Mercury speciation and biomagnification in the food web of Caddo Lake, Texas and Louisiana, USA, a subtropical freshwater ecosystem
Abstract
We studied the biomagnification of total mercury and methylmercury in a subtropical freshwater lake, Caddo Lake, Texas and Louisiana, USA. The present study is unique in that it not only included invertebrates (seven species) and fish (six species) but also an amphibian (one species), reptiles (three species), and mammals (three species). Nonfish vertebrates such as those included in the present study are often not included in assessments of trophic transfer of Hg. Mean trophic position (determined using stable isotopes of nitrogen) ranged from 2.0 (indicative of a primary consumer) to 3.8 (indicative of a tertiary consumer). Mean total Hg concentrations ranged from 36 to 3,292 ng/g dry weight in muscle and whole body and from 150 to 30,171 ng/g dry weight in liver. Most of the Hg in muscle and whole-body tissue was found as methylmercury, and at least 50% of the Hg found in liver was in the inorganic form (with the exception of largemouth bass, Micropterus salmoides). Mercury concentrations were positively correlated with trophic position, indicating that biomagnification occurs in the food web of Caddo Lake. The food web magnification factors (FWMFs; slope of the relationship between mean Hg concentration and trophic position) for both total Hg and methylmercury were similar to those observed in other studies. Because most of the total Hg in consumers was methylmercury, the FWMF for methylmercury was not significantly different from the FWMF for total Hg. Some vertebrates examined in the present study had low Hg concentrations in their tissues similar to those observed in invertebrates, whereas others had concentrations of Hg in their tissues that in previous studies have been associated with negative health consequences in fish. Environ. Toxicol. Chem. 2011; 30:1153–1162. © 2011 SETAC
INTRODUCTION
Mercury is a highly toxic metal that has detrimental effects on fish and wildlife 1-3. Although Hg is naturally occurring, concentrations in the environment have increased since the preindustrial era 4 as a result of a variety of anthropogenic activities, such as fossil fuel combustion and metal production that produce elemental and inorganic species of Hg 5. In aquatic ecosystems, bacteria convert inorganic Hg (i.e., Hg2+) to the more lipophilic and toxic species, methylmercury (MeHg), which readily bioaccumulates 6. Organisms at the base of the food web such as phytoplankton and periphyton concentrate MeHg (and, to a lesser extent, inorganic Hg) directly from water 7, whereas food represents an important route of exposure for invertebrate and vertebrate consumers 8, 9.
In vertebrates, MeHg and inorganic Hg are absorbed by the gut, although assimilation efficiencies can vary among species 10, 11. After absorption, MeHg is transferred to blood and distributed to other body tissues 10. Most MeHg is eventually redistributed to skeletal muscle tissue, where it accumulates bound to sulfhydryl groups in protein 12. Relative to MeHg, a larger proportion of inorganic Hg is retained in the gastrointestinal tract 7. Inorganic Hg can be sequestered by binding proteins in the liver 13, 14, and demethylation may occur in the liver, resulting in the accumulation of inorganic Hg 15-17. As a result of these processes, most Hg in the muscle of vertebrates is MeHg 18, 19, but the liver can contain elevated concentrations of inorganic Hg 20, 21. Methylmercury is also the predominant form of Hg in some invertebrate taxa, but the proportion of inorganic Hg in invertebrate tissues can be high (>90%), with considerable variation 12, 22.
Because MeHg biomagnifies, the number of trophic levels in a food web is one of the most important factors determining the concentration of MeHg in the tissues of consumers 23, 24. Stable isotope analysis, especially nitrogen isotopes, has become a powerful tool for studying contaminant biomagnification in wild animal populations, because it allows researchers to assign time-integrated, noninteger estimates of trophic position 25. The slope of the relationship between mean log-transformed Hg concentration and mean trophic position, referred to as the food web magnification factor (FWMF), is a measure of biomagnification and can be used to compare transfer efficiencies between MeHg and biomass in food webs 25-27. An FWMF greater than 0 indicates that MeHg is transferred more efficiently than biomass through the food web, in other words, that biomagnification is occurring 26. The inverse log of the FWMF represents the increase in MeHg concentration from one trophic level to the next averaged over the entire food web 25.
Most studies that have calculated FWMFs for Hg have examined total Hg in muscle tissue, assuming that total Hg roughly equals MeHg. This assumption is potentially problematic in studies that involve organisms feeding near the base of the food web, because the proportion of total Hg that is MeHg in an organism's tissues is hypothesized to increase with trophic position 28. Studies that have examined biomagnification of both total and MeHg report that MeHg increases 1.2 to 2.7 times more efficiently than total Hg from one trophic level to the next 29, 30. In addition, because Hg storage and detoxification is tissue specific 12, Hg accumulation, and therefore FWMFs, in muscle tissue may differ from that in other tissues, including liver.
