Complex evaluation of ecotoxicity and genotoxicity of antimicrobials oxytetracycline and flumequine used in aquaculture
Abstract
Ecotoxicity and genotoxicity of widely used veterinary antimicrobials oxytetracycline and flumequine was studied with six model organisms (Vibrio fischeri, Pseudomonas putida, Pseudokirchneriella subcapitata, Lemna minor, Daphnia magna, Escherichia coli). Overall median effective concentration (EC50) values ranged from 0.22 mg/L to 86 mg/L. Pseudomonas putida was the most sensitive organism (EC50 values for 16-h growth inhibition were 0.22 and 0.82 mg/L for oxytetracycline and flumequine, respectively), followed by duckweed Lemna minor (7-d growth inhibition, EC50 2.1 and 3.0 mg/L) and green alga Pseudokirchneriella subcapitata (4-d growth inhibition, EC50 3.1 and 2.6 mg/L). The least sensitive organism was Daphnia magna (48-h immobilization, lowest-observed-effect concentration [LOEC] of oxytetracycline of 400 mg/L). Oxytetracycline showed limited genotoxicity (SOS-chromotest with Escherichia coli, minimal genotoxic concentration of 500 mg/L), and flumequine was genotoxic at 0.25 mg/L. Based on the reported measured concentrations (MECs) and predicted no-effect concentrations (PNECs), oxytetracycline may be concluded to be of ecotoxicological concern (calculated risk quotient = 8), whereas flumequine seems to represent lower risk. Environ. Toxicol. Chem. 2011; 30:1184–1189. © 2011 SETAC
INTRODUCTION
During recent years, significant attention has been given to the occurrence of drugs in the environment. The use and disposal of pharmaceutical substances may have adverse effects in the environment. Drugs are specially designed to affect biological systems; thus, this is not surprising. Antibiotics are an important group of pharmaceuticals in present-day medicine. In addition to the treatment of human infections, they are used in veterinary medicine 1.
Veterinary antimicrobials are widely used both therapeutically and as growth promoters in intensive farming. Approximately 200 tons of antimicrobial agents are administered annually in Denmark, of that approximately 10 tons in fish farming 2, 3. Estimated antibiotic consumption worldwide is between 100,000 and 200,000 tons per year. In 1996, approximately 10,200 tons of antibiotics were used in the European Union, of which approximately 50% was applied in veterinary medicine 4. In the Czech Republic, 49 tons of veterinary antibiotics were used in 2003, and this amount increased to 80 tons in 2008, from which 45 tons were tetracyclines (http://www.uskvbl.cz/attachments/339_spot%C5%99eba%20ATB%202003-2008.doc).
In intensive fish farming, the antibacterial agents used to treat bacterial infection are administered directly to the water as feed additives, mostly as medicated feed pellets 2, 5, or by simple addition to water 1. Thus, most of these pharmaceuticals end up directly in the environment. As mostly hydrophilic compounds, antibiotics and their metabolites are highly mobile in the aquatic environment 4. Moreover, they often have a low biodegradability 2.
Although antibiotics are designed to kill bacteria, they may have effects on organisms from all trophic levels in the ecosystem. Antibiotic residues in the environment are also suspected to induce resistance in pathogenic bacterial strains, thus causing a threat to public health 1.
Two veterinary antimicrobials are commonly used in fish farming. Those are oxytetracycline (OTC) and flumequine (FLU) 6. In the Czech Republic, only two antibiotics preparations registered for the application to aquaculture, namely, Flumiquil, with flumequine as an active ingredient, and Rupin Special, with oxytetracycline as an active ingredient 7.
Oxytetracycline hydrochloride (CAS 2058-46-0) is a tetracycline broad-spectrum antibiotic with bacteriostatic action against various gram-positive and gram-negative bacteria. It is produced by Streptomyces spp. fungi. It inhibits protein synthesis by reversibly binding to the 30S ribosomal subunit of the microbial ribosome. Oxytetracycline has become the most widely used antimicrobial agent for the treatment of bacterial fish diseases 8. Flumequine (CAS 42835-25-6) is a quinolone broad-spectrum antibacterial agent with bactericidal action, especially against gram-negative bacteria, which is widely used in intensive aquaculture. The mode of action of quinolones is inhibition of bacterial growth by interfering with the enzyme DNA-gyrase (topoisomerase II), which is essential for coiling and uncoiling of DNA, and thus terminating the normal DNA synthesis 9. Both oxytetracycline and flumequine are persistent in surface sediments, with half-lives of approximately 150 d 10.
