Chesapeake Bay watershed pesticide use declines but toxicity increases
Abstract
Large areas of the Chesapeake Bay, USA, watershed are in agricultural land use, but there is no baywide program to track application rates of current-use pesticides in any of the watershed jurisdictions. Watershed studies demonstrate that several pesticides are present in surface and groundwater throughout the region. Between 1985 and 2004, the Maryland Department of Agriculture conducted surveys to estimate pesticide application within the state. Application rates of the dominant insecticides and herbicides were compiled over the survey period. Toxicity of the pesticides was tabulated, and the toxic units (TU) of applied active ingredients were calculated for several animal and plant species. The total mass of pesticides being applied to the watershed declined during the survey period. Due to increasing potency of the chemicals, however, total TUs applied have remained static or have significantly increased depending on the species of bioassay test organism used to assess toxicity. Applying estimates of pesticide transport into rivers in the Mississippi River basin show that significant quantities of pesticides may be entering Chesapeake Bay. Environ. Toxicol. Chem. 2011; 30:1223–1231. © 2011 SETAC
INTRODUCTION
The application of pesticides on agricultural lands introduces massive quantities of toxicants to watersheds and surficial water tables when viewed on a region-wide basis. The impact of the annual introduction of this stressor on receiving waters is unknown.
The quantity of undegraded pesticide that reaches water bodies via runoff or groundwater percolation is not well known. Likewise, the toxicity of the degradation products of many pesticides are not well studied, but some are known to be more toxic than the parent compound. Pesticide degradation byproducts are more likely to be found in groundwater than the parent compounds 1. Pesticides do reach local water bodies through spray drift, storm water runoff, and groundwater transport. Pesticides have been found in streams in agricultural areas of the Delmarva Peninsula (the eastern shore of Chesapeake Bay, USA) during base-flow conditions when the water source is mainly groundwater seepage 2, 3.
Most insecticides can be grouped according to their chemical structure, which in turn correlates with their mode of action. Most induce toxicity by interfering with the nervous system, although different types of compounds affect different components of neuronal function. For example, organophosphates interfere with neuronal and neuromuscular communication at the synaptic junction. Organochlorines and most carbamates affect selective ion channels on the axon. Other heavily used insecticides induce effects ranging from growth inhibition, to interference with molting, to pheromone effects, etc., but do not necessarily share the same chemical structure and therefore achieve effects through different mechanisms.
Herbicide toxicity is more complex in terms of mode of action. There are general classes of effect, such as auxin growth regulation (phenoxy compounds). Other compounds are used to inhibit root growth, but this may be achieved by a variety of mechanisms from inhibition of cell division to disruption of cell membranes. Some are metabolic toxicants that interfere with particular biochemical reactions such as formation of specific amino acids or lipids (glyphosate, imazapyr, thiocarbamates) but may elicit similar symptomatic responses in the plants. Similarly, some herbicides interfere with photosynthesis but at different steps in the biochemical process or by breaking down the chlorophyll directly. Thus, the potential synergistic and/or additive interactions of herbicides on plants are considerably more complex than in the case of insecticides on animals. Methods of herbicide application are another complicating factor related to the mode of action. Some herbicides must be applied to foliage to be effective. If they fall to the ground, they may or may not be taken up by the plant and therefore may or may not be effective. Other herbicides are applied to the ground as a pre-emergent poison acting on the seeds or the germinating plants. Herbicides may or may not be translocated through the plant even if they are taken up. This is both chemical specific and plant species specific. That the efficacy of a particular herbicide is related to its tendency to stay in the soil or not has implications for its potential to migrate to water bodies.
The mix of pesticides applied to the landscape is constantly changing. Different products are introduced on the market and others fall out of favor or are restricted by regulation. Crops are rotated between growing seasons, requiring different pesticides from year to year. Mixtures of pesticide active ingredients are marketed in different product formulations. Current-use pesticides are considerably more labile than older generations of pesticides. They break down in the environment more readily and do not pose as great a threat as compounds that are persistent and that may also bioaccumulate. This lack of persistence means that, to be effective at pest control, either they must be more toxic or they must be used in greater quantity. Pesticides are a significant expense to farmers, who do not want to apply more than is necessary. Therefore, an interplay exists among how much is applied, at what cost, and its effectiveness.
The constantly changing array of pesticides used in agriculture, and the relative lack of quantitative data on ultimate fate and transport, renders assessment of pesticide impact on receiving waters difficult. What is the long-term impact of fluctuating, but continuous, input of pesticides on receiving waters? What is the potential magnitude of the issue on a watershed scale? How has it changed over time? The present study attempts to address the latter two questions by examining the loading of pesticides, relative to their toxic effect, on a statewide basis in Maryland from 1985 to 2004.
MATERIALS AND METHODS
Outside of California, there is no current comprehensive statewide database for pesticide use. The U.S. Department of Agriculture (USDA) National Agricultural Statistical Service estimates pesticide use for specific crops in selected states. The U.S. Environmental Protection Agency (U.S. EPA) Office of Pesticide Programs estimates pesticide usage on a national scale. The most recent data available are for 2001 4. From 1985 through 2004, the Maryland Department of Agriculture (MDA) estimated pesticide use in the state of Maryland every two to four years by farm operators, certified applicators (commercial and private), and public agencies 5-11. The Maryland database is the most comprehensive long-term data set on pesticide usage in the entire Chesapeake Bay watershed. Data were gathered through a survey, but response was not mandatory. Response rates varied from 38 to 78%, depending on sector and year, so all the estimates reported in this paper are underestimates of actual usage. Furthermore, the U.S. EPA estimates that approximately 75% of all pesticide usage in the United States is agricultural. The other 25% is for home and garden use, industrial, commercial, and government sectors.