The primary objective of the present study was to assess the accumulation and biomagnifcation of both total Hg and MeHg in multiple tissues from a diverse assemblage of invertebrate and vertebrate consumers from Caddo Lake, a freshwater ecosystem located in northeastern Texas and northwestern Louisiana, USA (Fig. 1). Most previous biomagnification studies have focused only on fish and invertebrate communities 24, 31, 32, and those studies that have included other vertebrate taxa have been conducted only in estuarine or marine ecosystems 30, 33, 34. Therefore, data on biomagnification in food webs that include vertebrates other than fish, especially from freshwater ecosystems, are needed.

Map of Caddo Lake, located on the border of Texas and Louisiana, USA. Points within the sampling area represent sampling sites for fish and invertebrates. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
MATERIALS AND METHODS
Study site
Caddo Lake and its associated wetlands cover approximately 10,850 hectares and are composed of cypress swamps, marshes, bottomland hardwood forests, grasslands, and pine forests, much of which remains in a relatively undisturbed condition 35, 36. The limnology of the lake has been described elsewhere 24, 37. Briefly, the western portion of the lake is shallow (many areas <1 m) and characterized by wetland habitat dominated by bald cypress (Taxodium distichum), water elm (Planera aquatica), and other aquatic vegetation, including fanwort (Cabomba caroliniana), common waterweed (Elodea sp.), yellow pond lily (Nuphar lutea), and invasive water hyacinth (Eichhornia crassipes) 38. In contrast, the eastern portion of the lake is primarily open water habitat with an average depth of 1.4 m 39.
Fish from Caddo Lake contain some of the highest Hg concentrations recorded in Texas 40, and elevated Hg concentrations have also been documented in snakes and piscivorus birds 41, 42. Fish and invertebrates in the western portion of the lake contain higher concentrations of Hg than those in the eastern portion of the lake 24, 37. Mercury contamination in Caddo Lake is of particular concern because the lake supports a high level of biodiversity, including rare and threatened species 35 (see also Caddo Lake Institute, 2009, http://www.caddolakeinstitute.us/ramsar.html), which may be negatively impacted by Hg exposure. The most probable source of Hg loading into Caddo Lake is atmospheric deposition 43. The primary anthropogenic sources of Hg in the region are coal-burning power plants (U.S. Environmental Protection Agency, 2006, Toxic Release Inventory Program, http://www.epa.gov/tri/); however, a substantial proportion of the Hg deposited in the region may originate from sources outside of North America 44.
Sample collection and processing
To assess concentrations of Hg in Caddo Lake organisms and determine whether biomagnification occurs in the food web, we examined 20 different animal taxa (seven invertebrates, 13 vertebrates) hypothesized to represent multiple trophic levels (Table 1). All organisms were collected from May through August, 2007, with the exception of white-tailed deer, which were collected from October through December, 2005. The majority of the sampling effort occurred in an approximately 12-km2 sampling area located in the western region of the lake (Fig. 1). Crayfish, mussels, and all vertebrates except fish and white-tailed deer were collected opportunistically from within the sampling area. Fish and invertebrates other than crayfish and mussels were collected from six sampling sites within the sampling area (Fig. 1). White-tailed deer were harvested by recreational hunters from the Caddo Lake Wildlife Management Area adjacent to our sampling area.
| Common name | No. of samples (mean No. per composite) | Tissue analyzed | Total length (cm) | δ15N | Trophic positiona | Gut contentsb |
|---|---|---|---|---|---|---|
| Invertebrates | ||||||
| Grass shrimp (Palaemonetes kadiakensis) | 6 (44.5) | Whole | 3.29 ± 0.13 | 6.9 ± 1.0 | 2.6 ± 0.3 | ND |
| Dragonfly larvae (Libellulidae) | 6 (36.8) | Whole | 1.65 ± 0.06 | 4.6 ± 1.6 | 2.0 ± 0.4 | ND |
| Dragonfly larvae (Aeshnidae) | 2 (6) | Whole | 3.71 ± 0.39 | 6.9 ± 1.9 | 2.7 ± 0.4 | ND |
| Giant water bug (Belostoma sp.) | 6 (40.5) | Whole | 1.91 ± 0.08 | 4.6 ± 1.5 | 2.0 ± 0.4 | ND |
| Freshwater mussel (Unionidae) | 2 | Muscle | 6.8 ± 0.0 | 6.5 ± 0.4 | 2.0 ± 0.1 | ND |
| Crayfish (Cambaridae) | 4 (3.3) | Muscle | 7.47 ± 0.9 | 6.0 ± 1.5 | 2.4 ± 0.5 | ND |
| Rams-horn snail (Planorbidae) | 6 (6.7) | Muscle | 1.59 ± 0.09 | 3.2 ± 2.0 | 1.8 ± 0.4 | ND |
| Vertebrates | ||||||
| Fish | ||||||
| Bluegill (Lepomis machrochirus) | 8 (4.6) | Muscle | 9.97 ± 1.87 | 7.6 ± 0.6 | 2.8 ± 0.2 | Backswimmer (Notonectidae), giant water bug, unidentified invertebrate |
