Extrapolating mineralization rates from the ready Co2 screening test to activated sludge, river water, and soil
Abstract
The objective of this research was to derive empirical extrapolation factors by concurrently measuring mineralization rates of nine diverse chemicals in a Ready CO2 test (modified Sturm) and realistic 14C tests using activated sludge, river water, and sludge-amended soil while holding other variables constant. All nine chemicals were mineralized in the Ready test and each of the compartments, but no significant statistical relationships existed between biodegradation rates in the various tests. Mineralization rates in the Ready test were on average 8.1, 2.5, and 1.2 times lower than the rates in realistic activated sludge, river water, and soil tests, but variability in the scaling factors spanned up to 1.5 orders of magnitude. The scaling factors for extrapolating from ready CO2 data ranged from 1.7 to 19 for activated sludge, from 0.1 to 5.6 for river water, and from 0.3 to 2.8 for soil. Correlation analysis revealed that the scaling factors from the CO2 test to activated sludge and river water were related to the quantitative structure-activity relationship based solubility and log Kow estimates for the test chemicals.
INTRODUCTION
Biodegradability has always been considered a desirable attribute for a chemical, but until recently it was rarely incorporated in a quantitative manner into safety assessments. Recent concern about environmental quality and advances in computer models for predicting environmental concentrations, however, have significantly altered how biodegradation data are used and their importance [1, 2]. Models are used increasingly to estimate the removal of chemicals during sewage treatment and to calculate their concentration in various environmental compartments [3, 4]. While biodegradation rates are important input variables to these models, typically the only data available are from standardized biodegradation screening tests, and rates obtained under realistic conditions are nonexistent. The lack of appropriate input data decreases the accuracy and utility of existing models and limits the development of improved models. Thus, it would be highly useful to have a reliable procedure for extrapolating the results of standardized biodegradation tests to actual field conditions.
Although Gericke and Fischer [5, 6] compared the results of a large number of chemicals in various screening tests, there are no well-established theoretical or empirically derived approaches for extrapolating rates from a screening test to realistic activated sludge, river water, or soil. Struijs and Berg [7] proposed an approach to extrapolate results of the Organisation for Economic Cooperation and Development's (OECD's) “Ready” tests based on the assumption that half-life is inversely proportional to the total concentration of microorganisms. A first attempt at validation involved comparing the results of selected field studies, reported in the literature, with positive results in Ready tests. Boethling et al. [8] directly derived factors for extrapolating from one habitat to another based on ratio of literature-reported half-lives for a range of compounds. Reported half-lives covered a wide range, even under similar test conditions, and the calculated ratios varied widely depending on the chemical examined. Given the difficulties of estimating extrapolation factors using literature data obtained under highly variable conditions, there is a need for a consistent data set without confounding variables. Such a data set would be useful for statistically comparing biodegradation rates in various tests and compartments, deriving empirical extrapolation factors, and testing the assumption that half-life is inversely proportional to biomass.
The objectives of this study were (1) to concurrently test a diverse array of biodegradable chemicals in Ready CO2, 14C batch activated sludge, 14C river water, and 14C soil test systems and determine the rates of mineralization under each set of conditions; (2) to use regression and correlation analyses to determine the statistical relationships between mineralization rates obtained in the various test systems; and (3) to develop scaling factors based on these statistical relationships to extrapolate mineralization rates under one set of conditions to the other test conditions or compartments. To control biological variability, the Ready CO2 and 14C river water test samples were inoculated with microorganisms from the same sludge used in the 14C activated sludge test.
MATERIALS AND METHODS
Test chemicals
Aniline, cetyl alcohol, oleic acid, p-nitrophenol, and benzoic acid were obtained from Sigma Chemical Company (St. Louis, MO, USA). Alkyl ethoxylate and alkyl ethoxylate sulfate were commercial Neodols (C45E2.25 and C45E2.25S) obtained from Shell (Houston, TX, USA). Dodecyltrimethyl ammonium chloride (C12TMAC) and C12 glucose amide were synthesized at Procter and Gamble (Cincinnati, OH, USA). U-14C Aniline (62.1 mCi/mmol), 1-14C cetyl alcohol (2.5 mCi/mmol), U-14C p-nitrophenol (6.6 mCi/mmol), and 14C (ring) benzoic acid (13.3 mCi/mmol) were obtained from Sigma. 1-14C Oleic acid (1.85 mCi/mmol) was purchased from ICN Biomedicals (Irvine, CA, USA). 14C-(1,3 ethoxylate) C14E 3 alkyl ethoxylate (4.86 mCi/mmol), 14C-(1,3 ethoxylate) C14E3s alkyl ethoxylate sulfate (3.73 mCi/mmol), 14C-(methyl) C12TMAC (21.6 mCi/mmol), and 14C-(glucose) C12 glucose amide (3.9 mCi/mmol) were synthesized at Procter and Gamble. Radiochemical purity, based on high-performance liquid chromatography or thin-layer chromatography analysis, exceeded 98% for all chemicals except cetyl alcohol and C12TMAC, whose purities exceeded 95 and 91%, respectively.
