Acute Toxicity of Major Geochemical Ions to Fathead Minnows (Pimephales Promelas): Part A—Observed Relationships for Individual Salts and Salt Mixtures
Abstract
The results of a series of experiments on the acute toxicity of major geochemical ions (Na+, K+, Ca2+, Mg2+, Cl−, SO42−, HCO3−/CO32−) to fathead minnows (Pimephales promelas) are reported. Tests of individual major ion salts in various dilution waters demonstrated that the toxicities of Na, Mg, and K salts decrease as the overall ion content of the dilution water increases. For Na and Mg salts, this is attributable to Ca content as previously reported for Ceriodaphnia dubia. For K salts, the cause is unclear, but it is not due to Na as reported for C. dubia. In an unregulated test at high pH (9.3), NaHCO3 was also found to be twice as toxic compared to when the pH was reduced to 8.4. Experiments with binary salt mixtures indicated the existence of multiple independent mechanisms of action. These include K-specific toxicity and Ca/Mg-specific toxicity previously reported for C. dubia, but also apparent toxicities related to SO4 and to high pH/alkalinity in CO3/HCO3-dominated exposures. Previous work with C. dubia also suggested a general ion toxicity involving all ions that was correlated with osmolarity. For fathead minnow, similar correlations were observed, but multiple mechanisms were indicated. At higher Ca, this general toxicity could be attributable to osmotic effects, but at lower Ca, osmolarity may be more a covariate than a cause, with this toxicity being related to a combined effect of ions other than via osmolarity. Environ Toxicol Chem 2022;41:2078–2094. © 2022 SETAC. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
INTRODUCTION
Anthropogenic increases of major geochemical ions (Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−/CO32−) in freshwater systems are of great concern regarding impacts on aquatic communities (see reviews by Berger et al., 2019; Cañedo-Argüelles, 2020; Cañedo-Argüelles et al., 2019; Hintz & Relyea, 2019; Kaushal et al., 2021; Schuler et al., 2019). Substantial and widespread increases of ions from ambient levels, toxic levels of ions in effluents, runoff, and ambient waters, and impacts on aquatic ecosystems have been reported. Sources of these increases include road salting, irrigation water return, mining leachates and treatment of acid mine drainage, oil and gas production waters, various industrial process effluents, water softening, and saltwater intrusion. This ion enrichment involves complex mixtures that vary widely regarding ion ratios and dominant ions. However, current environmental standards focus on individual components, often Cl or SO4, rather than the suite of ions present.
The aquatic toxicity of major ions has been the subject of considerable research, which has established that toxicities of ion mixtures vary widely with their composition and with test water characteristics. Mount et al. (1997) established the importance of considering all major ions for assessing toxicities over the broad range of ion mixture compositions that might occur. For perhaps the most studied species, Ceriodaphnia dubia, Table 1 lists important toxicological interactions among ions that have been identified (Elphick, Bergh, & Bailey, 2011; Elphick et al., 2011; Erickson et al., 2017, 2018; Mount et al., 1997, 2016, 2019; Soucek, 2007; Soucek & Kennedy, 2005; Soucek et al., 2011). Based on the interactions reported in Table 1, Mount et al. (2016) and Erickson et al. (2017) suggested three primary independent mechanisms of action: (1) a K-specific toxicity, (2) a Ca/Mg-specific toxicity, and (3) a general toxicity related to all ions. Erickson et al. (2018) addressed how these mechanisms, and their dependence on dilution water composition, could be formulated into mathematical models for acute toxicity to C. dubia and Mount et al. (2019) did the same for chronic toxicity. Importantly, fully understanding and describing these relationships requires the use of molar rather than mass concentrations, and is often better based on chemical activities rather than concentrations.
| Salts | Exposure metric(s) related to toxicity | Important interactions |
|---|---|---|
| Na salts | Osmolarity |
|
| K salts | K activity |
|
| Mg salts | Mg activity |
|
| Ca salts | Ca activity |
|
| Na × K salt mixtures | Osmolarity K activity |
|
| Na × Mg salt mixtures | Osmolarity Mg activity |
|
| Na × Ca salt mixtures | Osmolarity Ca activity |
|
| K × Mg salt mixtures | K activity Mg activity |
|
| K × Ca salt mixtures | K activity Ca activity |
|
| Mg × Ca salt mixtures | Mg activity Ca activity |
|
Although there is considerable understanding of major ion toxicity to C. dubia, it is less clear whether the same principles govern responses of other freshwater species. Fathead minnows (Pimephales promelas) have been used in several studies of major ion toxicity, but the aggregate of these studies does not provide a comparable level of understanding of how this species responds to ion mixtures of widely varying composition. Mount et al. (1997) did extensive testing of the acute toxicity of 10 major ion salts to 2-day-old to 7-day-old fathead minnows, including both individual salt tests and 1:1 (by mass) mixture tests for 20 binary combinations of these salts. Although the findings of Mount et al. (1997) supported some of the mixture interactions in Table 1, they were unclear or incomplete in other regards, including not addressing the effects of dilution water chemistry. Other studies on acute toxicity of major ions to fathead minnow (Adelman et al., 1976; Birge et al., 1985; Elphick, Bergh, & Bailey, 2011; Meyer et al., 1985; Wang et al., 2016) were limited to single-salt testing of NaCl, KCl, Na2SO4, and MgSO4, and showed median lethal concentrations (LC50s) consistent with Mount et al. (1997), but did not explore the toxic interactions among the major ions or the influence of test water composition. For 7-day fathead minnow embryo-larval tests, Elphick et al. (2011) did show that the lethality of Na2SO4 is dependent on the hardness of the dilution water (although other ions varied in proportion to hardness), and Wang et al. (2016) found that the toxicity of Na2SO4 is exacerbated by low K concentrations.
Due to these limitations and uncertainties for existing data, the present study was designed to provide a more extensive and systematic evaluation of the acute toxicity of major ions to juvenile fathead minnow. Tests with single salts determined the effects of dilution water characteristics on toxicity and general potency differences among ions. Binary mixture experiments were used to infer the primary roles of different ions for determining mixture toxicity, in a manner similar to that used previously for C. dubia (Erickson et al., 2017; Mount et al., 2019). The relationship of salt toxicity to osmolarity was also investigated by testing the toxicity of mannitol and the interaction of this toxicity with major ions.
The present article provides the data from these experiments and the basic evaluations of ion interactions. In a companion article (Erickson et al., 2022), these findings are used to formulate mathematical models for major ion toxicity to fathead minnows, and we discuss some issues regarding their application to risk assessments.
MATERIALS AND METHODS
Test organisms
Fathead minnows were from the in-house culture at the US Environmental Protection Agency (USEPA) Great Lakes Toxicology and Ecology Division (Duluth, MN, USA), which is maintained at 25 °C in filtered Lake Superior water (LSW, obtained from an intake located offshore from the laboratory at 46.840°N, 92.004°W). This water typically has a pH of approximately 7.5, a dissolved organic carbon concentration of 1–2 mg C/L, and inorganic constituents as specified in Table 2. For each experiment, a cohort of newly hatched fry (<24 h old) from multiple spawns was isolated, fed brine shrimp nauplii ad libitum for 6 days, and used for testing when 7–8 days old. Preliminary efforts had evaluated the toxicities of NaCl and MgSO4 to fathead minnows <1, 3–4, 7–8, and 14–15 days old. The selection of organisms that were 7–8 days old was based on them not being significantly less sensitive than other ages, being robust compared with newly hatched fry regarding control survival in the absence of feeding, and being amenable to smaller chambers and less culture effort than older organisms. This work was conducted under an institution-approved animal care and use plan within the project plan (MED-QAPP-DM-2013-07).