| Largemouth bass (Micropterus salmoides) | 8 (4) | Muscle | 24.9 ± 6.33 | 9.9 ± 0.5 | 3.7 ± 0.1 | Backswimmer, crayfish, damselfly larvae (Zygoptera), dragonfly larvae, golden topminnow, giant water bug, grass shrimp, pickerel (Esox sp.), sunfish, unidentified fish, western mosquito fish (Gambusia affinis) |
| Golden topminnow (Fundulus chrysotus) | 9 (5.9) | Wholeb | 4.76 ± 0.52 | 7.4 ± 0.8 | 3.0 ± 0.2 | ND |
| Pirate perch (Aphredoderus sayanus) | 3 (11.7) | Wholeb | 3.89 ± 0.67 | 6.3 ± 1.8 | 2.3 ± 0.6 | ND |
| Red-ear sunfish (Lepomis microlophus) | 12 (3.8) | Muscle | 11.5 ± 1.86 | 7.6 ± 0.4 | 2.9 ± 0.1 | Amphipod (Hyalella azteca), dragonfly larvae, snail, unidentified invertebrate |
| Spotted gar (Lepisosteus oculatus) | 5 (4.6) | Muscle | 50.6 ± 2.88 | 10.1 ± 1.0 | 3.8 ± 0.3 | Crayfish, dragonfly larvae, grass shrimp, pickerel, pirate perch, sunfish, unidentified fish |
| Amphibian | ||||||
| Bullfrog (Rana catesbeiana) | 5 | Muscle | 14.6 ± 1.92 | 7.1 ± 0.8 | 3.0 ± 0.2 | Adult dragonfly, crayfish, giant waterbug, red wasp (Vespidae), water scorpion (Ranatra sp.) |
| Reptiles | ||||||
| American alligator (Alligator mississippiensis) | 2 | Muscle | 149 ± 52.2 | 5.1 ± 2.2 | 2.3 ± 0.8 | Crayfish, nutria, unidentified fish, unidentified insect, watersnake (Nerodia sp.) |
| Cottonmouth (Agkistrodon piscivorus) | 6 | Muscle | 67.6 ± 13.5 | 8.1 ± 0.8 | 3.2 ± 0.2 | Frog (Ranidae), watersnake |
| Red-eared slider (Trachemys scripta) | 7 | Muscle | 20.9 ± 1.30 | 4.3 ± 0.9 | 2.2 ± 0.4 | Unidentified vegetation |
| Mammals | ||||||
| Nutria (Myocastor coypus) | 4 | Muscle | 87.2 ± 6.79 | 6.2 ± 1.0 | 2.6 ± 0.3 | Unidentified vegetation |
| Raccoon (Procyon lotor) | 5 | Muscle | 76.8 ± 2.35 | 7.6 ± 0.7 | 3.2 ± 0.2 | All empty |
| White-tailed deer (Odocoileus virginianus) | 6 | Muscle | ND | 2.46 ± 0.7 | 1.7 ± 0.2 | ND |
- ND = Not determined.
- a Based on nitrogen isotope analysis.
- b Eviscerated and decapitated prior to analysis.
Invertebrates were collected using standard methods: by hand (mussels), minnow traps (crayfish), and by dip nets (all other invertebrate taxa). Immediately after collection, invertebrates were separated into individual labeled plastic bags containing ice and tap water, where they were held for at least 4 h before being frozen at −20°C. Invertebrate samples were later thawed and measured for total length. Invertebrate samples were processed whole except for crayfish, mussels, and snails, for which tail muscle, foot muscle, and soft tissues (whole body minus the shell), respectively, were processed.
All vertebrates except for white-tailed deer, which were harvested by hunters, were collected and euthanized using standard methods (hand collection [bull frogs], snake tongs [cottonmouths], capture noose [alligators], live capture traps [nutria, raccoons], hoop nets [red-eared sliders], and electrofishing [fish]) approved by the Institutional Animal Care and Use Committees of Texas Tech University and Texas Christian University (TCU). All vertebrates were euthanized immediately after capture. For all vertebrates except for small fish, morphometric data were recorded, gut contents examined, and muscle and liver samples collected in the field. Samples were placed on ice in labeled plastic bags and frozen at −20°C until processing. Small fish were placed whole in labeled plastic bags and frozen at −20°C before being processed in the laboratory. Golden topminnow and pirate perch were too small to collect muscle and liver samples, so we removed the head and gastrointestinal tract from these species and analyzed the remaining body tissues (primarily muscle). Hind-limb muscle samples were collected from deer by Texas Parks and Wildlife Department biologists, frozen at −20°C in labeled plastic bags, and donated to TCU for analysis. All samples were dried in a 60°C oven and homogenized to a flour-like consistency using a ball-mill grinder prior to Hg and stable isotope analysis.
After homogenization, fish and invertebrate samples from each of the six sampling sites were composited by taxa, resulting in up to 12 composite samples per species (Table 1). The number of composite samples per taxa is not consistent because not all species were captured at all six sampling sites, and more than one composite sample was produced for some species at some sites. For fish, equal weights of tissue from each individual were combined to create composites. In each composite, the smallest individual fish was no smaller than 75% of the total length of the largest individual fish. For invertebrates, all individuals collected from a given sample site were combined.