The top 20 herbicides and insecticides, in terms of total pounds applied, were compiled for each survey year. As the mix of pesticides applied changed over the years, the list of the top 20 evolved, but those that fell out of the top 20 were maintained in the data set to track changes in the use of pesticide types over time. Wood preservatives (e.g., chromium-copper-arsenic [CCA]), boat bottom-paint antifoulants (cuperous oxide), and pesticides that are not generally released by broadcast application (boric acid) were excluded. Applications of Bacillus thuringiensis in the early 1990s were not included. This biopesticide was applied in response to an outbreak of gypsy moths, and large swaths of urban and forested acreage were treated, in addition to use on agricultural land. Insecticides were grouped by chemical structure. Herbicides were grouped by toxic mode of action.
Bioassay lethal/effective concentration (LC50/EC50) data was gathered from the U.S. EPA Pesticide Ecological Effects Database 12. The data values and reports have been reviewed in a consistent program by the U.S. EPA. Other sources were surveyed: the Extension Toxicology Network (EXTOXNET, http://extoxnet.orst.edu/etn.txt.html), the U.S. Fish and Wildlife Service Contaminant Hazard Reviews 13 (http://www.pwrc.usgs.gov/infobase/eisler/CHR_all_1-35.pdf), and the Crop Protection Handbook 14, but most values from the various sources were picked up in the U.S. EPA database. Test species included rainbow trout (Oncorhynchus mykiss), bluegill sunfish (Lepomis macrochirus), the waterflea (Daphnia magna), and a marine mysid shrimp (Mysidopsis bahia). These are common test species for which large data sets are available. For fish and mysids, 96-h static-test LC50 data for larvae or juveniles or both were selected. For Daphnia, 48-h static EC50 data for juveniles were used. Other endpoints and test durations were not used. Only tests using 90% or greater active ingredient (a.i.) were included so that data could be related to active ingredient, which was how the MDA reported usage data. Plant species included a freshwater algae (Selenastrum capricornutum), a marine algae (Skeletonema costatum), and duckweed (a freshwater vascular plant, Lemna gibba). For algae and duckweed, 7- to 14-d EC50 data were used. Toxicity values were averaged for each pesticide for each species. If the values varied by more than one order of magnitude, the geometric mean was used. If static-test data were not available, data from flow-through tests were used. The resultant data sets are shown in Tables 1 and 2.
| Chemical | Type | Trout | Bluegill | Daphnia | Mysid |
|---|---|---|---|---|---|
| Aldicarb | CB | 560 | 83.4 | 553 | 13 |
| Bendiocarb | CB | 1,375 | 1,062.5 | 29.2 | 6.7b |
| Carbaryl | CB | 7,600 | 9,523.5 | 5.6 | 5.7b |
| Carbofuran | CB | 400 | 88 | 29 | 0.98 |
| Metam-sodium | CB | 34,100 | 510 | 2,360 | |
| Methomyl | CB | 2,065 | 16 | 230 | |
| Chlordane | OC | 69.5 | 74 | 240 | |
| Endosulfan | OC | 0.965 | 2.07 | 166 | |
| Heptachlor | OC | 7 | 13 | 3.4 | |
| Lindane | OC | 18 | 25 | 110b | 6.3b |
| Toxaphene | OC | 6.4 | 2.4 | 10 | |
| Acephate | OP | 110,000 | 2,000,000 | 16,400 | 3,800b |
| Azinphos-methyl | OP | 8.8 | 4.5 | 1.1 | 0.21b |
| Bromchlophos | OP | 270 | 2,200 | 0.3 | 8.8 |
| Chlorpyrifos | OP | 7.1 | 2 | 1.7 | 0.04b |
| Diazinon | OP | 90 | 168 | 1 | 4.2b |
| Dichlorvos | OP | 100 | 1,109.5 | 1 | 19b |
| Dimethoate | OP | 6,850 | 6,000 | 275c | 15,000b |
| Disulfoton | OP | 1,850 | 132.7 | 13 | 100b |
| Isofenphos | OP | 1,800 | 1,400 | 3.9 | 1.7b |
| Malathion | OP | 17.05 | 54 | 1 | 2.2b |
| Parathion | OP | 1,430 | 73.5d | 2 | 0.55b |
| Phorate | OP | 13 | 1.5 | 37 | 1.1b |
| Phosmet | OP | 167.5 | 72 | 5.6 | 16 |
| Temephos | OP | 8,956.6 | 32,400 | 0.011 | |
| Terbufose | OP | 7.6 | 1.9 | 1.07d | 0.22 |
| Trichlorfon | OP | 1,052.5 | 230 | 5.6b | 6.7b |
| Cyfluthrin | PY | 0.155b | 1.5 | 0.141 | 0.0023b |
| Cypermethrin | PY | 1.01b | 2.8b | 56.2d | 0.0055b |
| Esfenvalerate | PY | 0.07b | 0.23b | 0.15 | |
| Fenvalerate | PY | 0.73 | 0.26 | 0.333 | 0.008b |
| Lamda-cyhalothrin | PY | 0.165b | 0.21b | 0.14 | 0.0041b |
| Permethrin | PY | 6 | 8.1 | 1.76d | 0.033b |
| Tefluthrin | PY | 0.06b | 4.5b | 0.07 | 0.053b |
| Bifenazate | X | 760b | 580b | 500b | 58b |
| Captan | X | 73 | 310 | 445 | 8,400 |
| Chinomethionate | X | ||||
| Diflubenzuron | X | 140,000 | 182,500b | 3.2 | 2.1b |
| Fipronil | X | 142.5b | 54b | 106.3b | 0.14 |
| Halofenozide | X | 3,600 | 3,500b | ||
| Imidacloprid | X | 22,9100 | 85,200 | 38b | |
| Vorlex | X | ||||
| Median LC50s | |||||
| CB | 1,720.00 | 510.00 | 29.10 | 6.70 | |
| OC | 7.00 | 13.00 | 138.00 | 4.85 | |
| OP | 218.75 | 150.35 | 1.85 | 4.20 | |
| PY | 0.165 | 1.500 | 0.150 | 0.007 | |
| X | 760.00 | 445.00 | 472.50 | 48.00 |
- a Median toxicity values by chemical type are also shown at the bottom. CB = carbamate; OC = organochlorine; OP = organophosphate; PY = pyrethroid; X = other.