| Dilution water | Na (mM) | K (mM) | Ca (mM) | Mg (mM) | Cl (mM) | SO4 (mM) | Alkalinity (meq/L) |
|---|---|---|---|---|---|---|---|
| LSW | 0.070 | 0.015 | 0.349 | 0.120 | 0.042 | 0.035 | 0.859 |
| ALSW | 0.282 | 0.039 | 0.364 | 0.168 | 0.216 | 0.129 | 0.859 |
| ⅓×ALSW | 0.094 | 0.013 | 0.121 | 0.056 | 0.072 | 0.043 | 0.286 |
| 3×ALSWa | 0.845 | 0.116 | 1.09 | 0.488 | 0.648 | 0.440 | 2.54 |
| LoCa:Mg | 0.282 | 0.039 | 0.075 | 0.410 | 0.216 | 0.150 | 0.763 |
| HiCa:Mg | 0.282 | 0.039 | 0.475 | 0.053 | 0.216 | 0.150 | 0.849 |
| LoK:Na | 0.333 | 0.005 | 0.364 | 0.163 | 0.216 | 0.155 | 0.848 |
| HiK:Na | 0.082 | 0.256 | 0.364 | 0.163 | 0.216 | 0.155 | 0.848 |
| 1.6 mgNa/L | 0.070 | 0.039 | 0.364 | 0.168 | 0.042 | 0.110 | 0.859 |
| 10 mgNa/L | 0.435 | 0.039 | 0.364 | 0.168 | 0.407 | 0.110 | 0.859 |
| 30 mgNa/L | 1.31 | 0.039 | 0.364 | 0.168 | 1.278 | 0.110 | 0.859 |
| ⅓× Ca | 0.767 | 0.039 | 0.121 | 0.168 | 0.216 | 0.146 | 0.859 |
| 3× Ca | 0.282 | 0.039 | 1.09 | 0.168 | 1.67 | 0.129 | 0.859 |
| 9× Ca | 0.282 | 0.039 | 3.28 | 0.168 | 6.05 | 0.129 | 0.859 |
| 27× Ca | 0.282 | 0.039 | 9.84 | 0.168 | 19.2 | 0.129 | 0.859 |
| 81× Ca | 0.282 | 0.039 | 29.5 | 0.168 | 58.5 | 0.129 | 0.859 |
| 1/8× Ca | 0.919 | 0.038 | 0.046 | 0.168 | 0.216 | 0.152 | 0.858 |
| 1/4× Ca | 0.828 | 0.038 | 0.091 | 0.168 | 0.216 | 0.148 | 0.859 |
| 1/2× Ca | 0.645 | 0.039 | 0.182 | 0.168 | 0.216 | 0.142 | 0.859 |
| 2× Ca | 0.282 | 0.039 | 0.729 | 0.168 | 0.945 | 0.129 | 0.859 |
- a Major ion concentrations were increased to three times that in ALSW, except SO4 was increased 3.4 times. This was due to concentrations of ions other than these major ions not being altered from that in ALSW. Because these other ions are predominantly anionic, one of the major anions needed to be increased disproportionately.
- ALSW = amended Lake Superior water.
Test chemicals
Nine major ion salts were used to evaluate the relative toxicities of and the interactions among the major ions, including the chloride salts of all four cations, the sulfate salts for all cations except Ca (due to limited solubility), and the carbonate salts of Na and Mg. Gluconate salts of Na, Mg, and Ca were also tested to provide an anion expected to have limited interaction with the organisms to further evaluate correlation of toxicity to the cations. Mannitol was tested to provide a comparison of salt toxicity to that of a compound expected to only affect the external osmotic environment.
All chemicals were obtained from the Sigma-Aldrich Chemical Company or Thermo-Fisher Scientific Company. All compounds had a designated hydration and a purity of at least 98%, except for MgCO3 and Mg gluconate, for which the hydration was not specified. The certificates of analysis for these two salts were used to determine the ratio of the anhydrous salt to total salt weight for computing nominal concentrations. The certificate of analysis for MgCO3 also specified the Ca content to be 0.44% of the Mg content (on a molar basis), enough to appreciably affect the background Ca for tests on the toxicity of MgCO3, necessitating additional Ca measurements to document concentrations.
Test waters
The dilution water for most tests was amended Lake Superior water (ALSW), which was developed by Mount et al. (2016) to address concerns that ion ratios in some commonly used dilution waters are atypical of natural waters (e.g., unusually high Mg:Ca ratios in some synthetic waters, low Na in LSW relative to its hardness and alkalinity), which could cause misleading results. Amended Lake Superior water is prepared by amending sand-filtered and UV-treated LSW with major ion salts to provide ion ratios close to median values for United States freshwaters, while maintaining its alkalinity and other natural constituents. The major ion content of ALSW is provided in Table 2.
For some tests, the formula for ALSW was modified to create test waters with different concentrations for some or all ions (Table 2). This included three ways in which Ca was modified. First, Ca was changed in proportion to all other ions by diluting ALSW with deionized water so that all ion concentrations were reduced to a third of that in ALSW (⅓×ALSW) and by adding salts so that ion concentrations were increased to three times that in ALSW (3×ALSW). Second, Ca was changed inversely with Mg while keeping water hardness and other ions approximately constant so that the Ca:Mg molar ratio of 2.2 for ALSW was reduced to 0.2 (LoCa:Mg) and increased to 9 (HiCa:Mg). Third, several test waters were formulated with altered Ca concentrations ranging from 1/8× to 81× that in ALSW, reducing Ca by substituting Na for a portion of the Ca in the water formulation and increasing Ca concentrations by adding CaCl2, with other ions approximately constant. To test the effects of Na and K concentrations in dilution water, test waters were also formulated with different Na concentrations (1.6 mgNa/L, 10 mgNa/L, 30 mgNa/L), with concomitant changes to Cl, and with the K:Na molar ratio altered from 0.14 for ALSW to 0.015 (LoK:Na) and 3.1 (HiK:Na), with negligible changes to other ions.
Study design
Seventeen experiments, each comprising two to seven simultaneous toxicity tests (92 total tests), were conducted on the acute (96-h) lethality of single compounds to fathead minnows in various dilution waters. Table 3 specifies the experiments addressing the effects of different dilution waters on the lethality of the major ion salts and mannitol. Other experiments compared the toxicities, in ALSW only, of mannitol and of the gluconate salts of Na, Mg, and Ca to the chloride salts of these cations. An additional experiment compared the toxicity of NaHCO3 in ALSW with pH uncontrolled (9.3) to that with pH reduced to 8.4 by elevating CO2.
| Chemical | ⅓×, 1×, 3× ALSW | Varying Ca concentration | Varying Ca:Mg | Varying Na concentration | Varying K:Na |
|---|---|---|---|---|---|
| NaCl | 2× | 2× | × | - | × |
| MgCl2 | × | × | - | - | - |
| Na2SO4 | × | × | × | - | × |
| MgSO4 | × | × | × | × | - |
| NaHCO3 | × | - | - | - | × |
| MgCO3 | 2× | - | - | - | - |
| CaCl2 | × | - | - | - | - |
| KCl | × | - | - | × | - |
| K2SO4 | × | - | - | - | - |
| Mannitol | - | × | - | - | - |
- × denotes experiment conducted for that combination of chemical and dilution water characteristic; 2× denotes multiple experiments.
- ALSW = amended Lake Superior water.
Testing the toxicity of mannitol to fathead minnow in dilution waters with different [Ca] was important to the evaluation of the relationship of salt toxicity to osmolarity and to model development in the companion article (Erickson et al., 2022). For C. dubia the effects of Ca on mannitol were not tested by Mount et al. (2016), so an ancillary experiment was conducted to fill this data gap and is described in Supporting Information.
Fifteen binary mixture experiments (78 total tests) were conducted in ALSW. Each experiment consisted of multiple toxicity tests, each test having a fixed ratio of the two chemicals. A typical experiment had five tests with estimated toxic unit ratios of 1:0, 3:1, 1:1, 1:3, and 0:1, but ratios were sometimes changed or added to better establish chemical interactions. These mixture experiments included all possible combinations involving NaCl, Na2SO4, MgCl2, MgSO4, and CaCl2. The compounds NaHCO3 and MgCO3 were tested only in mixtures with their respective Cl salts. For K salts, the only mixture tests were of KCl with NaCl and MgCl2 due to the expectation of K-driven toxicity independent of other ions. A final mixture experiment was of NaCl and mannitol to test for concentration additivity on an osmolarity basis.