Mercury analysis
We examined MeHg in a subset of samples (Table 2). Methylmercury concentrations were determined by Quicksilver Scientific using Hg–thiourea complex liquid chromatography-cold vapor atomic fluoresence spectrometry (HgTu/LC-CVAFS) 45. Quality assurance included reference and duplicate samples. Samples of European Virtual Institute for Speciation Analysis and National Research Council Canada reference materials were analyzed approximately every 20 samples, and the mean percentage recovery was 98.5% (n = 7). Duplicate samples were analyzed approximately every 20 samples, and the mean relative percentage difference was 4.0% (n = 8).
| Common name | Total Hg in muscle or whole bodya,b | MeHg in muscle or whole body (No. analyzed)a,b | Percentage MeHg in muscle or whole body | Total Hg in liverb | MeHg in liver (No. analyzed)b | Percentage MeHg in liver |
|---|---|---|---|---|---|---|
| Invertebrates | ||||||
| Grass shrimp | 601 ± 114 | 435 ± 131 (3) | 72.4 | — | — | — |
| Dragonfly larvae (Libellulidae) | 174 ± 33.3 | 134 ± 52.7 (3) | 77.0 | — | — | — |
| Dragonfly larvae (Aeshnidae) | 314 ± 104 | 265 ± 116 (2) | 84.4 | — | — | — |
| Giant water bug | 346 ± 70.8 | 294 ± 68.0 (3) | 85.0 | — | — | — |
| Freshwater mussel | 160 ± 43.3 | 168 ± 26 (2) | 105.0 | — | — | — |
| Crayfish | 577 ± 260 | 405 ± 97.9 (3) | 70.2 | — | — | — |
| Rams-horn snail | 85.6 ± 44.5 | 48.2 ± 34.4 (3) | 56.3 | — | — | — |
| Fish | ||||||
| Bluegill | 640 ± 110 | 632 ± 171 (4) | 98.8 | — | — | — |
| Largemouth bass | 1,718 ± 386 | 1,442 ± 469 (6) | 83.9 | 997 ± 154 (5) | 736 ± 22.5 (3) | 73.8 |
| Golden topminnow | 570 ± 161 | 578 ± 158 (3) | 101 | — | — | — |
| Pirate perch | 605 ± 80.5 | 512 ± 63.5 (3) | 84.6 | — | — | — |
| Red-ear sunfish | 445 ± 54.7 | 385 ± 39.4 (4) | 86.5 | 414 (1) | 231 (1) | 55.8 |
| Spotted gar | 2,611 ± 478 | 2,224 ± 374 (4) | 85.2 | 30,171 ± 12,377 | 610 ± 309 (4) | 2.0 |
| Amphibians | ||||||
| Bullfrog | 620 ± 196 | 545 ± 288 (3) | 87.9 | 1,228 ± 535 | 367 ± 166 (3) | 29.9 |
| Reptiles | ||||||
| American alligator | 795 ± 10.0 | 649 ± 20.6 (2) | 81.6 | 2,263 ± 289 | 833 ± 182 (2) | 36.8 |
| Cottonmouth | 3,292 ± 2,186 | 3,075 ± 3,349 (3) | 93.4 | 7,456 ± 7,405 | 1,568 ± 2042 (3) | 21.0 |
| Red-eared slider | 225 ± 104 | 155 ± 149 (3) | 68.9 | 778 ± 338 | 112 ± 112 (4) | 14.4 |
| Mammals | ||||||
| Nutria | 36.3 ± 14.6 | 13.3 ± 3.85 (3) | 36.6 | 43.4 ± 17.0 | 16.1 ± 4.9 (3) | 37.1 |
| Raccoon | 2,439 ± 766 | 1,617 ± 697 (3) | 66.3 | 13,661 ± 4,204 | 2,618 ± 770 (3) | 19.2 |
| White-tailed deer | 35.8 ± 13.9 | 19.9 ± 17.4 (3) | 55.6 | 150 ± 215 | 8.0 ± 5.7 (3) | 5.3 |
We examined total Hg in all samples with a direct Hg analyzer (DMA-80; Milestone) that uses thermal decomposition, gold amalgamation, and atomic absorption spectrometry 46. Quality assurance included reference and duplicate samples similar to those described for previous studies of Hg contamination in Caddo Lake 24, 37. Samples of National Research Council Canada reference materials were analyzed approximately every 10 samples, and the mean percentage recovery was 99.2% (n = 21). Duplicate samples were analyzed approximately every 20 samples, and the mean relative percentage difference was 3.63% (n = 9). All Hg values are reported in nanograms per gram dry weight unless otherwise noted.
Trophic position and diet analyses
We used stable isotope analysis to estimate the food web position of all consumers. Stable isotope analysis has advantages over traditional dietary analyses involving gut contents in biomagnification studies, because it provides a time-integrated representation of assimilated food rather than a snapshot of recently ingested items 25, 47. Isotope turnover rates are on the scale of weeks and months for liver and muscle, respectively 48. Despite the integrative nature of stable isotope analysis, one-time collection and analysis of food web components, as in the present study, essentially presents a static measure of food web structure and contaminant flow that might not fully capture the dynamic nature of temporally variable food webs 25. We assume that sampling during one season was sufficient to assess biomagnification in Caddo Lake for two reasons. First, the diet of Caddo Lake organisms is not known to exhibit large-scale seasonal variation. Second, many of the organisms sampled in the present study are long-lived vertebrates with relatively slow tissue turnover rates. Thus the isotope values of vertebrate consumers sampled in the present study reflect the diet consumed over several weeks (liver) and months (muscle) and would be expected to exhibit minimal seasonal variation.