The present study is based on the hypothesis of potential adverse ecotoxic and genotoxic effects of the pharmaceuticals in aquatic ecosystems. The objective was to derive comparative data on the potential ecotoxicological impact of veterinary antibiotics flumequine and oxytetracycline at all three trophic levels, that is, producers (green algae, aquatic plant), consumers (freshwater crustacean), and decomposers (bacteria). Genotoxicity of the compounds also was evaluated by using a prokaryotic (bacterial) bioassay. The results serve for detailed evaluation and discussion of potential ecotoxicological risks of the investigated drugs that are being applied directly in the aquaculture.
MATERIALS AND METHODS
Chemicals
Veterinary antimicrobials, both flumequine and oxytetracycline hydrochloride, were obtained from the Institute for State Control of Veterinary Biologicals and Medicines, Brno, Czech Republic.
Bioassays
Experiments were conducted with a concentration series of the studied compounds prepared in appropriate test media. Evaluation of the results was based on the nominal concentrations (instrumental analyses not available). Nevertheless, low degradation during the experiments could be expected with respect to previous studies in which Robinson et al. 11 reported almost no degradation of flumequine during the toxicity tests, and Doi and Stoskopf 12 reported relatively long half-lives of oxytetracycline, ranging 4 to 14 d under conditions similar to those of the present experiments 12. Use of the nominal concentration is a limitation that was carefully considered, however, in the interpretation and discussion of the results.
Pseudomonas putida growth inhibition test was performed according to the International Organization of Standardization method ISO 10712:1995 13 in the miniaturized version 14. Cryopreserved cultures of Pseudomonas putida (stored at −80°C in glycerol) were used, and the growth inhibition was evaluated as the change in the absorption of bacterial culture (at 590 nm) after 16 h of exposure in the dark at 23 ± 1°C on a shaker. Each concentration was tested in three replicates. Three independent experiments were conducted to derive a median effective concentration (EC50).
Vibrio fischeri test on bioluminescence inhibition was performed according to the ISO norm 15. Freeze-dried cultures of V. fischeri were purchased from the Institute of Microbiology, Academy of Sciences of the Czech Republic. The decrease of bioluminescence of bacterial culture was evaluated after 15 and 30 min exposure. Evaluation of the EC50 value was based on three independent experiments, each using two replicates.
Algal growth inhibition test was performed according to the ISO 8692:1989 16, using the unicellular alga Pseudokirchneriella subcapitata. This assay was performed in a modified and miniaturized version, using 96-well microplates, as described by Rojickova et al. 17, using the 50% ZBB medium (prepared by mixing of Zehnder Z-medium and Bristol modified Bold medium and distilled water in the ratio of 1:1:2; http://www.butbn.cas.cz/ccala/index.php). Cells in the exponential growth phase were used, and the growth was evaluated as the change of absorbance (680 nm) determined after 96 h exposure. Three replicates were used for each exposure concentrations, and the assay was repeated three times.
Growth inhibition test with Lemna minor was performed according to the European standard 18. The growth inhibition was evaluated as the change of total frond number in comparison with the control. The test was performed in 50-ml polystyrene vessels in a culture room at 24 ± 2°C under continual light. Total duration of the exposure was 7 d. Each concentration was tested in three replicates; three independent experiments were conducted.
Acute immobilization test with Daphnia magna was conducted according to the European Standard EN ISO 6341:1996 19. Newly hatched neonates (less than 24 h old) were obtained from the continuous laboratory culture (in total 20 animals per concentration; two replicates each containing 10 organisms) and exposed to tested compounds for 24 and 48 h. Immobilized organisms were counted, and the results were expressed as a percentage of control. Toxicity assays were repeated three times.