Toxicity test procedures
Static, 96-h, unfed acute toxicity tests were conducted in accordance with ASTM International Method E729 (ASTM International, 2002). Exposures were in 5 oz (~150 ml) polystyrene cups (Fineline Settings) containing 80 ml of test solution and suspended in a temperature-controlled (25 °C) water bath using perforated floating foam boards, with glass sheets covering all cups. The test compounds were dissolved in the designated test dilution water (Tables 2 and 3) to produce the highest test concentration, with the dissolution of MgCO3 being CO2-assisted as described in Mount et al. (2016). This high test concentration was serially diluted with the test dilution water to produce a geometric series of 9–10 concentrations with a dilution factor of approximately 0.80× (i.e., 100%, 80%, 64%, 50% etc.) or 0.707× (i.e., 100%, 70.7%, 50% etc.). Solutions were prepared immediately prior to test initiation, except for two experiments involving NaHCO3 (NaCl × NaHCO3 mixture experiment, NaHCO3 toxicity at different pHs), for which solutions were prepared 3 days in advance to allow CaCO3 precipitation to occur and Ca concentrations to stabilize. In other, earlier experiments involving NaHCO3, CaCO3 precipitation caused Ca concentrations to decline by a factor of up to 20 over the course of the 4-day exposures, making it difficult to associate a specific {Ca} with the LC50 for this salt (Note that braces { } are used for chemical activities, vs. brackets [ ] for concentrations).
Each concentration was tested in duplicate cups, except quadruplicate cups were used for the control for the last two experiments (on NaCl × mannitol mixture interactions and the effects of Ca on mannitol toxicity) to provide better information on control survival. For the experiment on the effect of added CO2 on NaHCO3 toxicity, a third cup was added for the control and for three concentrations near the expected LC50 to provide samples for Na and Ca measurements at 48 h, but these destructively sampled cups were not included in effects assessment. Five fathead minnows were added to each cup at test initiation. The number of surviving organisms in each test chamber was recorded at the end of each 24-h period. Death was determined by the change in appearance from translucent to opaque or no reaction to gentle prodding. At the end of the exposures, surviving fish were euthanized with a buffered solution of nonpharmaceutical grade tricaine mesylate (Finquel).
Tests were conducted under ambient laboratory fluorescent lighting with a 16:8-h light:dark photoperiod. At the initiation of each test, alkalinity and hardness were measured in an aliquot of the control water, dissolved oxygen and pH were measured in aliquots of both the control water and the highest test concentration, and conductivity was measured for all test concentrations in one of the replicate test cups. Temperature was measured daily in one chamber of each test. Conductivity, dissolved oxygen, and pH were measured at 24, 48, and 72 h in one replicate of every test concentration reaching 100% mortality during the preceding time interval, and at 96 h in one replicate of all other concentrations. These measurements were only in test chambers being terminated to avoid the probes perturbing test organisms and/or test water chemistry, while still documenting the chemistry at concentrations causing mortality.
Exposure monitoring
Samples for analysis of the major ions were collected at the start of each experiment from the dilution water(s) and from the highest concentration of each toxicity test. In addition, samples were collected for each test at 24 h from the lowest concentration with 100% mortality, and at 96 h from the concentration nearest the apparent LC50. Additional samples were taken in some experiments to more fully characterize ion concentrations, especially Ca, in treatments near the LC50. The conductivity measurements made on all treatments confirmed that the intended gradient of exposure existed among treatments not sampled for cation analysis.
Dilution waters were analyzed for all four cations plus Cl and SO4. Other samples were analyzed for the test salt cation(s), and also for Ca in tests involving NaHCO3 and MgCO3 (which caused oversaturation of CaCO3), for the Na2SO4 × CaCl2 and MgSO4 × CaCl2 mixture tests (which caused oversaturation of CaSO4), and for the toxicity tests with NaCl, MgCl2, Na2SO4, and MgSO4 over the series of dilution waters with modified Ca.
Samples were filtered through an 0.45 µm nylon syringe filter (Grainger). For cation analyses, samples were acidified to 0.2% (v/v) with concentrated HNO3 and held at room temperature; for tests with NaHCO3 and MgCO3, this acid addition was increased by an amount calculated to neutralize the extra alkalinity. For Cl and SO4 analyses, samples were refrigerated. Analyses were by flame atomic absorption spectrophotometry for cations and ion chromatography for anions, using the methods and equipment described in Mount et al. (2016). For samples in which SO4 exceeded 20 mM, there was apparent interference with Ca analysis due to insufficient inhibitor for this interference, resulting in estimated Ca concentrations 10%–30% lower than nominal; such measurements were not used.
For measurements of ions in dilution waters (ALSW and the modifications thereof in Table 2), the ratios of measured to nominal concentrations had means (% relative standard deviation, sample size) of 1.03 (6%, n = 64) for Na, 1.03 (8%, n = 63) for K, 1.01 (6%, n = 63) for Ca, 1.07 (4%, n = 63) for Mg, 1.01 (4%, n = 57) for Cl, and 1.05 (7%, n = 60) for SO4. For measurements of test salt cations in test solutions near and above the LC50, the ratios of measured to nominal concentrations across all samples were 1.00% (4%, n = 402) for Na, 0.98% (3%, n = 74) for K, 1.02% (5%, n = 353) for Mg, and 0.96% (6%, n = 41) for Ca, excluding Ca analyses for tests in which CaCO3 or CaSO4 precipitation was a consideration and for tests with MgCO3 due to uncertain nominal Ca. For these test salt cation comparisons (n = 870), only 18 measurements (2.1%) deviated by more than 10% from nominal, with a maximum deviation of 19%. Such agreement was considered sufficient to warrant the use of nominal concentrations in the data analyses except as follows.
For the Na2SO4 × CaCl2 and MgSO4 × CaCl2 mixture experiments, most mixture ratios had visible precipitates and lower-than-nominal measured Ca concentrations at the highest exposure concentrations. At the LC50, precipitation was less evident, but test solutions were calculated to be oversaturated for CaSO4 and there were minor (~10%) reductions in measured Ca from nominal. Therefore, for these experiments, the Ca and SO4 concentrations to associate with the LC50 were based on interpolation of the average Ca measurements in treatments bracketing the LC50.
Measured Ca concentrations were also used in tests with NaHCO3 and MgCO3 due to precipitation of CaCO3 and due to the uncertain Ca content of the MgCO3 salt. At each measurement time, Ca measurements were used to estimate the Ca concentration at the LC50 by interpolation. If these estimates declined by ≤25% over time, their average was associated with the LC50. If the temporal variation was greater than this, both the nominal and final Ca concentrations are reported to designate a range for the Ca concentration to associate with the LC50. The same procedure was applied to the Na2SO4 toxicity test with the highest Ca elevation (81×), where substantial CaSO4 precipitation at the LC50 was evident.
Data analysis: LC50 estimation
Median lethal concentrations and their confidence limits were estimated similarly to Mount et al. (2016). For tests with at least two partial mortalities, a tolerance distribution analysis was conducted using a three-parameter log-logistic model. Parameters were estimated by maximum likelihood analysis using custom software written with Intel Visual Fortran Compiler XE 2015 (Intel Corporation) and Winteracter 13.0 (Interactive Software Services). Confidence limits (95%) were calculated using the likelihood ratio method (Williams, 1986). For tests with insufficient partial mortalities for this analysis, the same likelihood ratio method was used to calculate confidence limits for the LC50, which can be assigned in the absence of a unique point estimate for the LC50. For these tests, we assigned the geometric mean of these confidence limits to be the point estimate for the LC50. For cases in which there were no partial mortalities, these confidence limits are >95% and are the bracketing concentrations (i.e., the highest concentration with control survival and the lowest concentration with complete mortality), and the LC50 is equivalent to linear interpolation of survival versus log concentration between these two concentrations.
Median lethal concentrations were initially calculated as a percentage of the highest added concentration in the test and then converted to molar concentrations of the added salts. These concentrations were then added to the ion concentrations already present in the dilution water and the resultant total ion concentrations were input to the chemical speciation program Visual MINTEQ (Ver 3.1, https://vminteq.lwr.kth.se/visual-minteq-ver-3-1) to estimate the chemical activity of each chemical species at the LC50. The osmolarity at the LC50 was estimated based on MINTEQ activity estimates using the method described by Robinson & Stokes (1959). The sum of the concentrations of the major inorganic ions, mannitol, and gluconate was also calculated as a potential exposure metric. Confidence limits for LC50s expressed as these various metrics were assigned the same relative values as those for the percentage added concentration LC50s, and do not reflect any additional uncertainty associated with the speciation or other calculations.