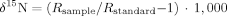
To calculate trophic position, δ15N values of consumers were first corrected for differences in basal δ15N using isotope values of gastropods and unionid mussels according to the method of Post 50. We then calculated trophic position for consumers from corrected δ15N values assuming an increase of 3.4‰ per trophic level 50. Chumchal et al. 37 provide a detailed description of methods used to calculate trophic position for consumers.
Gut contents were qualitatively examined from some larger vertebrates collected in the present study. These data were used to confirm that organisms were trophically related (i.e., exhibiting predator–prey relationships) and to aid in interpretation of isotope data 51.
Statistical analysis
We used linear regression (SPSS version 11.5.0) to examine the relationships between MeHg and total Hg concentrations in muscle, whole body, and liver; and between trophic position and MeHg and total Hg in these tissues. We were unable to determine Hg concentrations in livers of all organisms sampled in the present study because of insufficient amounts of tissue, and, because of high analytical costs, we were able to determine MeHg concentrations in only a subset of tissues. Therefore, in regression analyses that included data on liver or MeHg, we calculated taxa means using only the subset of individuals for which all data were available. To determine whether the FWMF for total Hg differed from the FWMF for MeHg, we used analysis of covariance (ANCOVA) to compare the slope of the relationship between MeHg and trophic postion and total Hg and trophic position. Statistical significance was determined at p < 0.05 for all analyses.
RESULTS
Trophic position of Caddo Lake biota
Trophic position exhibited substantial variation among the consumers collected from Caddo Lake (Table 1). Organisms spanned three trophic levels from primary to tertiary consumers. Many mid- and low-level consumers (small fish and invertebrates) were found in the guts of high- and midlevel consumers (large fish), indicating that the organisms examined in the present study were trophically related (i.e., they were feeding on one another).
Mercury concentration of Caddo Lake biota
Mercury was detected in tissues of all vertebrate and invertebrate species sampled in the present study, but both MeHg and total Hg were highly variable among tissues and species (Table 2). Mean MeHg concentrations ranged from 13.3 (nutria) to 3,075 (cottonmouth) ng/g in muscle and whole-body tissues and from 8 (white-tailed deer) to 1,568 (cottonmouth) ng/g in liver. Mean total Hg concentrations ranged from 35.8 (white-tailed deer) to 3,292 (cottonmouth) ng/g in muscle and whole-body tissues and from 43.4 (nutria) to 30,171 (spotted gar) ng/g in liver.
Methylmercury concentration in muscle was highly correlated with total Hg concentration in muscle (Fig. 2A). Most of the Hg in muscle and whole-body tissue was MeHg (mean percentage MeHg 81%); however, some organisms, including rams-horn snails (mean percentage MeHg 56%), white-tailed deer (55%), and nutria (36%), exhibited a relatively low percentage of MeHg in their tissues (Table 2). Methylmercury concentration in liver was highly correlated with total Hg concentration in liver (Fig. 2B), but, in contrast to muscle tissue, MeHg was not the predominant form of Hg in liver tissue for most species (mean percentage MeHg 31%). The percentage of total mercury present as MeHg was much lower in livers of spotted gar than in other species. Spotted gar was an outlier, so, although we plotted MeHg and total Hg data for spotted gar in Figure 2B for reference, we did not include these data in the regression analysis.
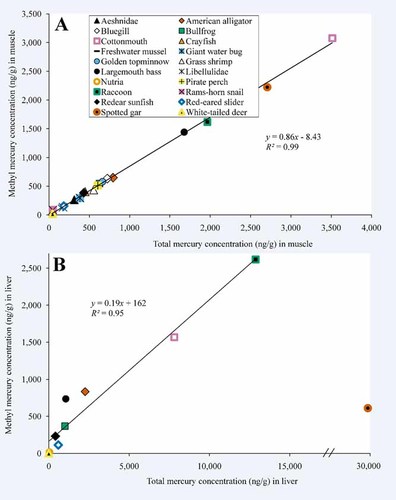
Relationship between mean methylmercury (MeHg) concentrations in muscle or whole-body tissues and mean total mercury (Hg) concentrations in muscle or whole-body tissues (A) and mean MeHg concentrations in liver and mean total Hg concentrations liver (B). Spotted gar is included on the figure for reference but was not included in regression analysis. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
The concentration of total Hg in liver was exponentially related to the concentration of total Hg in muscle (Fig. 3A). In species with greater than approximately 2,000 ng/g total Hg in the muscle, the concentration of total Hg in the liver tissue greatly exceeded the concentration of total Hg in the muscle tissue. There was an inverse relationship between the percentage of MeHg in the liver and the liver:muscle total Hg ratio (Fig. 3B).