| Test system | Test chemical concn. | Activated sludge solids (standard plate count) | CO2 trapping agent | CO2 detection |
|---|---|---|---|---|
| Ready CO2 | 20 mg/L | 2 mg/L (5-7 × 106 CFU/L) | 0.025 N Ba(OH)2 | Titration |
| 14C Activated sludge | 1 mg/L | 2,500 mg/L | 1.5 N KOH | LSC |
| 14C River water | 100 μg/L | 2 mg/L (1 × 106 CFU/L) | 1.5 N KOH | LSC |
| 14C Soil | 1 mg/kg | NA | 1.5 N KOH | LSC |
- CFU = colony-forming unit; LSC = liquid scintillation counting; NA = not applicable.
Environmental samples
Activated sludge was obtained from the Downingtown Regional Water Pollution Control Center (DRWPCC) in Downingtown, PA, USA, which receives primarily domestic waste-water. River water was collected from the East Branch of Brandywine Creek, just downstream from the DRWPCC. Soil was obtained from a corn field near Phoenixville, PA, USA. The soil was a silt-loam with a pH of 5.6 that had been last amended with sludge 6 months before sampling. It was classified as an Alfisol.
Experimental design
The nine chemicals were tested concurrently in a Ready CO2, 14C activated sludge, 14C river water, and 14C soil tests at 22 ± 3°C. The concentrations of test chemicals were 20 mg/L in the CO2 test, 1 mg/L in the batch activated sludge test, 0.1 mg/L in the river water test, and 1 mg/kg in the soil test. The inoculum for the Ready test, the activated sludge for the activated sludge test, and the simulated effluent for the river water test were obtained from the same activated sludge sample to control for biological variability. Each test was performed in duplicate, and production of CO2 was monitored over time. Table 1 summarizes key variables in the various tests. First-order rate constants were determined for each chemical in each test using nonlinear regression. Coefficients of variation between the duplicate treatments for these constants on average were 17% for the Ready test, 9% for the activated sludge test, 20% for the river water test, and 7% for the soil test. Scaling factors for each chemical were determined by calculating the ratio of the average first-order rate in one test compared to another. For example, the scaling factor for extrapolating from a Ready CO2 test to a batch activated sludge test equaled the average measured first-order rate in the batch activated sludge test divided by that in the Ready test.
Ready CO2 test
The modified Sturm test was conducted as described by the OECD guidelines [9] with minor modifications. Each test chemical was incubated at a concentration of 20 mg/L in 2 L of minimal salts medium inoculated with 1% settled homogenized activated sludge. The test system consisted of flasks (4 L) on a rotary shaker (110 ± 10 rpm) with the headspaces continually purged with CO2-free air. The headspace gas was subsequently passed through a series of 100-ml CO2 traps containing 0.25 N Ba(OH)2. These traps were titrated with 0.05 N HCl to determine total CO2 evolved at a minimum after 2, 4, 6, 9, 12, 16, 20, and 28 d or more frequently depending on the rate of evolution.
14C activated sludge test
Each test chemical was incubated at a concentration of 1 mg/L with freshly collected activated sludge (1 L) adjusted to a solids level of 2,500 mg/L. The test system consisted of flasks (2 L) on a rotary shaker (110 ± 10 rpm) with the headspaces continually purged with CO2-free air. The headspace gas was subsequently passed through a series of 100-ml CO2 traps containing 1.5 N KOH. Periodically, these traps were analyzed by liquid scintillation counting (LSC) to determine the amount of 14CO2 that had been evolved. Also, at each sampling, 15 ml of mixed liquor was removed, placed into a biometer flask with 2 ml of 1.5 N KOH in the sidearm, acidified with HCl, and incubated overnight. Dissolved 14CO2 was determined by analyzing the KOH in the sidearm of the biometer by LSC. At a minimum, samples were taken after 0.25 to 0.4, 1, 3, 5, 7, 10, 14, 21, and 28 d.
