is questioned whether the bioassay-derived TEQs represent Ah-receptor-mediated effects only. At present the chicken embryo hepatocyte bioassay as described by Kennedy et al. [9] is in use as a tool for measuring the potential toxicity of environmental samples. The assay is based on the cellular response on in vitro exposure to Ah-receptor-active compounds. 7-Ethoxyresorufin O-deethylase is used as the effect parameter. The dose-response relationships of the EROD assay in chicken primary hepatocyte cultures are biphasic. The EROD activity increases with an increasing concentration of a dioxinlike compound at first, followed by a decrease at higher concentrations. This decrease appears to be a common vertebrate response following exposure to high levels of diox-inlike compounds. The decrease has been shown in several studies carried out in a number of species and systems, including mammals, birds, fish, and cultured cells from each of these as reviewed by Hahn et al. [10]. Kennedy et al. [11] showed also a decrease in maximum enzyme activity with increasing median lethal concentration (EC50) for most of the compounds tested, which could not be explained by the structure-related in vitro activities of dioxinlike compounds. It was suggested that Ah-receptor-binding affinity is not the only factor determining the enzyme activity, but that additional factors play a role as well. Several groups have already noticed the biphasic course of the dose-response relationships for EROD activity induced by dioxinlike compounds [10, 12, 13]. However, none of them have been able to give a sound explanation for this biphasic course. Several explanations have been suggested. Gooch et al. [12] and Hahn et al. [10] suggested that a possible negative effect of the dioxinlike compound on the overall functioning of the cell could be responsible for the low enzyme activities observed at higher concentrations. Another explanation was postulated by Gooch et al. [12], who suggested that the inducing compound may compete with ethoxy-resorufin for the substrate binding places on the CYP1A1 enzyme. Indirect inhibition of the enzyme activity because of a blockage of heme synthesis has been suggested as well [13, 14]. The consequences of the various explanations are rather large. The dose-response curves and associated EC50 values are then the result of both Ah-receptor-related and nonrelated mechanisms. As a consequence, the assay-derived TEQs no longer represent only the “dioxinlike” potency of Ah-receptor-active compounds in the mixture.
In this study experiments have been performed to further define the cause of the decrease in enzyme activity at high concentrations and the decrease in maximum enzyme induction with increasing EC50 of dioxinlike compounds. The emphasis has been placed on possible cytotoxicity caused by TCDD during incubation; competitive inhibition of EROD activity by TCDD during the assay; and the consequences of coadmin-istration of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153), as a model compound for non-Ah-receptor-active PCBs, and TCDD with respect to the maximum activity level, and EC50 values.
MATERIALS AND METHODS
Chemical EROD assays
Krebs-Ringer buffer (KRB) contained NaCl (279 mg/L), KCl (8,970 mg/L), CaCl2(H2O)2 (149.5 mg/L), MgSO4(7H2O) (285 mg/L), KH2PO4 (157.5 mg/L), and NaHCO3 (210 mg/L) in double-distilled water. Albumin solution contained bovine serum albumin (BSA; 20 g/L) in KRB. The chemicals used for these solutions were from Sigma Chemical Co. (St. Louis, MO, USA). Red blood cell-lysing (RBL) solution was adjusted at pH 7.65 with 1 N HCl and contained Tris-base (Boehringer, Mannheim, Germany) (2.1 g/L), NH4Cl (Merck, Darmstad, Germany) (6.96 g/L), and KHCO3 (Boehringer, Mannheim, Germany) (1.0 g/L) in double-distilled water. The enzymatic breakdown of liver tissue was performed using a KRB solution containing hyalronidase and collagenase (Sigma Chemical Co.) (500 mg/L and 1,000 mg/L, respectively). Waymouth 705/1 medium from Gibco BRL (Life Technologies, Gaithersburg, MD, USA) was used (13.86 g/L in double-distilled water) and enriched with Na2CO3 (2.24 g/L), penicillin-streptomycin (Gibco BRL, Life Technologies) (100.000 units/L), 1-thyroxin (Sigma Chemical Co.) (1 mg/L), and insulin (Sigma Chemical Co.) (1 mg/L). All solutions were filter-sterilized using a 0.45-μm Milex-GV filter from Millipore Intertech (Malborough, MA, USA). Compounds administered to the cultured hepatocytes during incubation were TCDD (supplied by Schmidt, Amsterdam, The Netherlands), and PCB 153 (supplied by Promogem GmbH, Wesel, Germany).