(A) Relationship between mean total Hg (Hg) concentrations in muscle and mean total Hg concentrations in liver. (B) Relationship between total Hg liver:muscle ratio and the percentage of MeHg in the liver. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
Mercury biomagnification in the Caddo Lake community
We observed positive relationships between MeHg and trophic position and total Hg and trophic position (Figs. 4 and 5). For their trophic positions, white-tailed deer and nutria had lower concentrations of Hg in their muscle than were observed in the other organisms sampled in the present study. When white-tailed deer and nutria were not included in regression analyses, trophic position accounted for 69% and 73% of the variation in log-MeHg (Fig. 4A) and log-total Hg (Fig. 4B), respectively, in muscle and whole-body tissues among species. Tertiary consumers (spotted gar) exhibited mean MeHg and total Hg concentrations in muscle and whole body that were more than five times higher than in secondary consumers (redear sunfish; Table 1). For liver, trophic position accounted for 23% and 26% of the variation in log-MeHg (Fig. 5A) and log-total Hg (Fig. 5B), respectively. Concentrations of MeHg in liver were as much as 23 times higher in tertiary consumers (raccoon) than in secondary consumers (red-eared slider), and concentrations of total Hg were up to 73 times higher in tertiary consumers (spotted gar) than in secondary consumers (redear sunfish).
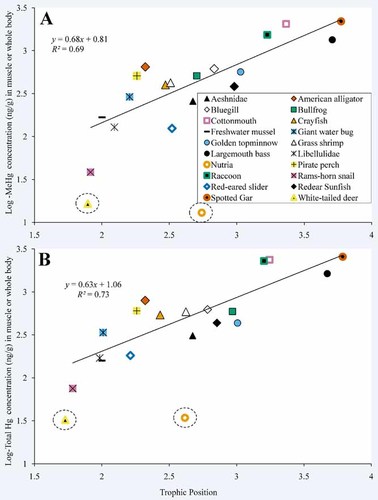
Relationship between mean log-transformed methyl Hg concentrations in muscle or whole-body tissue and mean trophic position (A) and mean log-transformed total Hg concentration in muscle or whole body tissue and mean trophic position (B). Terrestrial and semiaquatic white-tailed deer and nutria, respectively, are circled. These organisms are included in the figure for reference but were not included in regression analyses. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
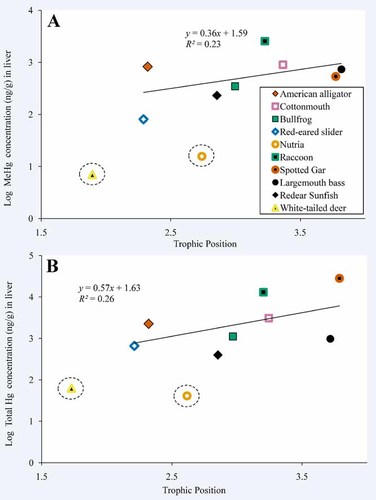
Relationship between mean log-transformed methyl Hg concentration in liver tissue and mean trophic position (A) and mean log-transformed total Hg concentration in liver tissue and mean trophic position (B). Terrestrial and semiaquatic white-tailed deer and nutria, respectively, are circled. These organisms are included in the figure for reference but were not included in regression analyses. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
The FWMF for MeHg in muscle and whole body was 0.68 (Fig. 4A). The inverse log of this value indicates that MeHg increased by a factor of 4.8 with each increase in trophic level in the Caddo Lake food web. The FWMF for total Hg in muscle and whole-body tissues was 0.63 (Fig. 4B), indicating that total Hg increased by a factor of 4.3. Although the FWMF for total Hg suggests that it was transferred approximately 1.1 times less efficiently than MeHg through the food web, this difference was not statistically significant (ANCOVA: degrees of freedom [df] = 1, 32; f = 0.13, p = 0.72). The FWMFs for MeHg and total Hg in liver were 0.36 and 0.57, respectively (Fig. 5), indicating that MeHg increased by a factor of 2.3 and total Hg increased by a factor of 3.7 per trophic level, a difference that was not statistically significant (ANCOVA: df = 1, 12; f = 0.19, p = 0.67).
DISCUSSION
Trophic position of Caddo Lake biota
In general, data on trophic position and gut contents for organisms sampled in the present study indicate that Caddo Lake organisms feed in a manner consistent with that observed in previous studies. For example, largemouth bass and spotted gar were found to feed as tertiary consumers in a previous study at Caddo Lake 24. However, the relatively low trophic position of alligators (<2.5) was surprising, because these reptiles are generally assumed to be the top predators in the ecosystems that they inhabit 52. This low trophic position suggests that the diet of the individuals sampled in the present study (one juvenile, one subadult) consisted largely of organisms that occupy lower trophic positions. Indeed, the gut contents of the two alligators sampled in the present study consisted of nutria, crayfish, and insects, all of which were found to occupy low trophic positions in Caddo Lake. Similarly, in a study in southern Louisiana, 36% of the diet of alligators of similar size to those collected in the present study consisted of small fish (Fundulus, Gambusia, Cyprinidon, and Lepomis), crayfish, and insects 53. Given our small sample size, more studies are needed to determine whether the low trophic position observed in the present study is typical for alligators of this size class in Caddo Lake.