14C river water test
Each test chemical was incubated at a concentration of 100 μg/L with river water (1 L) mixed with 1 ml of supernatant from settled homogenized activated sludge to simulate the biology of the mixing zone below a sewage treatment plant outfall. The final test system contained approx. 2 mg/L of activated sludge solids, which corresponds to a 10% dilution of effluent, which typically contains 10 to 30 mg/L suspended solids, into river water. The test system consisted of 2-L flasks on a rotary shaker (110 ± 10 rpm) with the headspaces continually purged with CO2-free air [10]. The headspace gases were subsequently passed through a series of 100-ml CO2 traps containing 1.5 N KOH. Dissolved and evolved 14CO2 were determined in the same way as described for the batch activated sludge test. At a minimum, samples were taken after 1, 3, 5, 7, 10, 14, 21, and 28 d.
14C soil mineralization test
Each test chemical was incubated at a concentration of 1 mg/kg with sludge-amended soil. The test chemicals were initially dosed to 1 g of dewatered digester sludge solids, which were subsequently mixed with 100 g of soil and adjusted to a 35% water content. The objective was to mimic the introduction of chemicals into soil on sludge. The test system consisted of 500-ml flasks incubated without mixing or agitation and the headspaces continually purged with CO2-free air. The headspace gas was subsequently passed through a series of 100-ml CO2 traps containing 1.5 N KOH. These traps were analyzed by LSC to determine the amount of 14CO2 that had been evolved after 1, 3, 5, 7, 10, 14, 21, and 28 d.
Kinetic analysis of mineralization data
Cumulative CO2 data were fitted to various first-order production equations using nonlinear regression. Regression analyses were performed using Jandel TableCurve 2D (version 2.00) software. These following equations were included:
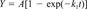
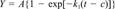
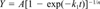
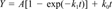
The equations that provided the best fit were identified based on statistical considerations (r2 and number of parameter estimates) and visual inspection of the fit and residuals.
Statistical analyses
Additional statistical analyses included simple linear regression and correlation as well as multiple and stepwise regression. These analyses were performed using Systat® for Windows (Evanston, IL, USA).
RESULTS
Curve fitting
Generally, the first-order logistic function provided the best fit to data generated in the Ready CO2 test. Without exception, this model resulted in an r2 always exceeding 0.99. In the case of the activated sludge and soil mineralization data, the best fit without exception was provided by the three-half-order model without growth [11]. In no case was r2 less than 0.97 for any activated sludge data set or less than 0.94 for any soil test. No single kinetic model accurately described CO2 evolution for all the chemicals in the river water test. The three-half-order model accurately described the mineralization of cetyl alcohol, benzoic acid, and oleic acid, while the mineralization of the remaining compounds was best described by the first-order logistic function. Once again, the fits were excellent, with r2 always exceeding 0.96. Figure 1 shows representative mineralization curves with the fitted functions using aniline as the example.
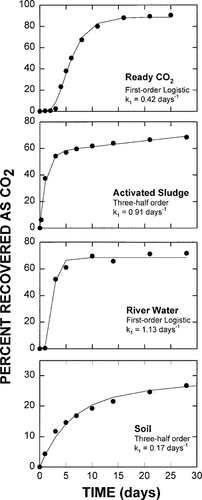
Mineralization data and kinetic functions that provided the best fit for aniline in the Ready CO2 test and in the realistic activated sludge, river water, and soil tests.
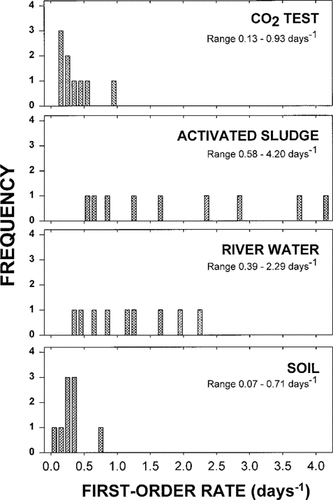
Frequency distribution of first-order rate constants observed for nine diverse chemicals in the Ready CO2 test and in the realistic activated sludge, river water, and soil tests.