Incubation of the eggs and preparation of the primary hepatocyte cultures
White leghorn chicken eggs were purchased from the National Institute for Public Health and Environmental Protection/RIVM (Bilthoven, The Netherlands). The eggs were artificially incubated in an automatic incubator (Olba, Oosterhesselen, The Netherlands) at 37°C and 55% humidity. The eggs were turned automatically every hour. After 19 d of incubation the chicken embryos were sacrificed. Following decapitation, the livers were dissected. The preparation of the primary hepatocyte cultures was performed according to Kennedy et al. [9]. Briefly, pooled livers were cut in small pieces and incubated for 45 min at 37°C with 75 ml of a hyalronidase-collagenase solution. After incubation, hepatocytes were separated from tissue debris by filtration, and washed using a freshly prepared KRB and albumin solution. The cells were then resuspended in 10 ml RBL solution, incubated for 5 min, and centrifuged for 5 min at 300 g using a Labofuge (Heraeus Sepatech GmbH, Osterode, Germany). After this treatment, the cells were washed with KRB and resuspended in medium. The cells: medium ratio was 1:19 (v/v). The cells were cultured in 48-well plates (Costar, Cambridge, MA, USA) containing 500 μl medium per well. Each well received 25 μl of the cell suspension. The plates were placed in a B 50/60 EC/02 incubator (Hereas Sepatech GmbH). Conditions were set at 37°C and 5% CO2. After 24 h preincubation, the medium was replaced and 2.5 μl dimethyl sulfoxide (DMSO) with the test compound(s) or 2.5 μl DMSO only (control) was administered. On each plate one compound was tested using a control and 14 different compound concentrations, all dosed in triplicate. The compound concentration in the culture medium ranged from 3.3 × 10−5 to 3.3 × 104 nM. For each hepatocyte culture two plates were dosed with only 2.5 μl DMSO per well for protein content measurement and resorufin fluorescence standards, respectively. After 24 h of incubation the medium was removed and plates were stored at −70°C until EROD analysis.
EROD assays
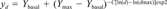
The method for the multiwell fluorometer protein assay was described by Lorenzen and Kennedy [15]. Briefly, fluoresce-min is added to each well, and fluorescence is measured using a 400/35-nm excitation and a 460/40-nm emission filter. Protein concentrations were calculated using BSA solutions in an empty plate as a standard. Protein content of wells exposed to dioxinlike compounds was assumed to be equal to the mean protein content of a DMSO-dosed plate.
Mixture interactions
To study interactions of TCDD and a non-Ah-receptor-active PCB during the incubation, mixtures of varying concentrations of TCDD and PCB 153 were made. The molar ratio between TCDD and PCB 153 was 1 to 10,000, based on ratios found in environmental samples and as reviewed by Bosveld and van den Berg [16]. For incubation 2.5 μl of the mixtures in DMSO were administered to the cells. Three plates were dosed with TCDD alone, and with a mixture of TCDD and PCB 153 (1:10,000 on a molar basis; seven concentrations, all dosed in triplicate ranging from 5 × 10−5 to 1.65 × 10−1 nM and 5 × 10−1 to 1.65 × 103 nM during incubation in the wells for TCDD and PCB 153, respectively). For both TCDD and TCDD + PCB 153-dosed cells, dose-response relationships were fitted to the TCDD concentration. The Ymax, dmax, and EC50 of the TCDD + PCB 153-dosed cells were compared to the parameters of the TCDD-dosed cells on the same plate.
Competition assays
The influence of the presence of TCDD as a competitive inhibitor of the metabolism of 7-ethoxyresorufin (7-ER) was studied using TCDD-dosed plates. Concentrations of TCDD in the medium during incubation ranged from 0 to 660 nM (seven concentrations). Each concentration was dosed in six-fold. During the EROD assay TCDD dissolved in DMSO was administered to three of the six wells of each of the seven concentrations. The TCDD solution was administered simultaneously with the 7-ER solution. Variations were made with respect to TCDD concentrations in the administered solution. The maximum amount of TCDD added can be compared to a concentration of 105 nM TCDD (0.8% DMSO) in the medium during incubation. This concentration is associated with a 60% reduction of EROD activity compared to the maximum EROD activity in normal TCDD dose-response relationships. Dimethyl Sulfoxide-dosed cells were used as controls. Following preincubation with 7-ER and the additional TCDD, the EROD activity was measured in each well. Separate dose-response curves were fitted for TCDD-dosed cells with and without additional TCDD added to the EROD reaction mixture.