- b Flow-through bioassay.
- c Lowest-observed-effect concentration (LOEC).
- d Geometric mean.
| Chemical | Action | Trout | Bluegill | Daphnia | Selenastrum | Skeletonema | Lemna |
|---|---|---|---|---|---|---|---|
| Acetochlor | Root inhib. | 0.67 | 1.47 | 9.80 | 0.0014 | 0.0051 | 0.0034 |
| Alachlor | Root inhib. | 17.33 | 42.00 | 21.00 | 0.0016 | ||
| Benefin | Root inhib. | ||||||
| CDAA | Root inhib. | ||||||
| Dimethenamid | Root inhib. | 61.58b | 8.20 | 14.00 | 0.0160 | 0.0700 | 0.0145 |
| Metolachlor | Root inhib. | 7.90 | 3.20 | 22.50 | 0.0090 | 0.0855 | 0.3510b |
| Naptalam | Root inhib. | 76.10 | 354.40 | 118.50 | |||
| Oryzalin | Root inhib. | 3.36 | 2.88 | 1.50 | 0.0420 | 0.0410 | 0.0154 |
| Pendimethalin | Root inhib. | 0.14 | 0.20 | 0.28 | 0.0054 | 0.0052 | 0.0125 |
| Prodiamine | Root inhib. | 3.18 | 10.33b | ||||
| Sulfometuron methyl | Root inhib. | 24.00c | 0.0046 | 0.0005 | |||
| Trifluralin | Root inhib. | 0.02 | 0.01 | 0.56 | 0.0280 | 0.0435 | |
| 2,4-D | Auxin mimic | 239.33 | 221.50 | 25.00 | 42.2000 | 2.0200 | 0.6950 |
| 2,4-DP | Auxin mimic | ||||||
| Chloramben | Auxin mimic | ||||||
| Clopyralid | Auxin mimic | 103.50 | 125.40 | 225.00 | 6.9000 | ||
| DCPA (Chlorthal) | Auxin mimic | 30.00 | 82.50 | ||||
| Dicamba | Auxin mimic | 81.70 | 135.30 | 110.70 | 0.4900 | ||
| Dithiopyr | Auxin mimic | 0.46 | 0.47 | 17.00 | 0.0200 | ||
| MCPA | Auxin mimic | 90.00 | 97.00 | 27.00c | 106.4500 | 6.9280b | 1.8750b |
| MCPP | Auxin mimic | 124.80 | 9.9640b | 0.0170 | 1.9000 | ||
| Triclopyr | Auxin mimic | 334.50 | 0.36d | 39.36b | 17.7000 | 8.0350 | 8.2000 |
| Aciflourfen | Metabolic | 35.50 | 31.00 | 52.55 | 0.3780 | ||
| Bensulide | Metabolic | 0.91 | 1.11 | 0.58 | 1.8000 | 0.1500 | |
| Butylate | Metabolic | 8.63 | 4.87 | 11.90 | 4.1000 | ||
| Chlormequat | Metabolic | ||||||
| Dinoseb | Metabolic | ||||||
| EPTC | Metabolic | 20.48 | 19.57 | 11.26 | 1.3800 | 6.1000 | 5.6000 |
| Glyphosate | Metabolic | 14.00 | 92.50 | 78.86b | 13.2700 | 3.3700b | 24.0000 |
| Imazapyr | Metabolic | 92.00c | 750.00 | 1.0000b | 92.0000 | 0.0227 | |
| Imazaquin | Metabolic | 280.00 | 420.00 | 280.00 | |||
| Maleic-hydrazide | Metabolic | 107.50 | 114.0000 | ||||
| Mesotrione | Metabolic | 840.00 | 1.9000 | 20.0000 | 17.7000 | ||
| Metam-sodium | Metabolic | ||||||
| Vorlex | Metabolic | ||||||
| Sodium chlorate | Desiccant | 919.30 | 133.0000 | ||||
| Atrazine | Photo. inihib. | 4.50 | 28.40 | 27.95 | 0.0740 | 0.0385 | 0.0800 |
| Clomazone | Photo. inihib. | 19.00 | 34.00 | 5.20 | 3.5000 | 1.0000 | 35.0000 |
| Cyanazine | Photo. inihib. | 9.00 | 22.50 | 45.50 | 0.0055 | 0.0178 | 0.0640 |
| Diquat dibromide | Photo. inihib. | 124.60 | 1.86b | 0.0094 | |||
| Diuron | Photo. inihib. | 1.95 | 3.00 | 8.40 | 0.0024 | ||
| Linuron | Photo. inihib. | 3.00 | 9.60 | 0.77 | 0.0670 | 0.0359 | 0.2730 |
| Metribuzin | Photo. inihib. | 60.92 | 83.98 | 14.56b | 0.0144 | 0.0088 | 0.1600 |
| Paraquat | Photo. inihib. | 33.84 | 84.50 | 0.3200 | 2.8400 | 0.0980 | |
| Simazine | Photo. inihib. | 48.37 | 58.00 | 0.1000 | 0.6000 | 0.1400 | |
| Median LC/EC50s | |||||||
| Root inhib. | 5.63 | 3.18 | 12.16 | 0.0054 | 0.0345 | 0.0145 | |
| Auxin mimic | 96.75 | 111.20 | 39.36 | 13.8320 | 2.0200 | 1.8875 | |
| Metabolic | 20.48 | 25.28 | 78.86 | 1.8000 | 13.0500 | 4.8500 | |
| Photo. inhib. | 14.00 | 34.00 | 8.40 | 0.0670 | 0.0385 | 0.1400 |
- CDAA = 2-chloro-N,N-diallylacetamide; 2,4-DP = 2-(2,4-dichlorophenoxy) propionic acid; DCPA = dimethyl tetrachloroterephthalate; MCPA = 2-methyl-4-chlorophenoxyacetic acid; MCPP = methychlorophenoxypropionic acid; EPTC = s-ethyl dipropylthiocarbamate.
- a Median values by mode of action are also shown at the bottom.