Mineralization rates
Figure 2 shows the frequency distribution of first-order mineralization rates observed with the diverse series of compounds in the various tests. First-order rates ranged from a low of 0.07 d−1 in the soil test to as fast as 4.2 d−1 in activated sludge. In the Ready CO2 test, the first-order mineralization rates for the vast majority of the compounds were clustered between 0.13 and 0.6 d−1. In contrast, rates in the activated sludge test were widely dispersed between 0.6 and 4.2 days−1. The rates in river water also were very dispersed, while those in soil were largely clustered between 0.07 and 0.4 d−1. While there was significant overlap in the distribution of rates observed in the Ready CO2 and soil tests, overlap between rates in the CO2 test and activated sludge or river tests was minimal, despite the tests sharing a common inoculum.
Empirical extrapolation factors
Scaling factors for extrapolating from the Ready CO2 to the realistic activated sludge, river water, and soil tests were derived for each compound by dividing the first-order rate in the realistic test by that observed in the screening CO2 test. The frequency distributions for these calculated scaling factors are shown in Figure 3. Mean scaling factors for extrapolating from the Ready CO2 test to activated sludge, river water, and soil were 8.1, 2.5, and 1.2, respectively. However, these scaling factors were not normally distributed and were highly variable, exhibiting eightfold to 35-fold differences in magnitude. The calculated scalers ranged from 1.7 to 19 when extrapolating from the Ready test to a realistic activated sludge from 0.1 to 5.6 when extrapolating to river water, and from 0.3 to 2.8 when extrapolating to soil. Correlation analysis revealed almost perfect noncorrelation between the rate observed in the CO2 test and those observed in the activated sludge (r = -0.06), river water (r = 0.04), and soil (r = 0.12) tests. Hence, there was no relationship between the mineralization rate observed in the Ready test and that determined in more realistic tests for this group of chemicals, resulting in wide variability in the extrapolation factors.
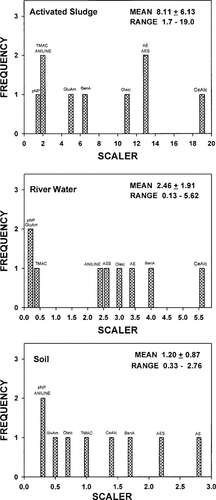
Frequency distributions of scaling factors for extrapolating first-order rates for nine chemicals from the Ready CO2 test to realistic activated sludge, river water, and soil test systems.
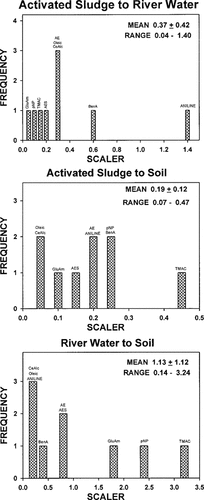
Frequency distributions of scaling factors for extrapolating first-order biodegradation rates for nine chemicals from one realistic test system to another.
| Scaling factor | Molecular weight | Log Kow | Solubility | Log solubility |
|---|---|---|---|---|
| Ready to activated sludge | 0.42 | 0.88** | -0.54 | -0.94** |
| Ready to river water | -0.03 | 0.82** | -0.45 | -0.69* |
| Ready to soil | 0.58 | 0.50 | -0.50 | -0.60 |
| Activated sludge to river water | -0.56 | -0.04 | 0.07 | 0.30 |
| Activated sludge to soil | 0.23 | -0.37 | 0.03 | 0.37 |
| River water to soil | 0.24 | -0.57 | 0.33 | 0.45 |
- * Significant (p ⩽ 0.05); ** Significant (p ⩽ 0.01).
The ability to predict mineralization rates in one realistic test based on another is shown in Figure 4, which contains frequency distributions for these various scalers. Linear regression analysis revealed only weak correlation between rates in the activated sludge and those in the river water test systems (r = 0.62) and no significant correlation between rates in activated sludge and soil (r = 0.38). Rates in soil and river water also were only very weakly correlated (r = 0.53). Mean scaling factors for extrapolating from activated sludge to river water and soil were 0.37 and 0.19, respectively. The mean scaling factor for extrapolating from river water to soil was 1.1. Once again, these scaling factors were not normally distributed and varied seven-fold to 35-fold depending on the tests being related.