Viability tests
Viability of the cells was tested using the lactate dehydrogenase (LDH) test and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) test. Lactate dehydrogenase is a cytosolic enzyme that is unable to cross the intact cell membrane. The ratio between LDH inside the cells and in the medium is a measure for membrane disruption and associated loss of viability. Metabolism of MTT can be used as an index for mitochondrial respiration and associated cell viability. The MTT is metabolized to formazane, which can be measured with a spectrophotometer. The LDH test was performed basically according to Bergmeijer et al. [17] and was adapted for use with primary hepatocytes in 48-well plates. Lactate dehydrogenase was measured by its catalytic effect on the metabolism of pyruvate to lactate. Reduced nicotinamide adenine (NADH) (Sigma Chemical Co.) was used as a cofactor.
After the usual 24-h incubation of the hepatocytes with various concentrations of TCDD, 300 μl of the 500 μl of medium was taken from the wells. The remaining 200 μl of medium was discarded from the wells and 500 μl of phosphatebuffered saline (PBS) with EDTA and Triton X-100 (Sigma Chemie GmbH, Diesenhofen, Germany) (16.81 mg/L and 500 mg/L, respectively) was added to every well. The plate was shaken at 4°C for 2.5 h, followed by centrifugation of the incubation mixture. Of the supernatant, 300 μl was taken for the measurement of LDH activity. To 300 μl of the supernatant and 300 μl of the medium, respectively, 300 μl of 50 nM phosphate buffer containing 0.6 nM sodium pyruvate and 0.18 nM NADH was added. These mixtures were vortexed. Lactate dehydrogenase activity was quantified by the consumption of NADH over 5 s. Extinction of NADH at 340 nm was measured during 5 s using a Shimadzu (Shimadzu Scientific Instruments, Columbia, MD, USA) spectrophotometer. The results of five successive measurements were averaged. The relative LDH leak was defined as the ratio LDH activity in medium to LDH activity in medium + cells.
Viability tests after incubation of chicken hepatocytes with 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD). The effect of increasing concentrations of 2,3,7,8-TCDD on 7-ethoxyresorufin O-deethylase (EROD) activity (▴), percent lactate dehydrogenase (LDH) leakage (•), and percent 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltrazoliumbromide (MTT) metabolism (○) compared to blank.
The MTT test was performed according to the method of Mosmann [18] as modified by Denizot and Lang [19] and further adapted for use with primary chicken hepatocytes in 48-well plates. After the usual 24-h incubation of the hepatocytes with various concentrations of TCDD, the medium was removed and 500 μl of PBS with 1 mg/ml MTT (Janssen Chimica, Tilburg, The Netherlands) was added. The plate was incubated at 37°C for exactly 45 min. The reaction was stopped by putting the plate on ice. The PBS solution was removed and 500 μl of isopropanol was added to extract the formazane from the cells. The extraction solution was transferred to an Eppendorf cup and diluted with another 500 μl of isopropanol. Extinction of formazane was measured at 560 nm with a Shimadzu spectrophotometer. Isopropanol was used as a blank.
RESULTS
Viability
Both LDH and MTT tests showed no dose-related alterations in viability. Cells dosed with TCDD showed activities comparable to cells dosed with the carrier (DMSO) only. Figure 1 shows the relative LDH leakage and MTT metabolism in combination with the EROD activities as observed in similar TCDD-dosed cells. As shown, the decrease in EROD activity is not associated with any loss of cell viability.
Competition assay
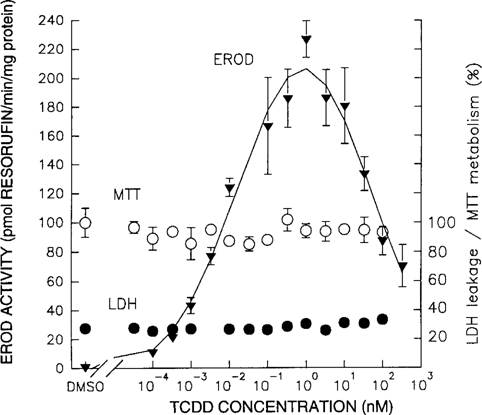
Additional TCDD in the reaction mixture did not inhibit EROD activity compared to the control. Figure 2 shows the dose-response relationships of both the EROD assay in which the maximum amount of TCDD was added, as well as the EROD assay in which just the carrier (DMSO) was added as a control. Similar results were observed when lower TCDD concentrations were administered (data not shown).