Data analysis: Evaluating relationships in binary salt mixture experiments
As discussed in Erickson et al. (2017) and references therein, binary toxicity experiments can be used to infer certain information about toxicity mechanisms, specifically whether the toxicities of the two chemicals show concentration addition (suggesting a shared toxicity mechanism), independent action, synergism, antagonism, or other interactions. Binary mixture toxicity experiments in Erickson et al. (2017) contributed to the inferences about major ion toxicity to C. dubia summarized in Table 1, using isobolograms that compared the observed mixture LC50s to isoboles for concentration addition or independent action fitted to the individual salt LC50s for the experiment. In the present study, isoboles are similarly fitted for some data comparisons, but mixture experiment LC50s are also compared with isoboles predicted based on prior results with individual salts. Furthermore, the prior data was just for Cl salts, so that the predictions address consistency of relationships across different anions. Details on the methods for both fitting and predicting isoboles are provided in Supporting Information.
RESULTS AND DISCUSSION
Supporting Information, Tables S1 and S2, provide detailed information for single salt and mixture tests, including control survivals, LC50s (with confidence limits) as total added concentrations of each salt, and estimates at the LC50s for pH, activities for selected ionic species, osmolarity, and total concentrations of solution components (total osmoles). Across all tests, temperature measurements averaged 24.6 °C, ranging from 24.1 to 25.1 °C. Dissolved oxygen concentrations were >8 mg/L at test start and were no lower than 7.5 mg/L for treatments with surviving organisms at test end. Additional exposure effects and water quality data are available at USEPA's Environmental Dataset Gateway (https://edg.epa.gov/metadata/catalog/main/home.page).
For four tests, the mortality was <40% at the highest exposure or >50% at the lowest exposure, so the LC50s in Supporting Information, Tables S1 and S2, are reported as greater than or less than concentrations, and the ion concentrations and activities are those for the limiting exposures. For six tests, there was substantial (>25%) loss of Ca from the start to the end of exposure due to precipitation of either CaCO3 or CaSO4, and Supporting Information, Table S1, includes chemistries for both the start and end of these tests. The initial test of MgCO3 in 3×ALSW (Experiment 13–21) produced both a greater than LC50 and substantial loss of Ca, so this experiment was repeated (Experiment 15–32). However, the repeat test for 3×ALSW proved even more problematic and is not reported due to a nonmonotonic and very shallow concentration/exposure relationship apparently caused by irregular trends of CaCO3 precipitation across treatments.
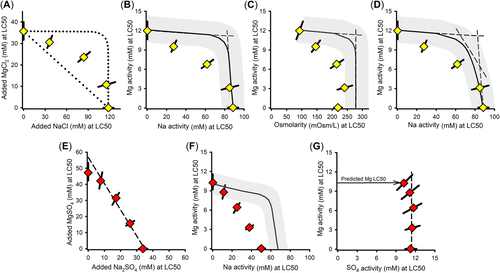
Effects of dilution water on the toxicity of Mg salts
Effects of dilution water Ca
For all modifications of Ca in dilution water (Tables 2 and 3), Figure 1A provides LC50s for MgCl2, MgSO4, and MgCO3 based on added salt molarity versus Ca concentration. Median lethal concentrations in Figure 1A increase substantially with Ca concentration for all Mg salts and for all methods for modifying Ca concentration. This adds to a consistent pattern of such effects on major ion toxicity, including Ca effects on major ion toxicity to C. dubia (Mount et al., 2016), effects of Ca on Na salt toxicity in the present study, and effects of hardness reported by other authors (e.g., Elphick, Bergh, & Bailey, 2011; Elphick et al., 2011).
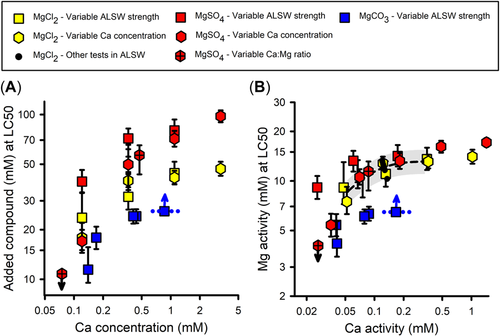
The LC50 for the MgCO3 test in 3×ALSW is uncertain because this is a “greater than” value; however, because there was 30% mortality at the highest concentration (Supporting Information, Table S1), the LC50 should be no more than 20% higher based on typical slopes of the concentration/effect curves. Ca also changed during this test due to CaCO3 precipitation. The Ca concentration to associate with this LC50 is unlikely to be at either extreme of this Ca range, but could be lower than the indicated midpoint of the range to the extent that mortality reflects increased potencies (due to lower Ca) at the end of the test. Regardless of these uncertainties, this test supports a Ca effect on Mg toxicity.
Effects of other dilution water characteristics
In contrast to the effects of Ca, LC50s for MgSO4 did not change substantially when just Na and Cl were modified in the dilution water (Supporting Information, Table S1, Experiment 13–31). Furthermore, if the consistent effects on LC50s of the three methods for changing Ca are due to changes to ions other than Ca, then these LC50 changes should be correlated to these other ions. Instead, increasing LC50s for Mg salts in Figure 1A are associated with (1) increasing Na for the ⅓×/1×/3×ALSW series (see Na concentrations in Supporting Information, Table S1, for Experiments 13–20, 13–21, and 15–32); (2) decreasing Na for the ⅓×/1×Ca tests (Experiments 15–06 and 15–18); and (3) constant Na for the tests with altered Ca:Mg ratio and for Ca elevated above ALSW (Experiments 13–28, 15–06, and 15–08). Similar inconsistencies exist for Cl. This further argues against Na and Cl being modifiers for Mg toxicity.
However, there are some indications of effects of dilution water on toxicity other than from Ca. At the lowest Ca concentration, the smallest LC50 for MgSO4 in Figure 1A is for a dilution water with a very low ratio of Ca to other ions and is more than three times smaller than the LC50 for ⅓×ALSW, for which ion ratios are more typical of natural waters. It is uncertain whether this variation in toxicity is due to the different relative ion concentrations or due to other consequences of testing at very low Ca, where variability among tests was also high for C. dubia (Erickson et al., 2018; Mount et al., 2016). However, these variations are of limited practical importance because toxic Mg concentrations are unlikely to co-occur with such low Ca concentrations (Erickson et al., 2018).
Implications of single salt tests on exposure metrics for Mg salt toxicity
Exposure metric for MgCl2 and MgSO4 toxicity
In addition to addressing effects of dilution water composition, the data in Figure 1 inform the issue of what exposure metric is best related to Mg toxicity. Based on total added molar concentrations (Figure 1A), MgSO4 is approximately half as toxic as MgCl2 for tests at Ca concentrations from 0.3 to 3 mM. In contrast, based on ion activities (Figure 1B), MgCl2 and MgSO4 have similar toxicities for these same tests, their LC50 confidence limits usually overlapping and differences being less than interexperimental variability. This illustrates how chemical speciation (e.g., complexation of Mg by SO4) can reduce toxicity on a total chemical basis and how an exposure metric such as {Mg} can better relate to toxicity. At lower Ca concentrations (<0.3 mM), the relative toxicities of MgSO4 and MgCl2 are less clear due to limited data and the increasing and variable toxicity as Ca becomes extremely low.
Exposure metric for MgCO3 toxicity
The toxicity of MgCO3 relative to MgCl2 shows a different pattern than MgSO4. Despite Mg complexation by HCO3/CO3, MgCO3 is more toxic than MgCl2 on a molar concentration basis (Figure 1A) and becomes even more toxic for the 1× and 3×ALSW dilution waters when expressed as activities (Figure 1B). These LC50s are associated with high pH (9.2) and high alkalinity (≥36 meq/L; Supporting Information, Table S1), which might adversely impact respiratory CO2 excretion and acid/base balance (Truchot & Forgue, 1998; Wood, 1992) in addition to any Mg-related toxicity. Ceriodaphnia dubia did not exhibit such enhanced toxicity (Erickson et al., 2017, 2018; Mount et al., 2016), which may be due to interspecies differences in respiratory relationships or to alkalinity not reaching these high levels due to the greater sensitivity of C. dubia to Mg. An effect of pH relevant to this was demonstrated for NaHCO3 toxicity, which is discussed in Effects of dilution water chemistry on toxicity of Na salts. Due to the limited data and multiple confounding factors (pH, alkalinity, Mg), it is uncertain how best to relate exposures and effects for MgCO3-dominated solutions.