- b Geometric mean.
- c Lowest-observed-effect concentration (LOEC).
- d Flow-through bioassay.
All application rates were converted from pounds to kilograms. Toxicity units (TU) were calculated for each year for each chemical, based on the application rate and the LC/EC50 for each species (TU = kg/LC50). The total TU of herbicides and insecticides was summed for each species by year. The total kilograms applied versus total applied in terms of TU was plotted over time to assess trends in application rates and potential toxic impact. None of the species had complete toxicity data for all pesticides. Also, the mix of pesticides for which toxicity data were available for each species was different, so the mix of data differs between species to some degree. The insecticide data are nearly complete for the animal species, but more data exist for fish than for invertebrates. Data on herbicide toxicity to animals are less complete, but even fewer data are available for nontarget plant species (Table 2); thus the true patterns of application rate versus toxicity for plants and algae are less certain. Data for EC50s for insecticide toxicity to plants are largely unavailable.
RESULTS
The toxicity of the different classes of insecticides varies greatly between animal type and insecticide class (Table 1). Carbamates are generally the least toxic to all species but are considerably more toxic to invertebrates than to fish. Organochlorines are considerably more toxic to fish than carbamates. Organophosphates vary widely in toxicity. They are less toxic to fish than the organochlorines but are orders of magnitude more toxic to arthropods than to fish. The more recently introduced pyrethroids are the most toxic type among all insecticides, especially to mysids.
Insecticide application ranged from a high of over 1,000,000 kg in 1985 to a low of 194,000 kg in 1997, followed by an increase to over 385,000 kg in 2004. The amount of organochlorines has been greatly reduced because of restrictions or bans on their use (Fig. 1). Only endosulfan and lindane were reported in the most recent years of the survey. Carbamate and organophosphate application rates have declined greatly, but the use of organophosphates was up to 159,000 kg in 2004. The rise and fall of organophosphates are due almost entirely to changes in the application of chlorpyrifos, which accounted for 69% of all organophosphates and 29% of all insecticides applied in 2004. The decline in carbamates is primarily attributable to the 1991 ban on granular application of carbofuran, which accounted for more than 58% of all carbamates in 1988 and 80% in 1985. Carbofuran has since been banned for use on food crops. Pyrethroids are becoming more heavily used because of their extreme toxicity to arthropods, but with relatively low toxicity to mammals. Changes in the use of permethrin have driven the changes in pyrethroids in the past, but cypermethrin and esfenvalerate were the dominant pyrethroids used in 2004. The insecticides listed as miscellaneous in Table 1 exhibit a variety of effects, from neurotoxicity to hormone mimics that interfere with proper molting. The introduction of the neurotoxin imidacloprid in 2000 is primarily responsible for the increase in this group of compounds.
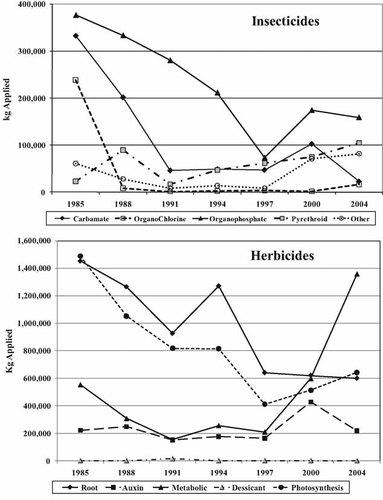
Trends in pesticide application in Maryland from 1985 to 2004. Insecticides are grouped by chemical type. Herbicides are grouped by mode of action (root = root growth inhibition; auxin = auxin mimic; metabolic = enzyme/membrane disruption; desiccant = desiccant; photosynthesis = electron transfer/chlorophyll disruption).