Daphnia magna reproduction test was performed according to the Organisation for Economic Co-operation and Development standard 221 20. Newly hatched neonates (younger than 24 h old, only females) obtained from the continuous laboratory culture were used (10 animals for each concentration and control, each individual exposed separately in 50 ml standard medium). Total duration of the exposure was three weeks. Organisms were fed with a mixture of green algae three times a week. The medium was periodically changed, and offspring produced by parent animals were counted and removed. Survival of parent animals and number of live offspring were evaluated and expressed as a percentage of control. Results of an experiment conducted in 10 replicates per concentration are presented.
Bacterial genotoxicity test (so-called SOS-chromotest) was performed using genetically modified bacterial tester strain Escherichia coli PQ 37. The test was performed without metabolic activation in the 96-well plate as described previously 21. After 2 h incubation with compounds of interest, activity of β-galactosidase (reporter enzyme for genotoxicity induced along with the DNA repair system) was measured using a chromogenic substrate ortho-nitrophenyl-β-D-galactopyranoside. At the same time, the activity of alkaline phosphatase, marker of viability/cytotoxicity, was assessed using p-nitrophenyl phosphate chromogenic substrate. Cytotoxic effect was quantified as a percentage of inhibition of the alkaline phosphatase in comparison with the negative control, and the concentrations causing more than 50% inhibition were excluded from genotoxicity evaluations. The SOS induction factor was then calculated for each tested concentration, and the minimal genotoxic concentration (MGC; the concentration at which the induction factor reaches the critical value 1.5) was determined. Each concentration was tested in three replicates, and the experiments were repeated three times.
Data analysis
For all bioassays, experiments included initial testing of a wide range of concentration (1:10 dilutions) followed by a detailed assessment at approximately 50% median effective concentration values (EC50). Full dose–response curves for all bioassays were repeated independently at least three times. To determine values of the highest tested concentration with no observable effects and LOEC (the lowest tested concentration causing statistically significant effect), data were pooled and analyzed by the multivariate analysis of variance followed by the Dunnett's test. The factors in the analysis of variance were tested concentration, replicate values for each concentration from individual experiments, and three independent tests. Homogeneity of variance was controlled by Levene's test. Data that did not fulfill the criteria of homogeneity and normality were analyzed using nonparametric Mann-Whitney U test. Concentrations eliciting 50% effects were estimated using sigmoidal nonlinear dose–response regression in the GraphPad Prism 4.0 software (GraphPad Software). For each independent experiment, the EC50 value was calculated, and the final reported value is the mean of three independent tests. For the genotoxicity assay, MGC for each test was derived according to standard procedure as the mean concentration that exceeded the induction factor value of 1.5. The final reported MGC value is the arithmetic mean of three independent experiments. Calculations were performed with Microsoft Excel and Statistica for Windows 7.1 (StatSoft).
Risk characterization
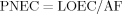
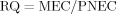
If a risk quotient is calculated to be less than 1, ecological risk is not expected 22.
RESULTS
Effects of both tested compounds in all ecotoxicological and genotoxicity tests, that is, values of the EC50, the LOEC, the highest tested concentration with no observable effects, and MGC, are summarized in Table 1. Both antimicrobials showed significant effects in most of the bioassays, with effect concentrations in a wide range from 0.04 mg/L (LOEC of OTC in the growth inhibition test with P. putida) to 500 mg/L (MGC of OTC in the SOS-chromotest). The more toxic compound was OTC, with the lowest EC50 value of the present study in the P. putida growth inhibition test, but FLU elicited major toxicity in most of the other tests, including the genotoxicity test. In the tests on producers, the toxicities were similar for both tested compounds. In the tests with D. magna and SOS-chromotest, the differences between the two antimicrobials were more obvious. Table 1 also presents calculation of risk quotients. The risk quotient of OTC reached the value of 8.5, which exceeds the critical value of 1 (because of a very low LOEC of 0.04 mg/L). Full dose–response curves are presented in Figures 1-3. Further, Table 2 shows available literature results on OTC and FLU ecotoxicity and genotoxicity.