Other statistical analyses
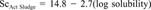
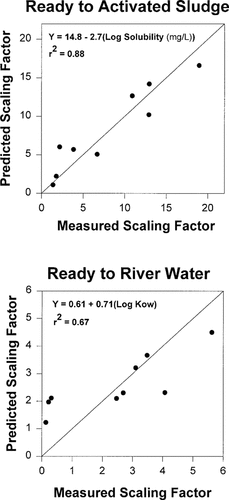
Comparisons of measured and predicted scaling factors for extrapolating from the Ready CO2 test to activated sludge (top) and river water (bottom). Predicted scaling factors were determined using regressions with physical properties of the test chemicals as independent variables.
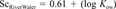
Figure 5 shows graphic comparisons between measured and predicted scaling factors based on these two equations. The relationship between log solubility and the scaling factor for extrapolating from the Ready test to activated sludge was particularly strong, thereby showing value as an approach for extrapolating Ready test data to this key habitat.
| Test system | Molecular weight | Log Kow | Solubility | Log solubility |
|---|---|---|---|---|
| Ready CO2 | -0.63 | -0.59 | 0.92** | 0.67* |
| 14C Activated sludge | 0.02 | 0.61 | -0.27 | -0.63 |
| 14C River water | -0.46 | 0.36 | -0.23 | -0.16 |
| 14C Soil | -0.05 | 0.02 | -0.10 | -0.02 |
- * Significant (p ⩽ 0.05); ** significant (p ⩽ 0.01).
Table 3 shows the correlation between first-order mineralization rates in the various tests and the physicochemical properties of the test chemicals. Mineralization rates in the Ready CO2 test were highly correlated (r = 0.92) with increasing solubility. In contrast, correlations between physicochemical properties and the mineralization were the opposite but not significant in activated sludge and nonexistent in river water and soil. Thus, it appears that while rates in the Ready CO2 test are heavily influenced by a chemical's solubility, this factor is less important under more realistic conditions that occur in the field. This importance of solubility in the Ready test but not the other tests probably relates to the elevated test material concentration unique to the Ready test in this study.
DISCUSSION
All nine compounds tested in this study were mineralized in the Ready test as well as in the activated sludge, river water, and soil. While the Ready test accurately predicted whether biodegradation would occur in these important environmental compartments, there was surprisingly no statistical relationship between the mineralization rates in the Ready test and those in the actual environmental compartments. This absence of correlation would suggest that the Ready test cannot independently predict mineralization rates under realistic field conditions. This inability to extrapolate rates from the Ready test appears to be related to the role that a chemical's physicochemical properties, particularly solubility, play in determining mineralization rates under conditions in which the test material concentration is elevated far beyond that observed in the environment. While not correlated with rates in the more realistic test systems, that in the Ready test was highly correlated with test substance solubility. In contrast, this physicochemical parameter had little or no relationship with mineralization rates in activated sludge, river water, and soil systems, where test material concentrations were much lower. Furthermore, factoring test material solubility or Kow, a related parameter, into the scaling factor, permitted more accurate extrapolation of Ready test results to activated sludge and to a lesser degree river water.
Previous investigators also have found that results from screening tests do not correlate with those from realistic systems. Larson [12] showed that biodegradation rates of nitriloactetic acid and linear alkylbenzene sulfonate (LAS) in a CO2 screening test were similar to those in natural waters. In contrast, mineralization rates of octadecyltrimethyl ammonium chloride and dioctadecyldimethyl ammonium chloride were much slower in a CO2 screening test than in natural waters. The former compounds were highly soluble, while the latter were poorly soluble or insoluble. Gledhill et al. [13] examined the effect of LAS and methyl ester sulfonate concentration on results in the modified OECD screening test and related poor results to toxicity at high concentrations. Furthermore, they showed much more rapid degradation of these compounds in river water/sediment microcosms. Thus, both solubility and toxicity issues influence results observed in standardized tests that utilize unrealistically high test chemical concentrations.