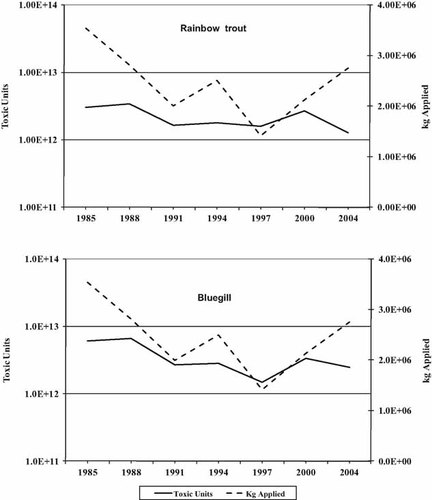
The calculated TUs of applied insecticides, relative to the mass of insecticides applied, are shown in Figure 2 for fish. Although the mass of pesticides declined in the 1980 s and early 1990s, the TUs declined as well. As insecticide use leveled off in the late 1990s and the early part of the new century, the toxicity of the chemicals used increased up to or greater than the levels seen in 1985. For both species of fish, the increase in toxicity is due to cyfluthrin, cypermethrin, and esfenvalerate, all pyrethroids. A different picture is seen for the arthropods (Fig. 3). In this case, TUs never decreased significantly with the decline in application rates and dramatically increased for Daphnia in 2004. Also, the total TUs for the invertebrates are in the range of 1015 to 1016 as opposed to 1014 for the fish. The increase in TUs for Daphnia is due primarily to cyfluthrin. Cypermethrin, telfluthrin, and the organophosphate temophose also contribute to the increase over earlier years. Similarly, cyfluthrin, cypermethrin, and lambda-cyhalothrin are the main sources of TU increases for the mysids. It should be noted that most data for Mysids are from flow-through tests, which yield lower values than static tests. Most values (but not all) are lower for Mysids than for Daphnia.
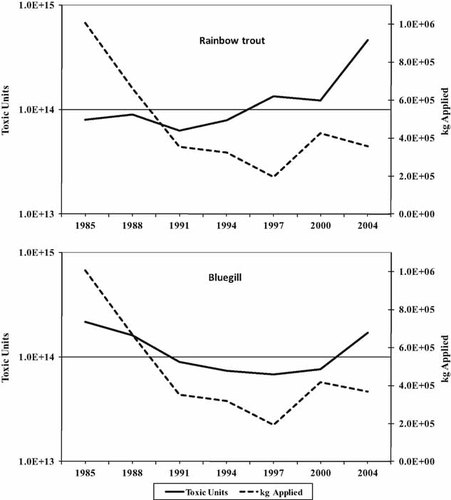
Total kilograms of insecticide applied in Maryland versus toxic units for rainbow trout (Oncorhynchus mykiss) and bluegill sunfish (Lepomis macrochirus).
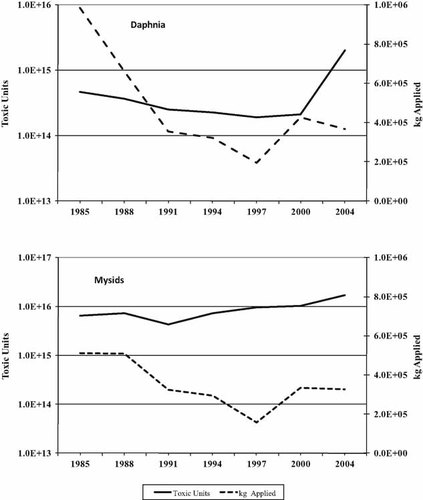
Total kilograms of insecticide applied in Maryland versus toxic units for the cladoceran Daphnia magna and the shrimp Mysidopsis bahia.
Herbicide application fluctuated, ranging from 3,700,000 kg in 1985 to 2,800,000 kg in 2004 (Fig. 1), roughly an order of magnitude higher than insecticide application rates. The primary root growth inhibitors used were metolachlor and alachlor, but use of the latter declined from over 450,000 kg to less than 16,000 kg from 1985 to 2004. Metolachlor has always been the dominant herbicide used in this category and in 2004 accounted for 86% of root growth inhibitors. The dominant auxin mimic used throughout the entire period was 2,4-dichlorophenoxyacetic acid (2,4-D). In 2000, more than 210,000 kg of 2-methyl-4-chlorophenoxyacetic acid (MCPA) was applied versus 102,000 kg of 2,4-D. Chemically, they are almost identical. Except for that year, 2,4-D constituted 40 to 70% of all auxin mimics. Products containing 2,4-D as the primary active ingredient are also used extensively in home-use broad-leaf-weed–control products. The rise in the use of metabolic poisons is due entirely to glyphosate and the introduction of genetically engineered Roundup®-ready crops that are tolerant to it. Glyphosate is the most heavily used herbicide in the data set, with nearly 1,280,000 kg reported applied in 2004. It is also marketed heavily in the home-use sector. Sodium chlorate is the only desiccant on the list that was ever in the top 20 herbicides. Its reported use was sporadic, and it was in the top 20 herbicides only in 1991, when it was used almost exclusively in Dorchester County on the eastern shore of the Chesapeake Bay. It is a nonselective herbicide that will kill all vegetation, generally used for clearing land or maintaining transportation corridors. Atrazine was the most heavily used photosynthesis inhibitor accounting for 50 to 80% of this category. Nationally, atrazine is one of the most widely used herbicides. The dominant herbicides, atrazine, glyphosate, and metolachlor, amounted to 44 to 81% of all herbicides applied. In 2004, more than 2,200,000 kg of these three chemicals alone were reportedly applied to the landscape. A plot of the dominant herbicides used in each mode of action class is shown in Figure 4.