Competitive effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) administered during the 7-ethoxyresorufin O-deethylase (EROD) assay. The effect of coadministration of ethoxyresorufin (ER) and 2,3,7,8-TCDD (4.7:1) (□) during the EROD assay on EROD activity, compared to administration of only ER (○).
Mixture interactions
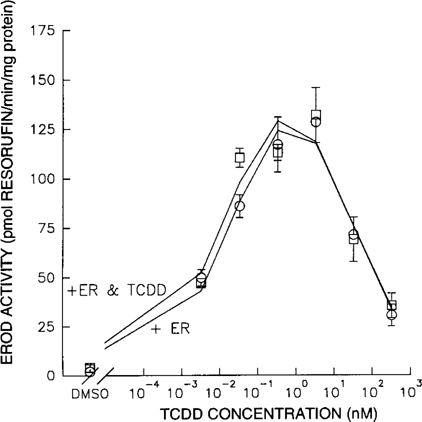
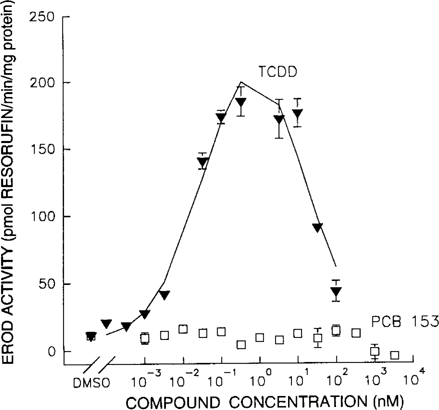
Both TCDD and PCB 153 were individually tested in the EROD assay. Cells exposed to TCDD showed a dose-related effect on EROD activity, and an average EC50 of 0.02 nM was calculated for the observed relationships. Cells exposed to PCB 153 did not show any dose-related increase of EROD activity (Fig. 3). Coadministration of TCDD and PCB 153 (TCDD:PCB 153 = 1:10,000) compared to TCDD alone significantly altered the TCDD dose-response relationship (Fig. 4). Coadministration of PCB 153 reduced the EC50, Ymax, and dmax values of TCDD by 59%, 31%, and 77%, respectively (Table 1).
Typical dose-response curves for 7-ethoxyresorufin O-deethylase (EROD) activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) or 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153). The effect of increasing 2,3,7,8-TCDD concentrations (▴) or PCB 153 concentrations (○) on EROD activity.
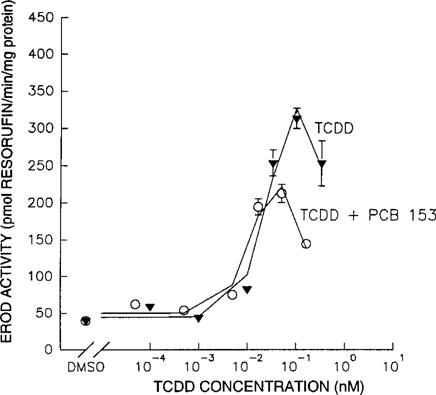
Mixture interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) and 2,2′4,4′,5,5′-hexachlorobiphenyl (PCB 153). The effect of coadministration of 2,3,7,8-TCDD and PCB 153 (1:10,000) (○) on 7-ethoxyresorufin O-deethylase (EROD) activity, compared to administration of only 2,3,7,8-TCDD (▴).
DISCUSSION
The LDH test indicates that cell viability is not affected by the test compounds in the dose ranges used in our experiments. Similar results were observed when MTT metabolism was used as an indicator for cell viability. These results suggest that the decrease in enzyme activity at high TCDD concentrations is not due to cytotoxicity. This conclusion is in agreement with studies by Gooch et al. [12]. They found that decreased induction of EROD activity in microsomes of PCB 77-exposed scup occurs in the presence of highly induced levels of CYP1A1 mRNA. Comparable results were observed by Rodman et al. [13]. They exposed primary cultures of chicken hepatocytes to PCB 126 and PCB 153 and found no significant LDH release. Even at the highest doses, at which a decrease in EROD activity was observed, no LDH release was found. The results of our study and the ones mentioned above, together with those summarized by Hahn et al. [10], indicate that the decrease of EROD activity at high doses of dioxin like compounds is not caused by cytotoxicity.