Mercury concentration of Caddo Lake biota
Most of the Hg in whole-body and muscle tissues was MeHg for all but the lowest level (i.e., primary) consumers (rams-horn snails, white-tailed deer, nutria; Table 2). This is consistent with the hypotheses that consumers accumulate inorganic Hg less efficiently than MeHg 12 and that the proportion of MeHg in the tissues of consumers is positively related to their trophic position 28. Total Hg is often used as a proxy for MeHg concentrations in high-trophic-level fish 18 and other vertebrates 19, but our data suggest that total Hg is a suitable proxy for MeHg in muscle and whole-body tissue for all but primary consumers.
Interestingly, unionid mussels (also a primary consumer) had one of the highest proportions of MeHg in their muscle tissue. Other studies have reported high proportions of MeHg in the tissue of freshwater mussels but also high individual and ecosystem variation in MeHg concentrations 54, 55. Malley et al. 54 reported an increase in the proportion of MeHg in mussel foot muscle from approximately 70% to over 90% after the flooding of an experimental wetland in Ontario, Canada. Malley et al. 54 hypothesized that the percentage of MeHg in muscle tissue increased because mussels were exposed to MeHg bound to particles of decaying organic matter during flooding 54. The mussels examined in the present study were collected from the downstream end of a seasonally flooded wetland, and it is possible that they were exposed to MeHg via organic particles suspended in flood waters 54.
Unlike the case in muscle tissue, MeHg was not the dominant form of Hg in liver. Low proportions of MeHg in liver relative to muscle or other tissues have been reported in previous studies 20, 21. The difference in Hg concentrations between muscle and liver is indicative of the different transport and assimilation mechanisms in the two tissues. Fish and other vertebrate consumers are exposed to MeHg and inorganic Hg through the diet 12, 21. After crossing the gut, Hg is transported via the hepatic portal system to the liver. Binding proteins, including metallothioneins and selenoproteins, have a high affinity for inorganic Hg but not MeHg 14, 21. Thus, MeHg in liver declines after exposure and eventually relocates to skeletal muscle, where it accumulates bound to sulfhydryl groups in protein 12, whereas inorganic Hg is sequestered in the liver 21. The high proportion of inorganic Hg in liver may also result from hepatic demethylation 15-17.
Several authors have reported that the concentration of total Hg in the liver is less than or similar to that in muscle tissue when the concentration in muscle is <2,500 ng/g dry weight (500 ng/g wet wt) 21, 56, 57. Conversely, when the concentration of total Hg in muscle is from 2,500 to 5,000 ng/g dry weight, total Hg concentrations in liver are greater than those in muscle. Organisms in the present study with total Hg concentrations greater than approximately 2,000 ng/g dry weight in their muscle had total Hg concentrations in their liver that were greater than would be expected if the relationship between total Hg concentration in liver and muscle was 1:1. Drevnick et al. 21 hypothesized that this phenomenon is a result of accumulation of inorganic Hg in the liver. Our data support this hypothesis; we observed an inverse relationship between the liver:muscle total Hg ratio and the percentage of MeHg in liver. This indicates that, when the concentration of total Hg is high in liver tissue relative to muscle tissue, the majority of Hg in the liver is in an inorganic form. This pattern of high concentrations of inorganic Hg and low concentrations of MeHg in the livers of organisms with high concentrations of total Hg in muscle could be evidence of hepatic demethylation 57. Alternatively, a similar pattern of high concentrations of inorganic Hg and low concentrations of MeHg in the liver would be expected if organisms had a significant dietary source of inorganic Hg (e.g., invertebrates with elevated inorganic Hg concentrations) 21.
Mercury biomagnification in the Caddo Lake community
We observed Hg biomagnification in the Caddo Lake food web. The FWMF for total Hg observed in the present study is similar to FWMFs reported in other studies that included nonfish vertebrates in addition to fish and invertebrates (Table 3). To our knowledge, this is one of the few studies examining Hg biomagnification outside of the temperate or subarctic regions (for review see Riget et al. 30). In addition, the present study is one of the most comprehensive (number and diversity of organisms) studies of Hg biomagnification in a freshwater ecosystem. The rate of Hg biomagnification has been hypothesized to be greater in freshwater than in marine ecosystems 58. However, the similarity between the FWMF observed in the present study and FWMFs in marine ecosystems throughout the world do not support the hypothesis that Hg biomagnification is more efficient in freshwater ecosystems than in marine ecosystems. Mercury biomagnification may be a process that is not dependent on ecosystem type or location 29, 30. Additional studies are needed to determine whether the FWMF observed in the present study is unique to Caddo Lake or freshwater ecosystems in general.