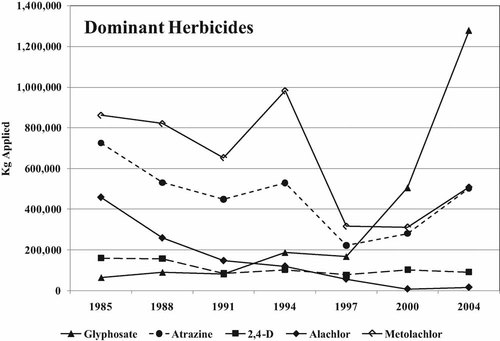
Total kilograms of the dominant herbicides applied in Maryland from 1985 to 2004.
Although these three herbicides were important contributors to the sum of TUs toxic to animals by virtue of their application rates, the most toxic compounds to fish and invertebrates were the dinitroanilines, trifluralin, pendimethalin, and oryzalin (Table 2). The organophosphate bensulide is also relatively toxic to fish and Daphnia and exhibits the same neurotoxic mode of action in animals as other organophosphate insecticides. The total TUs of the herbicides to fish and invertebrates were one to several orders of magnitude less than the TUs of the insecticides (Figs. 5 and 6), which were applied at much lower rates. The drop in TUs for fish (Fig. 5), Daphnia (Fig. 6), and Skeletonema (Fig. 7) between 2000 and 2004 is due primarily to an order of magnitude drop in the use of pendimethalin between those years.
Total kilograms of herbicide applied in Maryland versus toxic units for rainbow trout (Oncorhynchus mykiss) and bluegill sunfish (Lepomis macrochirus).
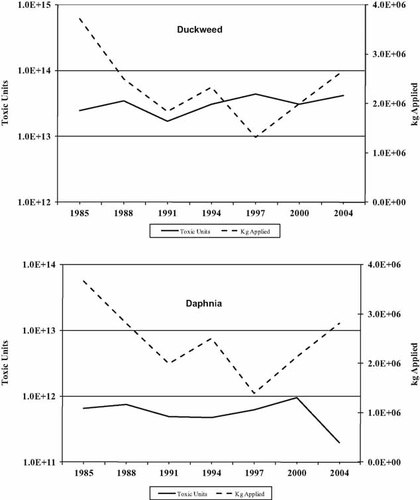
Total kilograms of herbicide applied in Maryland versus toxic units for duckweed (Lemna gibba) and the cladoceran Daphnia magna.

Total kilograms of herbicide applied in Maryland versus toxic units for a freshwater algae (Selenastrum capricornutum) and a marine algae (Skeletonema costatum).
The most toxic herbicides to duckweed and algae were the root growth and photosynthesis inhibitors (Table 2). Atrazine and metolachlor were considerably more toxic than glyphosate. The specific mode of action of the root growth inhibitors on the algae is not known, but these chemicals generally affect cell membrane function or cell division, effects that would also impact algae. What effect the auxin mimics might have on algae and why the diatom is as sensitive as the duckweed are other unknowns. Overall, for the algae and duckweed, the total TUs of herbicides applied over the reporting period did not change substantially.
DISCUSSION
The Potomac River is the second largest tributary into the Chesapeake Bay. It drains a large proportion of Maryland and parts of Pennsylvania and northern Virginia, including the Shenandoah Valley. The Potomac subestuary is roughly the same size as San Francisco Bay. The average flow rate for the Potomac River is 13,000 cubic feet per second (cfs). For the sake of illustration, at that flow rate, if all the insecticides released onto the landscape in 1997 were metered into the river, it would be enough to make the river toxic to D. magna for 16.5 years, to Mysids for 837 years, to rainbow trout for 11.6 years, and to bluegill for 5.8 years. For herbicides, the figures are 3.8 years for duckweed and 5.2 and 14.3 years for Skeletonema and Selenastrum, respectively. The 1997 data comprise information from 209 (100%) public agencies, 831 (58.6%) licensed commercial applicators, 2,540 (58.4%) certified private applicators, and 971 (59.7%) sampled farm operators 9. There were toxicity data for 89% of the insecticides but only 56% of the herbicides (for the plant species). No accounting was made of household use by citizens on lawns or other private land. Thus, if the data set were actually complete, the calculated TU values would be considerably higher.
Clearly, the calculations with regard to the Potomac River (including the inherent assumption of additivity) are contrived, but they do illustrate the magnitude of the release of toxic substances into the landscape. The Potomac is the second largest tributary in the entire Chesapeake Bay system, and the capacity of a large river being inadequate to dilute what is an underestimate of the annual pesticide application in the watershed is pertinent. Aquatic wildlife in the smaller rivers and tributaries in southern Maryland and on the Delmarva Peninsula (on the east side of Chesapeake Bay) that are literally surrounded by farm fields cannot help but be impacted by the constant input of pesticides. In a compilation of U.S. Geological Survey (USGS) data from streams in the mid-Atlantic, Ferrari et al. 15 reported that concentrations of nine pesticides were found to be greater than Federal Ambient Chronic Water-Quality Criteria for the Protection of Aquatic Organisms in a small number of samples. Consequences to the open Chesapeake Bay are unknown.