Mg toxicity model for isobole predictions
Interactions in binary mixtures of Mg salts
MgCl2 × MgSO4 binary mixture experiment
Figure 2A presents the isobologram for LC50s based on molarity of added salt. The closeness of the data to a straight line indicates a strong degree of additivity, supportive of Mg-driven toxicity. However, [Mg] at the LC50 steadily increases as the proportion of MgSO4 in the tests increases, reaching nearly 60% greater for the MgSO4-only test than for the MgCl2-only test, indicating total concentration is not an appropriate exposure metric for Mg-based toxicity.
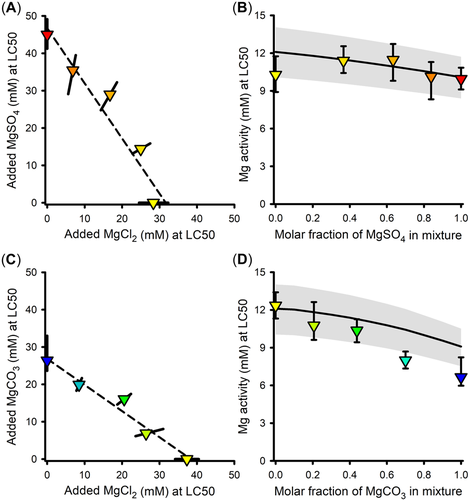
In contrast, LC50s for the MgCl2 × MgSO4 mixtures expressed as {Mg} (Figure 2B) are less variable and are consistent with predicted values from the {Mg}-based toxicity model (Equation 1). This prediction includes a small (~10%) reduction of LC50 as the molar fraction of MgSO4 increases, due to complexation of Ca by SO4. Due to the unusually low toxicity for the MgCl2-only exposure in this experiment (which is the lowest black dot in Figure 1B), this Ca effect is not clear in these data. However, a Ca effect consistent with model predictions is evident in mean LC50s in ALSW across the entire study (Supporting Information, Tables S1 and S2), which on the basis of {Mg} drop from 12.1 mM for MgCl2 tests (n = 9) to 10.9 mM for MgSO4 tests (n = 6).
MgCl2 × MgCO3 binary mixture experiment
Figure 2C presents the isobologram for LC50s based on molarity of added salt. Again, the closeness of the data to the linear regression line suggests considerable additivity. However, the Mg concentration at the LC50 for this mixture is 30% lower for MgCO3 than MgCl2, in the opposite direction of that expected from increased complexation and again indicative of total concentration not being a good exposure metric.
However, unlike for the MgCl2 × MgSO4 mixtures, when LC50s are expressed as {Mg}, some MgCl2 × MgCO3 mixtures deviate from the {Mg}-based toxicity model predictions (Figure 2D). The observed LC50s are reasonably consistent with predicted LC50s up to an equimolar mixture, but show increasingly greater toxicity than expected at higher mole factions of MgCO3. This deviation is associated with pH ≥ 9.1 and {HCO3} > 16 mM, compared to pH ≤ 9.0 and {HCO3} < 13 mM for mixtures with lower mole fractions of MgCO3 (Supporting Information, Table S2). This is consistent with the deviations of MgCl2 LC50s from MgCO3 LC50s for 1× ALSW in Figure 1B, for which pH was 9.2 and {HCO3} was 18 mM (Supporting Information, Table S1). This again suggests some effect of the combined high pH and alkalinity in MgCO3-dominated exposures other than that expected just from effects of Ca activity on Mg-driven toxicity. Equation 1 with {Mg} as the exposure metric is thus useful for mixtures containing up to at least half MgCO3, but the metric for MgCO3-dominated exposures is uncertain. Addressing toxicity in high carbonate exposures is further discussed in the companion modeling article (Erickson et al., 2022).
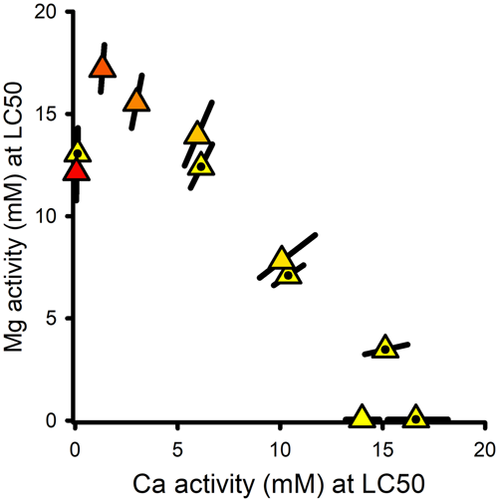
Interactions in binary mixtures of Mg and Ca salts
Figure 3 provides a combined isobologram for the MgCl2 × CaCl2 and MgSO4 × CaCl2 mixture experiments based on Mg and Ca activities, showing good agreement between the Cl-only and combined Cl/SO4 mixtures, thereby supporting cation-driven toxicity. The linearity of {Mg} versus {Ca} for all but the lowest level of Ca indicates concentration addition, with effects of Ca on Mg toxicity (Figure 1B) causing the LC50 at the lowest Ca to deviate from the line. As for C. dubia (Erickson et al., 2017, 2018), this additivity indicates a common mechanism with {Mg} and {Ca} as the exposure metrics. A prediction for this isobole is not provided in the present study because Equation 1 does not address the complete Ca range; rather, this relationship is further addressed in the companion modeling article (Erickson et al., 2022).
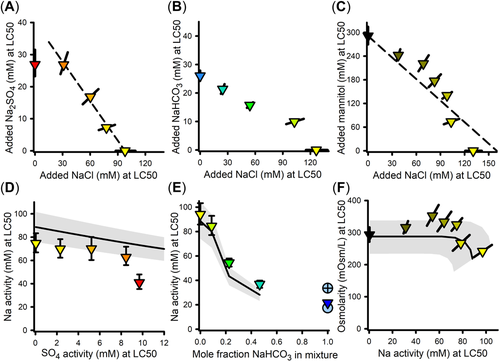
Effects of dilution water chemistry on toxicity of Na salts and mannitol
Effects of dilution water Ca
For all modifications of Ca in dilution water (Tables 2 and 3), Figure 4A provides LC50s for NaCl, Na2SO4, NaHCO3, and mannitol based on added compound concentration versus Ca concentration. Figure 4A also provides the two tests of NaHCO3 toxicity in 1×ALSW for which test solutions were equilibrated prior to exposure with either ambient air or with 1% CO2.
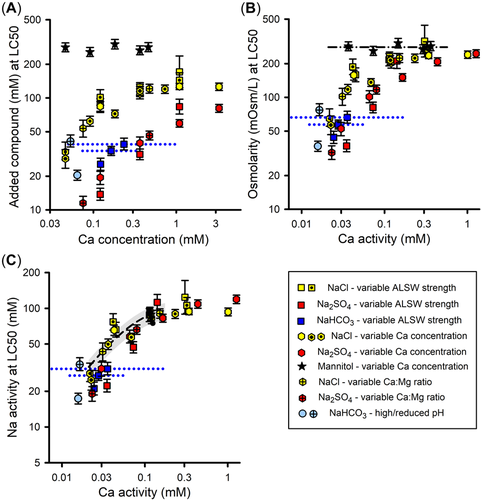
Median lethal concentrations for NaCl and Na2SO4 increase substantially with Ca concentration for all methods for modifying Ca concentration. Relative to the other salts, the data for NaHCO3 are more limited, consisting of three nonequilibrated tests in the ⅓×/1×/3×ALSW series and the pre-equilibrated test at high pH in 1×ALSW, and more uncertain due to the large changes in Ca concentration during the nonequilibrated tests in 1× and 3×ALSW. Nevertheless, based on mid-range Ca concentrations, the Ca dependence of NaHCO3 is similar to that for NaCl and Na2SO4. Only if the LC50s for the nonequilibrated 1× and 3×ALSW tests are related to the lowest Ca concentrations (at the end of the tests) would a Ca dependence not be evident. However, the pre-equilibrated test at high pH in 1×ALSW argues against such an interpretation because it was run at a constant Ca concentration similar to those at the end of the nonequilibrated 1× and 3×ALSW tests, and indicates the LC50 to be considerably lower at this Ca concentration.