| Ecotoxicity | Oxytetracycline | Flumequine | ||||
|---|---|---|---|---|---|---|
| EC50 (mg/L) | LOEC (mg/L) | NOEC (mg/L) | EC50 (mg/L) | LOEC (mg/L) | NOEC (mg/L) | |
| Pseudomonas putida | 0.22 (0.14–0.25) | 0.04 | < 0.04 | 0.82 (0.81–1.1) | 0.2 | < 0.2 |
| Vibrio fischeri | 21.0 (13–35) | 5.0 | 2.5 | 11 (4.4–28) | 0.63 | 0.31 |
| Pseudokirchneriella subcapitata | 3.1 (1.5–6.3) | 1.6 | 0.78 | 2.6 (0.45–15.0) | 1.6 | < 1.6 |
| Lemna minor | 2.1 (1.7–3.0) | 1.0 | < 1.0 | 3.0 (0.5–7.0) | 1.0 | < 1.0 |
| Daphnia magna acute test | — | — | 400 | 59 (16–227) | 25 | 12.5 |
| Daphnia magna reproduction test | 86 (48–155) | 20.0 | < 20.0 | 1.2 (0.44–3.1) | 0.75 | < 0.75 |
| Genotoxicity | MGC (mg/L) | MGC (mg/L) | ||||
| SOS-chromotest | 500 | 0.25 | ||||
| Risk evaluation | ||||||
| PNEC (µg/L)b | 0.04 | 0.2 | ||||
| MEC (µg/L)c | 0.34 | 0.032 | ||||
| MEC/PNEC | 8.5 | 0.16 | ||||
- a All toxicity values are in mg/L (values in parentheses show the range of the 95% confidence intervals); EC50 = concentration causing 50% effect; LOEC = lowest-observable-effect concentration; NOEC = the highest concentration causing no-observable effect; MGC = minimal genotoxic concentration.
- b PNEC (predicted no-effect concentration) was calculated by applying factor of 1000 to the lowest LOEC value.
- c MEC (measured environmental concentration) is the highest value reported (for oxytetracycline 31, for flumequine 34).
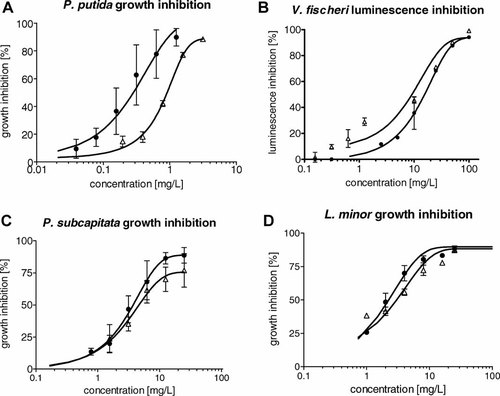
Ecotoxicity (concentration–response curves) of the studied antimicrobial drugs. (A) Pseudomonas putida growth inhibition test. (B) Inhibition of luminescence of Vibrio fischeri. (C) Growth inhibition test with Pseudokirchneriella subcapitata. (D) Growth inhibition test with Lemna minor. OTC = oxytetracycline hydrochloride (black circles), FLU = flumequine (white triangles). The symbols represent mean and standard deviations of three independent experiments.
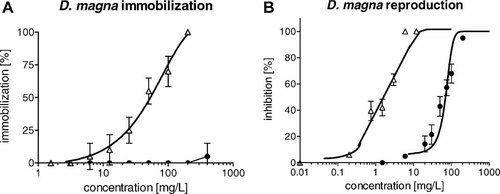
Comparison of toxicity of the studied antimicrobial drugs in the acute and reproduction test with Daphnia magna. (A) Acute immobilization test with D. magna. (B) Reproduction test with D. magna. OTC = oxytetracycline hydrochloride (black circles), FLU = flumequine (white triangles).
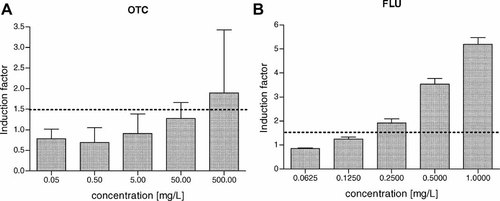
Comparison of genotoxicity of the studied antimicrobial drugs in the SOS-chromotest. OTC = oxytetracycline hydrochloride, FLU = flumequine.