At the present time, particularly in Europe, chemicals are classified on the basis of their ability to pass a Ready test. Success in these tests is defined by completeness of biodegradation and the ability of the chemical to be degraded during a 10-d time window. The latter criteria is included to ensure that the chemical will not only biodegrade completely but also quickly in all relevant environmental compartments. The results of the current study, which is the first to compare biodegradation of several chemicals in the Ready test and realistic systems in a systematic and rigorous fashion, indicate that for biodegradable compounds, there is no relationship between the rate of biodegradation in the Ready test and the actual environment even with an inoculum from the source. Furthermore, this work shows that the best predictor of a chemical's biodegradation rate in the Ready test is its solubility, which became less important under realistic activated sludge and river conditions. It appears, therefore, that for biodegradable chemicals the rate in the Ready test is a reflection of the chemical's solubility rather than an indication of its true biodegradation rate in the environment, thus placing the value of the 10-d time window under serious question. Despite this limitation, Ready tests remain very powerful tools for demonstrating the complete biodegradation of a chemical and the existing distribution of degradative microorganisms.
Recently, investigators have attempted to derive quantitative factors for extrapolating results from one test or compartment to another. Boethling et al. (8) attempted to derive these factors on the basis of data in the literature and found wide variability in the factors. In the present study, there also was wide variability in extrapolating not only from the Ready test to various environmental compartments but also from one realistic test to another. For example, the calculated scaling factor for extrapolating from a realistic activated sludge test to a realistic river water test varied 35-fold, even though the river water was inoculated with liquor from the same activated sludge used in the former. While biodegradation rates for eight of the nine chemicals were slower in river water than in activated sludge and one could qualitatively conclude that rates in river water are slower than those in activated sludge, there was no way to quantitatively predict biodegradation rates in river water on the basis of rates in activated sludge using data generated in this study. This lack of quantitative linkage of compartments probably reflects the complexity and wide variability that exists in the biomass, the activity and composition of degrader communities, as well as differences in the concentration and environmental form or speciation of the test chemicals in these habitats.
Struijs and van den Berg [7] proposed an approach for deriving biodegradation rates in various aerobic habitats based on the outcome in the Ready test and the relative concentration of microorganisms in a habitat. In the present study, there were not strong relationships between biomass concentration and biodegradation rate that could justify extrapolating biodegradation rates on the basis of biomass. While rates in the activated sludge test were consistently faster than those in the Ready test, the magnitude of this difference in rates was not consistent with the more than 100-fold higher level of biomass in activated sludge. Observed scaling factors varied from 1.7 to 19, and most of this variability was explained by solubility. In addition, although soil contains 100 times more biomass per unit than the Ready test, the mean scaling factor was 1.2. Likewise, while river water contained approx. 10 times less biomass, biodegradation rates in river water were on average twice as fast as in the Ready test. Even factoring in the differential biodegradability of sorbed and free chemical in these habitats did not reveal a proportional relationship between biomass and biodegradation rates across tests. This inability to scale biodegradation rates on the basis of biomass again reflects the predominant role of physicochemical properties in determining biodegradation rates at the high test substance concentrations in the Ready test.
While illustrating the difficulties of extrapolating Ready test results using single multipliers or relationships with biomass, this study shows that reasonable extrapolations from the Ready test to activated sludge and to a lesser degree river water may be possible when the physicochemical properties of a chemical are taken into consideration. Scaling factors derived from a regression equation with log solubility as the independent variable greatly improved the ability to extrapolate Ready test rates to activated sludge. Likewise, scaling factors derived from a regression with log Kow as the independent variable somewhat improved extrapolations to river water. Because these regressions are based on only nine chemicals, caution should be exercised in their use. Nevertheless, their existence provides hope that understanding not only the different biology of test systems but also the effect of environmental concentration and form on biodegradation will one day allow us to confidently link the laboratory to the field.
In summary, this work shows the absence of a clear relationship between biodegradation rates in the Ready test and tests that more accurately simulate conditions in the various habitats for a diverse range of chemicals. This inability of the Ready test to predict real-world kinetics is related to issues associated with a chemical's physicochemical properties (e.g., solubility), which are manifested at high concentrations in the Ready test that are not found in the real world. Understanding the role of these physicochemical factors in the Ready test could potentially improve the value of Ready test data in exposure assessments.
Acknowledgements
We thank Kay Marks of Roy F. Weston, Inc., for her technical assistance and Robert J. Larson, Tom Feijtel, and Patrick Masscheleyn for their helpful comments and critical review of the manuscript.