In contrast to the Ca-dependence of inorganic Na salt toxicity, there is no effect of Ca on mannitol toxicity (Figure 4A). The ancillary experiment for the Ca-dependence of mannitol toxicity C. dubia similarly showed no effects (see Supporting Information, Figure S5). If mannitol toxicity is due to osmotic effects on passive water movement, Ca apparently has little effect on this process, versus its impact on other ion toxicity processes. In any event, these results suggest different mechanisms of toxicity for mannitol and the Na salts at lower Ca concentrations.
Effects of other dilution water constituents
In contrast to Ca, Mg has no apparent effect on the toxicity of Na salts. Increasing LC50s for Na salts in Figure 4 are associated with (1) increasing Mg for the variable ALSW tests (see Mg concentrations in Supporting Information, Table S1 for Experiments 13–19, 13–22, and 13–23); (2) decreasing Mg for variable Mg:Ca ratio tests (Experiment 13-28); and (3) constant Mg for the variable Ca tests (Experiments 15–02, 15–16, and 18–14). These same experiments also showed inconsistent correlations of NaCl salt toxicity to SO4 in the dilution water and of Na2SO4 toxicity to Cl in the dilution water (Supporting Information, Table S1), suggesting little or no effect of these anions. Changes in K had no effects on the toxicity of NaCl, but reduced that of Na2SO4 by 17% and that of NaHCO3 by 30% (Supporting Information, Table S1, Experiment 13–24). However, this effect of K on Na2SO4 toxicity is of uncertain statistical significance and this effect of K on NaHCO3 toxicity is confounded by uncertainties regarding Ca effects and the impact of high pH/alkalinity.
There is an effect of pH on NaHCO3 toxicity, as evidenced by the test with an unregulated pH of 9.3 having an LC50 approximately half of that in the test under an enriched CO2 atmosphere with pH 8.4 (Figure 4A). This pH effect is also pertinent to the toxicity of MgCO3 being greater than that for MgCl2 and MgSO4 (Figure 1).
Implications of single salt tests on exposure metrics for Na salt toxicity
Added molar salt concentration as exposure metric
In addition to addressing the effects of dilution water composition, the data in Figure 4 inform the issue of what exposure metric is best related to Na salt toxicity. Using added molar concentration as the exposure metric (Figure 4A) results in considerable differences among the chemicals. Depending on [Ca], Na2SO4 is two to five times more toxic than NaCl. When pH is not regulated (9.3–9.5), NaHCO3 is two to three times more toxic than NaCl, although similar when pH is reduced. In contrast, mannitol is substantially less toxic than NaCl, by a factor ranging from approximately 2.5 at higher Ca to 10 at lower Ca.
Many of these differences in toxicity based on added molar concentration have plausible explanations. The inorganic compound Na2SO4 has more Na per mole than NaCl and SO4 would complex Ca more strongly than Cl, both contributing to greater toxicity than NaCl. For NaHCO3, complexation of Ca would also increase toxicity relative to NaCl. Because NaHCO3 toxicity when pH is reduced corresponds well to that of NaCl and because pH alone up to 9.5 does not cause direct toxicity to fathead minnows (J.R. Hockett, unpublished data), the greater toxicity of NaHCO3 at high pH suggests some joint action of high pH (9.2–9.4) and alkalinity (>25 meq/L), consistent with that for MgCO3 (Supporting Information, Table S1). If osmolarity is a factor in Na salt toxicity at higher Ca, mannitol would be less toxic on a molarity basis due to having only one osmole per mole compared to two for NaCl. All these explanations indicate a need for better exposure metrics than total concentration.
Osmolarity as exposure metric
Because prior work with C. dubia indicated osmolarity to be a useful exposure metric for Na salt toxicity, Figure 4B plots LC50s based on osmolarity versus {Ca}. Interchemical variability is greatly reduced relative to Figure 4A, but does not achieve the same degree of concordance among chemicals as for C. dubia (Figure 7C in Mount et al., 2016). At {Ca} > 0.2 mM, LC50s for mannitol, NaCl, and Na2SO4 do converge within interexperimental variability and are similar to internal osmolarities of fish (Hoar & Randall, 1969), so that osmolarity is a plausible exposure metric in this Ca range for Na salt toxicity.
However, at lower Ca, osmolarity-based LC50s for NaCl and Na2SO4 become increasingly smaller with declining Ca, whereas mannitol LC50s remain constant. This indicates that osmotic effects cannot be the cause of Na salt toxicity at these lower Ca activities, so that osmolarity is not a good mechanistic exposure metric for this range and other exposure metrics must be considered. Furthermore, the osmolarity-based LC50s of NaCl and Na2SO4 in Figure 4B also diverge from each other at lower Ca, apparently due to SO4 toxicity when it is the sole or nearly sole anion (see Interactions in binary Na salt mixtures), and differences between NaHCO3 and NaCl toxicities also still exist, further underscoring the need for other exposure metrics.
Na activity as exposure metric
One interpretation for the divergence of Na salt toxicities from mannitol toxicity is that Na salts have specific ion-related toxicities at low Ca that decrease with increasing Ca until the LC50s are high enough to reach an osmolarity-related toxicity and coincide with the LC50 for mannitol. As a first consideration for a different exposure metric at low Ca, Figure 4C replots the data based on {Na}, this being the common ion for these salts. This further reduces differences in LC50s among the salts at lower Ca compared with Figure 4B, but even on this basis, Na2SO4 and NaHCO3 remain more toxic than NaCl at lower Ca, albeit by modest amounts. Although the idea that {Na} is a causal factor independent of anions parallels the inference that the toxicities of Mg, K, and Ca salts are cation-driven, this lack of concordance among the Na salts argues against it. How best to express exposure is further informed by the binary mixture experiments discussed in subsequent sections.
Na toxicity model for isobole predictions
Interactions in binary mixtures of Na salts
NaCl × Na2SO4 binary mixture experiment
Figure 5A presents the isobologram for LC50s based on molarity of added salt. Starting at the NaCl-only LC50 on the abscissa, the data for all the mixtures follow a straight line indicative of additivity, but then abruptly deviate horizontally to the Na2SO4-only LC50 on the ordinate. This deviation is consistent with the divergence of NaCl and Na2SO4 toxicities at low Ca in Figure 4. Because the only component that increases with this deviation is SO4, this suggests a SO4-specific toxicity once SO4 (or the ratio of SO4 to another ion) reaches some critical level. Below this critical level, the additive relationship between Na2SO4 and NaCl suggests a different mechanism involving only Na or multiple ions.
To illustrate this further, Figure 5D presents the data as {Na} versus {SO4}, with predictions from the {Na}-based toxicity model (Equation 2). The NaCl-only LC50 for this experiment is the lowest in our study (lowest black dot on Figure 4C) and the mixture data also follow the lower limit of the uncertainty band, declining gradually as {Ca} decreases due to complexation by SO4. However, for the Na2SO4-only test, the LC50 abruptly deviates from predictions as SO4 apparently reaches a toxic level. It is important to note that the adherence of the data to the model line at lower SO4 does not provide proof that {Na} is the toxicity driver, but only that it is a good correlate; this toxicity could be due to the combined effect of multiple ions as was the apparent case for C. dubia (Erickson et al., 2017; Mount et al., 2016). Toxicity of SO4 is also indicated by study-wide mean LC50s in ALSW from Supporting Information, Tables S1 and S2, which on the basis of {Na} drop from 89 mM for NaCl (n = 13) to 55 mM for Na2SO4 (n = 6), twice the drop from 89 to 72 mM predicted by the {Na}-based model.
NaCl × NaHCO3 binary mixture experiment
Figure 5B presents the isobologram for LC50s based on molarity of added salt. Although the general linearity of the data suggests a considerable degree of additivity, the low LC50s for NaHCO3-dominated test solutions indicate impacts of other factors. More clarity regarding this is provided by Figure 5E, which plots {Na}-based LC50s versus the mole fraction of NaHCO3 in the mixture, along with predictions from the {Na}-based model of Equation 2. There is considerable CaCO3 precipitation once the mole fraction reaches 20% (Supporting Information, Table S2), causing increased toxicity due to low {Ca}. The model does not predict this precipitation, but does predict the general drop of the LC50s when measured Ca concentrations are provided to it. The NaHCO3-only LC50 is not included in the prediction because the {Ca} for this test is beyond the range for Equation 2 and because of the possible role of elevated pH/alkalinity in such NaHCO3-dominated exposures. Equation 2 with {Na} as the exposure metric is thus appropriate for NaCl × NaHCO3 mixtures containing considerable NaHCO3, but the appropriate metric for NaHCO3−dominated exposures is uncertain.