| Parameters | Plates | TCDD | TCDD + PCB 153 (1:10,000) | % Decrease |
|---|---|---|---|---|
| EC50 (nM) | 1 | 0.026 | 0.010 | 61.5 |
| 2 | 0.033 | 0.015 | 54.5 | |
| 3 | 0.022 | 0.010 | 54.5 | |
| Average decrease in EC50 | 56.8 ± 4.0 | |||
| Ymax (pmol resorufin/min/mg protein) | 1 | 323.6 | 206.9 | 36.1 |
| 2 | 339.1 | 255.6 | 24.6 | |
| 3 | 326.9 | 224.5 | 31.3 | |
| Average decrease in Ymax | 30.7 ± 5.8 | |||
| dmax (nM) | 1 | 0.183 | 0.026 | 85.8 |
| 2 | 0.356 | 0.060 | 83.1 | |
| 3 | 0.110 | 0.041 | 62.7 | |
| Average decrease in dmax | 77.2 ± 12.6 |
- a The different parameters, median effective concentration (EC50), maximum 7-ethoxyresorufin O-deethylase (EROD) activity (Ymax), and TCDD concentration for Ymax (dmax), are given for three replicate plates on which mixture interactions were tested.
The results of the competition study suggest that competitive inhibition of the inducing compound on the active site of CYP1A1 also is not the cause of the decrease in 7-ER metabolism at high doses of dioxin like compounds. Adding TCDD to the reaction mixture of the EROD assay did not result in a decrease in EROD activity compared to the normal EROD assay. In contrast to these results, Gooch et al. [12] did find competitive inhibition of EROD activity in microsomes of scup liver when PCB 77 was added to the reaction mixture. However, they also note that James and Little [20] did not find inhibition by PCB 169 of arylhydrocarbon hydroxylase (AHH) activity in vitro in the sheepshead, a teleost species closely related to scup. It can be concluded that competitive inhibition of EROD activity by a dioxinlike compound may occur. However, at the conditions used in the chicken embryo hepatocyte assay, this inhibition is not found to influence the final outcome.
As is shown in Figure 3, TCDD induces CYP1A1 activity in primary chicken hepatocytes, whereas CYP1A1 activity is shown to be unaffected by PCB 153. Based on the additivity of the effects of dioxinlike compounds, the effect of a mixture of TCDD and PCB 153 should just reflect the enzyme induction of TCDD. However, coadministration of PCB 153 with TCDD significantly reduced the Ymax, dmax, and EC50 values of TCDD. Compared to TCDD, the dose-response curves for the mixture show quantitatively similar relationships at the low dose levels. However, maximum EROD activity is reached at a lower TCDD concentration (Fig. 4). The decrease appears to have an earlier onset at higher concentrations of dioxinlike compounds, as previously discussed. This suggests that there is an additional factor, in addition to Ah-receptor-binding affinity, that determines the final outcome measured in this CYP1A1 induction assay.
The results of our study suggest that when extracts of environmental samples are tested in a hepatocyte in vitro CYP1A1 induction assay, the estimated TEQ concentration in the environmental sample will be overestimated when high levels of PCB 153 or related compounds are present in the extract. Environmental mixtures contain relatively high concentrations of di-ortho compounds, such as PCB 153. The PCB-induced inhibition of EROD activity may obscure the determination of the amount of TEQs in environmental samples. This finding may have serious consequences for the use of this bioassay in toxicity testing and risk assessment.
CONCLUSION
The biphasic course of EROD activity is seen in a number of vertebrate species and cultured cells of these species exposed to dioxinlike compounds. From our results it can be concluded that the decrease in EROD activity observed at higher concentrations of the inducing compound is not due to cytotoxicity caused by the administered compounds. Neither is this decrease caused by competitive inhibition of the metabolism of 7-ER by binding of the administered compound to the active site of the CYP1A1 enzyme. In addition, coadministration of PCB 153 and TCDD was found to significantly reduce EC50, Ymax, and dmax values, compared to administration of TCDD alone. Following exposure to a mixture of TCDD and PCB 153, the maximum enzyme activity occurs at a lower TCDD concentration compared to TCDD alone. It is suggested that the dose-related decrease at higher concentrations is, at least in part, induced via non-Ah-receptor-related mechanisms. This may obscure bioassay-derived TCDD-like potencies of environmental mixtures.