The properties that govern pesticide transport from the site of application are persistence and mobility. These are influenced in the field by soil characteristics, local climate, topography, and agricultural management practices. Persistence is a function of the rate of degradation under field conditions. Degradation may result from chemical transformation processes, photochemical reactions, and microbial metabolism. For example, the half-life for diazinon ranges from 3 to 54 d, with 3 to 13 d considered representative of actual field conditions 16. In general, the time taken for 90% of a pesticide residue to dissipate is four times the field dissipation half-life (see USDA, http://www.arsusda.gov/). Properties that affect pesticide movement are water solubility, sorption coefficient, and Henry's law constant 17-19. The tendency of a pesticide to bind to soil particles is a function of its organic carbon normalized adsorption coefficient (KOC). Pesticides with relatively high KOC tend to remain in the soil or attach to soil particles entrained in flowing water. Pesticides with relatively low KOC values and/or higher solubility are more likely to be leached from the soil and transported by surface or groundwater. The tendency of a pesticide to volatilize is expressed by Henry's law constant. Compounds with values less than approximately 1.2 Pa-m3/mol are considered to have low volatility. For example, the value for diazinon predicts that it will tend to remain in solution rather than volatilize to the atmosphere 20, 21. Volatilization rates from ground surfaces are complicated by competing processes, such as sorption of the pesticide to the organic matter of plants and/or soil, as well as the amount of sunlight and wind 18.
Presumably, farming practices in Maryland are similar to those in other states bordering the Chesapeake, including Virginia to the south, most of which drains into the Chesapeake. The Susquehanna River also drains large agricultural watersheds in central Pennsylvania to the north, where orchards are more important than in Maryland, and the watershed extends well up into New York State.
The USGS has conducted a number of water quality studies on selected watersheds in the mid-Atlantic region, including some of the major watersheds to the Chesapeake 15, but a comprehensive assessment of the entire watershed or the Bay itself has not been done. In the mid-Atlantic study 15, pesticides were detected in 75% of surface water samples and at more than 90% of the sites sampled over several studies. The authors reported that the 16 pesticides most commonly detected in the mid-Atlantic region are found in streams year-round. Concentrations of herbicides in particular were highest in the spring and summer. Median concentrations of herbicides were higher in basins where agriculture was the dominant land use, and median concentrations of insecticides were higher in basins where the major land use was urban, indicating significant nonagricultural sources. Concentrations of selected pesticides in small streams increased during high flows in the growing season, up to 30 times the concentration observed during low-flow conditions.
The total mass of pesticides reaching most of the tributaries and the Chesapeake Bay itself is unknown. Herbicide loading rates to the Gulf of Mexico via the Mississippi River were estimated to be 640, 320, 215, 53, and 50 metric tons for atrazine, cyanazine, metolachlor, simazine, and alachlor, respectively, in 1993 22. The authors estimated that this amounted to 1 to 2% of the atrazine and cyanizine annually applied to the drainage basin, and less than 1% of the others. Presumably the loading in regional watersheds in the Mississippi River system would be higher because degradation would have occurred throughout the system as the rivers flow to the Gulf of Mexico. Larson et al. 23 assessed herbicide and insecticide concentrations throughout major tributaries of the Mississippi River basin. Their calculations of the flux of 25 individual pesticides to the river ranged from 0 to 5.2% of total application (excluding diazinon [up to 20%], because the authors concluded that the concentrations in the rivers were a consequence of nonagricultural use, consistent with the findings reported by Ferrari et al. 15). The average flux ranged from 0.30 to 0.46% of use depending on watershed. With these flux rates applied to the maximum and minimum quantity of insecticide TUs applied, the average annual delivery of insecticides in Maryland to the Chesapeake Bay would have ranged from 584 to 4,754 kg active ingredient between 1985 and 2004. Going back to the Potomac River illustration, if this mass of insecticides was metered into the river, it would be enough to make the river toxic to D. magna and M. bahia for 0.14 to 0.2 and 2 to 3.6 years, respectively. This input occurs on an annual basis. For fish, the numbers are between 0.03 and 0.06 years. However, it should be noted again that these values are based on incomplete reporting and apply only to those insecticides for which LC50s exist. Also, the endpoints are for acute lethality, not a sublethal endpoint.
For herbicides, a flux of 0.3 to 0.46% from the watershed would have delivered 4,275 to 17,108 kg active ingredient between 1985 and 2004. For duckweed, this translates into less than 0.01 to 0.013 of a year of Potomac River flow. For Skeletonema and Selenastrum, respectively, the periods are 0.013 to 0.02 and 0.06 to 0.08 years. The results for fish are two orders of magnitude less.
The Chesapeake Bay is not a toxic soup because of pesticides. The well-documented decline and rise of striped bass is a prime example. In the 1970 s and 1980 s, the population of striped bass collapsed. The decline was attributed primarily to overfishing, which might have made the striped bass population more susceptible to pollution and other stresses, including pesticides in the spawning streams. However, after Maryland and Delaware imposed fishing moratoria from 1985 through 1989 to protect the dominant year class and Virginia imposed a one-year moratorium in 1989, the population rebounded and with strict harvest controls has remained healthy to the present time.