Interactions in binary mixtures of NaCl and mannitol
A mixture experiment involving NaCl and mannitol (Figure 5C) was conducted to further address whether osmolarity is a useful exposure metric for some aspects of ion toxicity. The dashed line connects the molarity of the mannitol-only LC50 to an estimated molarity of NaCl needed to produce the same level of osmolarity, so represents additive toxicity for an exposure metric of osmolarity. Importantly, mixture toxicities are being elicited at combinations of mannitol and NaCl concentrations which individually would not be toxic, demonstrating considerable additivity (i.e., NaCl contributes to mannitol's mechanism of toxicity). There are upward deviations of mixtures from this line, but these are <20%. At higher proportions of NaCl there is a modest trend toward LC50s with lower osmolarities, suggesting a shift away from osmolarity-driven toxicity.
The relationships in Figure 5C are consistent with the data in Figure 4, which suggest that NaCl has a mechanism that is specifically ion-related and more toxic than mannitol at low Ca, but, as this toxicity decreases with increasing Ca, NaCl LC50s converge with that of mannitol and transition to a mechanism correlated with osmolarity. The mixture test in Figure 5C was conducted at Ca levels near but slightly below this transition, which would explain both the substantial degree of additivity on an osmolarity basis and the deviation to lower osmolarities for the NaCl-dominated tests.
Figure 5F plots LC50s on the basis of osmolarity versus {Na} to better illustrate the interaction of these two suggested mechanisms, with predictions based on the Ca-dependent {Na}-based model (Equation 2) and an average study-wide, Ca-independent, osmolarity-based LC50 for mannitol-only tests (278 mOsm/L, n = 7; Supporting Information, Tables S1 and S2). There are undoubtedly complexities to these toxicity relationships that are not fully resolved and data trends are modest relative to uncertainties, but these predictions are reasonably consistent with the data, namely, osmolarities at the LC50 are roughly constant and similar to mannitol for most mixtures and a transition to a specific ion-related mechanism is indicated at the highest proportions of NaCl. If this mechanistic interpretation is correct, the shape of this isobole would be expected to change at different dilution water [Ca], showing more independence at lower Ca and more complete additivity at higher Ca, which would be a useful element of future study.
Given the lack of environmental relevance of exposure to mannitol, the results in Figure 5C,F might seem to be of only academic interest. However, there is a need to address interactions of Na salts with salts of other cations, and interpreting such interactions will be informed by these interactions of NaCl and mannitol.
Interactions in binary mixtures of Na and Ca salts
Figure 6 provides a combined isobologram for LC50s from the NaCl × CaCl2 and Na2SO4 × CaCl2 mixture experiments, as osmolarity versus {Ca}. It also includes predicted LC50s at {Ca} > 3 mM for independent action of osmolarity-based toxicity (the aforementioned study-wide average of 278 mOsm/L for mannitol toxicity) and Ca-specific toxicity (a study-wide average of 15 mM {Ca} for CaCl2 toxicity from Supporting Information, Tables S1 and S2). At these higher {Ca}, the observed LC50s are consistent with independent action of these mechanisms, that is, starting with the CaCl2-only tests at the lower right, Ca activities at the LC50 first remain constant as Na salts are added and then transition to osmolarity-based LC50s similar to those for mannitol. As was the case for C. dubia (Erickson et al., 2017), there is an indication of greater-than-expected toxicity when the Ca-specific and osmolarity mechanisms are at roughly equal toxicity (the bend in the prediction line), but the deviation is only for one test and is <20%.
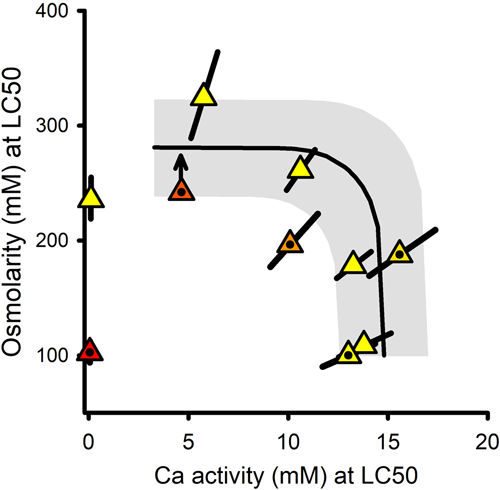
The Ca-dependent toxicity at lower Ca is not addressed by the predictions in Figure 6 because it involves only two data points and these have different mechanisms of toxicity. More complete consideration of this part of the relationship is provided in the companion modeling article (Erickson et al., 2022).
Interactions in binary mixtures of Na and Mg salts
NaCl × MgCl2 binary mixture experiment
Figure 7A compares LC50s based on added concentrations of NaCl and MgCl2 to fitted isoboles for concentration addition and independent action. The mixture with the highest NaCl fraction does fall on the independent action line, but the data then veer off to be intermediate to the two isoboles (i.e., “partial addition”). Plotting the data as {Mg} versus {Na} and using the independent action isobole predicted from Equations 1 and 2 has no appreciable effect on this pattern (Figure 7B), which was expected due to the strong correlation between added concentrations and cation activities. A primary implication of this is that there is a mechanism of toxicity to which both MgCl2 and NaCl contribute, instead of or in addition to any mechanisms related just to the individual salts.
For C. dubia, similar plots (Figure 3A–C in Erickson et al., 2017) showed LC50s to also be intermediate between concentration addition and independent action, although closer to the latter. When LC50s were expressed as {Mg} versus osmolarity there was close adherence to independent action (Figure 3D–F in Erickson et al., 2017). This was interpreted as Na salt toxicity involving a mechanism involving multiple ions, to which the Mg salts also contribute. Osmolarity was selected as the metric for this mechanism because of the close concordance of osmolarity-based salt LC50s with mannitol LC50s, at least at the {Ca} for the mannitol tests; these are also in accord with D. magna internal osmolarities indicated in Morris et al. (2021). However, this does not mean that effects are from osmotic stress across all {Ca}.
Because of such relationships for C. dubia, Figure 7C compares LC50s on the basis of {Mg} and osmolarity to a model of independent action for {Mg} (using Equation 1) and osmolarity (278 mOsm/L). The LC50s for the three tests with the highest mole fraction of NaCl have approximately the same osmolarity, consistent with toxicity being related to multiple ions, but are offset from the predictions by 15%–25%, indicating osmolarity is not the best metric. This deviation from the predicted osmolarity is consistent with this mixture experiment having {Ca} low enough that NaCl LC50s are reduced compared with higher {Ca}, where they closely agree wth mannitol LC50s (Figure 4B).
Because this deviation from mannitol LC50s is not great, osmolarity might still contribute to toxicity, jointly with other mechanisms of toxicity. This possibility is considered in Figure 7D, in which LC50s on the basis of Mg and Na activities are compared to a three-mechanism model that includes not only {Mg}-based and {Na}-based toxicity based on Equations 1 and 2, but also osmolarity-driven toxicity using the study-wide average mannitol LC50 of 278 mOsm/L. This moves the isobole closer to the observed LC50s, but falls short enough of the data that this is not a convincing mechanistic explanation.
NaCl × MgSO4 and Na2SO4 × MgCl2 binary mixture experiments
These experiments have results similar to that for NaCl × MgCl2, with LC50s expressed as Mg and Na concentrations or activities showing relationships intermediate to concentration addition and independent action. Because these mixed anion experiments had lower {Ca} due to complexation by SO4, osmolarity is expected to be even less explanatory of the LC50s. More details on these experiments are provided in Supporting Information.
Overall, the results of the NaCl × MgCl2, NaCl × MgSO4, and Na2SO4 × MgCl2 binary mixture experiments demonstrate partial additivity, or some other interaction, between these Na and Mg salts. Therefore, further model development at {Ca} lower than that at which toxicity is credibly related to osmolarity (≈0.2 mM; Figure 4) requires a metric instead of, or in addition to, the {Na} used in Equation 2 which can address interactions of NaCl toxicity with other ions. Based on the information in the present study, a suitable mechanistic metric based on the activities of specific ions is not clear. The implication of this to the development of models for ion toxicity is further addressed in the companion modeling article (Erickson et al., 2022).