| Bioassay | Endpoint | Toxicity | Reference |
|---|---|---|---|
| Oxytetracycline | |||
| Activated sludge bacteria growth inhibition | 48-h EC50 | 0.08 (0.06–0.1) | |
| Pseudomonas sp. | 24-h MIC | 1.0 | |
| Vibrio fischeri luminescence inhibition | 30-min EC50 | 64.50 (47.2–88.3) | |
| 120 (112–130) | |||
| 15-min EC50 | 87.0 (50.8–148.9) | ||
| Microcystis aeruginosa growth inhibition | 7-d EC50 | 0.21 (0.18–0.25) | |
| Chlorella vulgaris growth inhibition | 48-h EC50 | 6.4 (4.9–8.4) | |
| Pseudokirschneriella subcapitata growth inhibition | 72-h EC50 | 4.5 (2.3–86) | |
| 0.342 (0.321–0.364) | |||
| 0.17 (0.11–0.25) | |||
| Rhodomonas salina growth inhibition | 72-h EC50 | 1.6 (0.4–6.1) | |
| Lemna minor growth inhibition | 168-h EC50 | 4.92 (3.6–6.8) | |
| D. magna immobilization | 48-h EC50 | 22.64 (17.19–29.81) | |
| 621.2 (437.71–804.8) | |||
| 48-h LOEC | 100 | ||
| Ceriodaphnia dubia immobilization | 48-h EC50 | 18.65 (15.96–21.79) | |
| D. magna reproduction | 21-d EC50 | 46.2 | |
| Brachionus calyciflorus population growth inhibition | 48-h EC50 | 1.87 (1.19–2.96) | |
| C. dubia population growth inhibition | 7-d EC50 | 0.18 (0.11–0.26) | |
| Flumequine | |||
| V. fischeri luminescence inhibition | 30-min EC50 | 12.7 (12.0–13.5) | |
| Microcystis aeruginosa growth inhibition | 7-d EC50 | 0.16 (0.066– 0.38) | |
| 5-d EC50 | 1.96 (1.76–2.16) | ||
| P. subcapitata growth inhibition | 72-h EC50 | 5.0 (1.6–16) | |
| 5.0 (4.8–5.2) | |||
| Rhodomonas salina growth inhibition | 72-h EC50 | 18 (10–31) | |
| Lemna minor growth inhibition | 7-d EC50 | 2.47 (1.65–3.3) | |
| Artemia salina mortality (ArToxKit) | 72-h EC50 | 96.0 (39– 240) | |
- a All toxicity values in mg/L. EC50 = median effective concentration; MIC = minimum concentration inhibiting growth; LOEC = lowest-observable-effect concentration.
DISCUSSION
For both tested antimicrobials, P. putida was the most sensitive organism, which was expected, because antibiotics are primarily designed to kill bacteria. The LOEC derived in the present study was nearly two orders of magnitude lower than the minimum growth inhibitory concentration previously reported for Pseudomonas sp. 23 (Table 2).
The test with marine bacteria V. fischeri was much less sensitive; however, other authors also reported low sensitivity of this test to antibiotics 24. Isidori et al. 25 studied toxicity and genotoxicity of six antibiotics, including OTC, and reported an EC50 value of 65 mg/L for V. fischeri, which is comparable to the results of the current study. However, the 30-min EC50 of OTC for V. fischeri reported by Lalumera et al. 6 was 10 times higher. The results of the same authors for FLU 6 were in accordance with the current study. Lower sensitivity of V. fischeri to the antimicrobials, namely OTC, can be explained by its mode of action on protein synthesis, which is not of importance during short-term bioluminescence testing. However, a drastic increase in the V. fischeri sensitivity was shown during the prolonged 24-h incubation 26.
Both representatives of producers—green alga P. subcapitata and aquatic vascular plant L. minor—demonstrated more or less the same sensitivity for both tested compounds. Three different values for algal growth inhibition tests with OTC were found in the literature (Table 2), and they were in general comparable with the results of the current study 5, 27.