Blue crabs (Callinectes sapidus) are another important fishery which is in decline in the Chesapeake Bay. According to surveys conducted jointly by Maryland and Virginia, significant decline has been observed in the spawning stock, recruitment, larval abundance, and female size since 1992 (Virginia Institute of Marine Science, http://www.vims.edu/research/units/programs/bc_winter_dredge/index.php). Stocks are down by more than 80%. Postlarval recruitment has declined by an order of magnitude. It is thought that the initial decline resulted from poor recruitment in 1991, and the population has not had a chance to recover since, because of overfishing. The population is known to go through cycles, but the current decline has persisted for more than a decade. Because crabs are arthropods, they are more sensitive to current-use insecticides than to older generations of insecticides. A relationship exists between crab harvest and TUs of insecticides applied (Fig. 8). However, the relationship is composed of only seven points, so a demonstrated cause-and-effect relationship is tenuous. Also, although spawning occurs throughout the bay, female crabs migrate to the mouth of the Chesapeake Bay to release their eggs, where the bay water is highly diluted with Atlantic sea water and larvae from multiple sources mix. An effect of pesticides on eggs and larvae would likely require an indirect effect on gametes while adults are living up in the shallows or a direct effect on larval recruitment during spring pesticide application when the larvae are migrating up the perimeter of the bay. Results from the most recent (2008–2009) Winter Blue Crab Dredge Survey (Maryland Department of Natural Resources, http://www.dnr.state.md.us/fisheries/crab/winter_dredge.html) indicate that adult populations have increased compared with the previous year as a result of fishing restrictions but that juvenile populations had not increased.
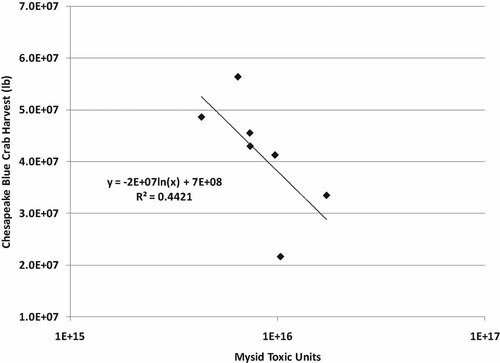
Plot of blue crab (Callinectes sapidus) harvest in Maryland versus toxic units of insecticides applied, calculated for Mysidopsis bahia between 1985 and 2004.
Another important component of the Bay ecosystem is composed of several species of vascular plants collectively called submerged aquatic vegetation (SAV). Submerged aquatic vegetation is considered an indicator of water quality; it traps sediment and affords shoreline erosion protection, provides food for waterfowl, and provides sheltering habitat for young fish and crabs. Large-scale declines of SAV occurred in the late 1960 s and early 1970 s throughout the bay 24. These declines have been blamed on point and nonpoint source inputs of nutrients as well as sediments resulting from development of the bay's shorelines and watershed. Recently it was estimated that only 15% of the bay's historical SAV distribution still remains 25. A substantial effort is underway to re-establish SAV beds in the Chesapeake 26. What, if any, impact the flux of herbicides might have on recovery of submerged vegetation around the perimeter of the Chesapeake is a complete unknown. It is thought that the failure of SAV recovery in the bay is a consequence of excessive turbidity from sediment and algae blooms 27, but little research has been done on SAV bioassays 26, 28, 29. Clearly, herbicides are not inhibiting phytoplankton growth in this system. Annual algae blooms and subsequent hypoxia episodes have been a problem in the Chesapeake for years 30. The system is so heavily overfertilized that effects of residual pesticides might very likely be masked.
CONCLUSIONS
The Chesapeake Bay watershed is annually subjected to massive quantities of toxicants from agricultural and urban pesticide application. An adequate accounting of current-use pesticides is unavailable from any of the watershed jurisdictions. Watershed studies demonstrate that a variety of pesticides is present in surface and groundwater throughout the region. Partial information from just one state indicates that significant quantities of active ingredients potentially enter the tributaries and the bay. The makeup of the pesticide mixture has changed over the years and continues to do so. Although the mass of pesticides being applied to the watershed declined during the reporting period, their increasing potency results in static or significantly increased TUs applied to the watershed. Insecticide trends indicate that chemicals in current use are dramatically more toxic to crustaceans than previously used chemicals. Historical blue crab harvests have declined in proportion to insecticide toxicity, but the data are not comprehensive enough to conclude a direct cause-and-effect relationship. Changes in the toxicity of herbicides are not as significant, probably because herbicides have not created as well-publicized environmental problems as insecticides (e.g., DDT). However, the magnitude of herbicide use is far larger than insecticide use. The recovery of SAV beds in the bay has not occurred despite large-scale efforts to re-establish them. Virtually no data are available on the sensitivity of various SAV species to herbicides or the concentrations of pesticides in the shallows of the Bay that may be affecting them.
Acknowledgements
All pesticide use data compiled in this article was diligently gathered and organized by Mary Ellen Setting or Dennis Howard and their staff at the Maryland Department of Agriculture. Eric Durell of the Maryland Department of Natural Resources assisted in compiling toxicity data from various sources.