Na2SO4 × MgSO4 binary mixture experiment
In contrast to the three other Na salt × Mg salt mixtures, the isobole for Na2SO4 × MgSO4 on an added salt basis (Figure 7E) is nearly linear, indicative of additive toxicity, although some deviation from the line is suggested at the MgSO4-only test. On a cation activity basis (Figure 7F), the isobole is also largely linear, deviating greatly from the predicted isobole for independent action using Equations 1 and 2, except for the MgSO4-only test. This linear isobole is supportive of the apparent SO4 toxicity noted regarding Figures 4 and 5. Figure 7G shows how toxicity in this experiment is associated with constant {SO4}, although the LC50 in the MgSO4-only test suggests a transition to Mg-driven toxicity.
Interactions of KCl with NaCl and MgCl2
The LC50s for both KCl and K2SO4 were similar on the basis of {K} and showed monotonic increases over the ⅓×/1×/3× ALSW dilution water series (Supporting Information, Table S1, Experiments 13–19 and 13–23). For C. dubia (Mount et al., 2016), similar increases were shown to be related to the Na concentration in the dilution water, based on tests in waters with variable NaCl concentrations (Figure 8A). However, fathead minnows differ from C. dubia in showing no such dependence on Na (Figure 8A). Such interspecies differences might reflect differences in K transporters in fish versus invertebrates (Griffith, 2017). The reason for the effect of different ALSW strengths on K salt toxicity to fathead minnows is unexplained and warrants further testing regarding other attributes of the dilution water, such as Ca concentration.
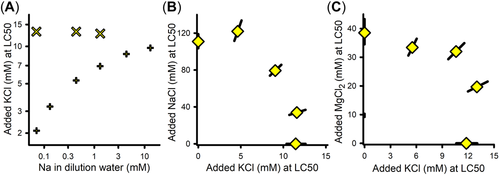
Figure 8B,C shows that, as for C. dubia (Erickson et al., 2017), K appears to involve a specific mechanism of toxicity that is independent of the toxicity of both NaCl and MgCl2. For D. magna, Morris et al. (2021) reported increased K concentrations in the hemolymph as K exposures approached lethality. This would represent disturbance of the normal K gradients between intracellular and extracellular fluid, consistent with a specific action for K that should be independent of other ions. Such independence of K toxicity means that, in the event that K exposure does get to toxic levels, toxicity data for K salts could be used independently of models for the toxicities of Na, Mg, and Ca salts.
SUMMARY
A total of 170 toxicity tests were conducted regarding the acute toxicities of major geochemical salts to fathead minnows, evaluating the impact of dilution water characteristics on these toxicities and the toxicity interactions in binary mixtures of these salts. The relationships among test results supported several inferences regarding mechanisms of toxicity and useful exposure metrics.
Mg and Ca salt toxicities
An important dilution water characteristic affecting the toxicity of Mg salts is Ca content, consistent with previous results for C. dubia (Erickson et al., 2017; Mount et al., 2016). Toxicities of Mg and Ca salts are best related to their cation activities and are concentration additive with each other, indicating a shared mechanism, as was also the case for C. dubia. However, in contrast to C. dubia, the toxicity of MgCO3−dominated exposures is greater than that of MgCl2 and MgSO4 on the basis of {Mg}, this enhanced toxicity possibly reflecting direct effects of combined high pH and alkalinity, requiring separate consideration in risk assessments.
K salt toxicities
In contrast to findings for C. dubia (Mount et al., 2016), the toxicity of KCl to fathead minnows is not affected by Na, but is reduced modestly by increasing all ions, with the specific cause of this being uncertain. Toxicities of K salts are also best related to cation activity and are independent of the toxicities of other salts, as was the case for C. dubia (Erickson et al., 2017; Mount et al., 2016).
Na salt toxicities
An important dilution water characteristic affecting the toxicity of Na salts is Ca concentration, also consistent with previous results for C. dubia (Erickson et al., 2017; Mount et al., 2016). Another notable effect of dilution water is reduction of NaHCO3 toxicity when the high unregulated pH (9.3) in tests with this salt is reduced to 8.4 by increasing CO2 concentration.
The toxicities of Na salts and mixtures are not simply related to Na but involve mechanisms related to multiple ions. For C. dubia, this was addressed across all Ca concentrations using an exposure metric of osmolarity (Erickson et al., 2017, 2018; Mount et al., 2016), although osmolarity per se was not claimed to be causative at all [Ca]. The present study indicates the need to consider multiple mechanisms. Osmolarity is possibly causative at [Ca] above that of ALSW, where osmolarity-based toxicities of Na salts are similar to that of mannitol. At lower Ca, Ca-dependent Na salt toxicities deviate from Ca-independent mannitol toxicity, indicating different mechanisms. Similar considerations are needed for further development of the models for C. dubia toxicity, given the Ca independence of mannitol toxicity for this species reported in Supporting Information.
The toxicities of NaCl, Na2SO4, and NaHCO3 at lower Ca also indicate different mechanisms among these salts. For Na2SO4-dominated exposures, toxicity is best attributed to {SO4}, but becomes additive with NaCl in mixtures that are not SO4-dominated, suggesting Na2SO4 also contributes to a mechanism in common with NaCl. Similar to MgCO3, the toxicities of NaHCO3-dominated exposures toxicity are greater than expected based on NaCl toxicity, apparently due to the combined effects of high pH and alkalinity as evidenced by the effect of pH on toxicity. The toxicity of NaHCO3 also becomes additive with that of NaCl in mixtures that are not HCO3-dominated, suggesting NaHCO3 also contributes to a mechanism in common with NaCl.
For Na salt toxicities not attributable to high pH/alkalinity, SO4, or osmolarity, the appropriate exposure metric is unresolved, despite good correlations with {Na} and osmolarity. Because mixture tests of Na salts with Mg salts indicate some degree of additivity, the exposure metric should not simply be {Na}, but include contributions of other ions. Using osmolarity to address these multi-ion effects would also not be advisable because it suggests that the mechanism involves osmotic effects, which likely is not the case.
Toxicity modeling implications
These results for fathead minnow present a more complicated picture than in Erickson et al. (2018) because C. dubia shows neither enhanced toxicity from high pH/alkalinity nor SO4-related toxicity, and because different mechanisms at different Ca were not then evident for general ion toxicity to C. dubia. Application of this information for fathead minnow to toxicity models thus presents more challenges, both in how models should be formulated and what range of exposure conditions should be included. Development of these models and certain implications to risk assessment are addressed in the companion article (Erickson et al., 2022).
Supporting Information
The Supporting Information is available on the Wiley Online Library at https://doi.org/10.1002/etc.5390.
Acknowledgment
The authors wish to thank K. Lott for supervising the culture of test organisms used in the present study and J. Nichols, J. Lazorchak, and three anonymous referees for helpful reviews.
Disclaimer
The views expressed in the present study are those of the authors and do not necessarily reflect the views or policies of the US Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author Contributions Statement
Russell J. Erickson: Conceptualization; Formal analysis; Methodology; Software; Visualization; Writing—original draft. David R. Mount: Conceptualization; Project administration; Supervision; Investigation; Methodology; Writing—review & editing. Terry L. Highland: Investigation; Methodology; Data curation; Writing—review & editing; Validation. J. Russell Hockett: Investigation; Methodology; Formal analysis; Data curation; Writing—review & editing; Validation. Dale J. Hoff: Investigation; Methodology; Supervision; Writing—review & editing. Correne T. Jenson: Investigation; Methodology; Data curation; Writing—review & editing; Validation. Teresa J. Norberg-King: Investigation; Methodology; Writing—review & editing. Brandy Forsman: Investigation; Methodology; Data curation; Writing—review & editing; Validation.
Open Research
Data Availability Statement
Data, associated metadata, and calculation tools are available through the USEPA Environmental Dataset Gateway (https://edg.epa.gov/metadata/catalog/search/resource/details.page?uuid=%7BBB921115-0AF4-4B11-905D-C8C584BF2247%7D) or from the corresponding author ([email protected]).