The least sensitive organism was D. magna, for which OTC in acute test demonstrated no toxicity up to 400 mg/L, and only low toxicity was observed in the chronic reproduction test. Similar differences in sensitivity of rotifers and crustaceans vs algae were previously reported by Isidori 25. However, toxicity of FLU to D. magna was much higher in the reproduction test (two orders of magnitude lower LOEC). Low toxicity of OTC for D. magna was also previously reported by Wollenberger et al. 2; however, other authors observed higher toxicity with the EC50 value of 22.6 mg/L 25. Robinson et al. 11 reported minor toxicity of FLU for D. magna with the highest tested concentration, with no observable effects of 10 mg/L, which is similar to the result presented here; however, no study previously addressed chronic toxicity of FLU in the reproduction test with D. magna.
The present study seems to indicate parallels in responses of the D. magna (during the reproduction test) and E. coli in the SOS-chromotest. Both Daphnia and E. coli were markedly more sensitive to FLU than to OTC, which might be related to the mode of action of both antimicrobials. Although OTC has an effect on the protein synthesis in bacteria, FLU acts directly on the DNA by inhibiting the topoisomerase II enzyme 8, 9.
Low genotoxicity of OTC was observed in the current study (very high and environmentally nonrelevant MGC of 500 mg/L), which corresponds to results of the study by Isidori et al. 25, who reported no mutagenicity of OTC, neither in the Ames nor in the SOS-chromotest. However, genotoxicity of FLU was markedly higher. Interestingly, genotoxicity of FLU was not previously reported, although studies with other quinolone antibiotics are available. For example, Itoh et al. 28 showed DNA alterations induced by various quinolone antimicrobials in the comet assay and in vitro micronucleus test. Significance of the quinolone compounds was suggested by Hartmann et al. 29, who concluded that genotoxicity detected in hospital wastewaters is caused mainly by fluoroquinolone antibiotics. Moreover, Lancieri at al. 30 demonstrated teratogenic effects of FLU during acute exposures of the early stages of fish Danio rerio.
Many antibiotics have been found in sewage influent and effluent samples, surface waters, and even groundwater and drinking water. In Italy, flumequine and oxytetracycline were indicated as priority chemicals with possible side effects in aquaculture 6. Because of their precipitation, tetracyclines are not expected to be present dissolved in the aquatic environment; however, they sorb to the organic matter and may accumulate in sediments. Nevertheless, OTC was detected in the United States surface water samples at concentrations of 0.34 µg/L 31. Higher concentrations (55.7 µg/L) were reported in the overland flow water 32. In the Czech Republic, concentrations of OTC in the fish farm bottom sediments reached up to 1,516 µg/kg two months after application 33.
Flumequine was detected in concentrations up to 32 ng/L at five sampling locations in the Seine River inner estuary, France 34. Reported concentrations were compared with the observed ecotoxicity LOECs, and risk quotients were calculated (Table 1).
For OTC, some risk assessments were previously reported but led to contradictory conclusions. In part, Park and Choi 35 reported the risk quotient of 2, using an MEC of 0.34 µg/L for the calculation. Their PNEC was derived from the EC50 value of 0.17 mg/L in the P. subcapitata growth inhibition test. However, Isidori et al. 25 reported a risk quotient lower than 1, using an MEC of 0.05 µg/L and the same value of PNEC as in the study of Park and Choi 35. Results of the current study are comparable to the findings of Park and Choi 35: Using an MEC of 0.34 µg/L, we have calculated the risk quotient to be 8.5.
No previously reported risk assessment for FLU has been found in the literature; however, according to the results of the present study, rather lower risk can be predicted (the risk quotient of 0.16), but significant genotoxicity of FLU should not be overlooked.
CONCLUSIONS
The present study provides comprehensive data on aquatic ecotoxicity and genotoxicity of two veterinary antimicrobials frequently used in aquaculture. Pseudomonas putida was found to be the most sensitive species, with EC50 values lower than 1 mg/L. Algae and vascular plants also were affected by studied drugs, with EC50 in the low milligrams per liter range. Minor effects were observed in D. magna; however, FLU had significant effects during the chronic reproduction study. Risk quotient for OTC highly exceeded 1, thus indicating possible environmental risk, whereas FLU seemed to have only minor impact. The results indicate potential risk for nontarget organisms resulting from exposure to low levels of veterinary antimicrobials.
Acknowledgements
The research was supported by the Czech Ministry of Education CR (project INCHEMBIOL 0021622412) and by project CETOCOEN (CZ.1.05/2.1.00/01.0001) from the European Regional Development Fund.