| Tissue | Location | FWMFa | Reference | |
|---|---|---|---|---|
| Total Hg | MeHg | |||
| Whole/muscle | Fly Estuary, Papua New Guinea | 5 | — |
Yoshinaga et al. 33 |
| Whole/muscleb | Lancaster Sound, Canada | 4.8 | — |
Atwell et al. 34 |
| Whole/muscle | Northwater Polyna | 4.7 | 5.7 |
Campbell et al. 29 |
| Whole/muscle | Davis Strait, West Greenland | 1.2 | 3.2 |
Riget et al. 30 |
| Whole/muscle | Caddo Lake, Texas, USA | 4.3 | 4.8 | This study |
- a FWMF = 10b · α or eb · α depending on whether the study used log or ln transformation, respectively. Where b is the slope of the relationship between log(or ln)-transformed Hg concentration and δ15N and α equals a trophic enrichment value of 3.4‰. Calculated in this manner the FWMF represents the increase in Hg concentration from one trophic level to the next averaged over the entire food web.
- b Mercury analyzed in wet tissues.
Methylmercury is transferred more efficiently than total Hg in food webs 59; however, because few biomagnification studies have examined both total and MeHg, it is not well understood how the mean FWMF of MeHg and total Hg differs over the length of food chains. In the present study, the FWMF for MeHg was not significantly different from the FWMF for total Hg, because most of the total mercury in the consumers examined was methylmercury. Campbell et al. 29 found that MeHg was transferred only slightly more efficiently than total Hg (×1.2) in the Northwater Polyna food web, but they did not statistically compare FWMFs. Riget et al. 30 found that MeHg was transferred through the food web >2.5 times more efficiently than total Hg in the Davis Strait of West Greenland. The enhanced FWMF rates for MeHg in the latter study likely resulted from the inclusion of a greater proportion of low-trophic-level organisms rather than a fundamental difference in the transfer of Hg in the food chain. Riget et al. 30 sampled more organisms at the base of the food chain with low MeHg:total Hg ratios than in the present study or the study by Campbell et al. 29. This indicates that studies that include a large number of primary consumers with low MeHg:total Hg ratios may underestimate MeHg FWMF if they use total Hg as a proxy for MeHg. Future comparisons of FWMFs between studies should take this potential confounding factor into consideration.
We observed biomagnification of both MeHg and total Hg in the livers of Caddo Lake vertebrates. The FWMFs for total Hg and MeHg in liver were not significantly different. The FWMF for total Hg in liver was similar to that observed for muscle, but the FWMF for MeHg in liver was about two times lower than that observed for muscle. In one of the few other studies to assess FWMFs in liver, Dehn et al. 60 reported that total Hg in liver increased by a factor of 4.2 from one trophic level to the next, similarly to studies that have examined only muscle tissue. The ability of Hg to biomagnify in liver tissue is potentially significant from a wildlife health standpoint, because elevated Hg concentrations in liver have recently been found to be related to biomarkers indicative of oxidative stress 21. However, it is worth noting that, although the FWMF for total Hg in liver was similar to that observed in muscle, the speciation of Hg in the two tissues differed. Methylmercury was the predominant species in muscle, whereas inorganic Hg was the predominant species in liver. More research is needed on the roles of MeHg and inorganic Hg in liver toxicity 21.
Nutria and white-tailed deer had lower concentrations of Hg and a lower percentage of MeHg in their tissues than most of the aquatic consumers examined in the present study. We hypothesize that this pattern occurs because nutria and white-tailed deer are part of a terrestrial–semiaquatic food chain that is not contaminated with high levels of MeHg. White-tailed deer consume terrestrial vegetation but feed on emergent aquatic vegetation in some habitats, whereas nutria prefer floating and emergent aquatic vegetation but consume some terrestrial vegetation 61-64. Terrestrial vegetation becomes contaminated with inorganic forms of Hg that are deposited directly from the atmosphere onto the surface of vegetation 65. Because terrestrial vegetation contains low concentrations of inorganic Hg, which does not typically biomagnify, organisms such as nutria and white-tailed deer feeding in terrestrial food chains would be expected to have low concentrations of total Hg and MeHg.
Implications for fish and wildlife health
The toxic effect of MeHg on vertebrates has been well documented 2, 19, but the level of risk posed to wildlife by concentrations of MeHg frequently encountered in the environment is not clear 2. For example, raccoons in the present study did not contain Hg concentrations in their liver considered harmful to adult mammals (10,000–20,000 ng/g wet wt or ∼50,000–100,000 ng/g dry wt 66). However, few studies have examined sublethal effects of MeHg in wild mammals 12. Even less is known about the sublethal effects of Hg on the health of reptiles and amphibians 1.
Several attempts have been made to assess the risk posed to piscivorous wildlife by Hg in fish 67-69. Hinck et al. 69 proposed 100 to 300 ng/g wet weight in whole bodies of prey fish (∼500–1500 ng/g dry wt in whole bodies and ∼800–2,400 ng/g dry wt in muscle tissue) as the threshold level at which piscivorous fish and wildlife are at risk of adverse health effects. Based on this standard, fish from Caddo Lake may pose a threat to exclusively piscivorous organisms.
During the past decade, evidence has accumulated that physiology and reproduction of fish are negatively impacted by MeHg at concentrations similar to the concentrations observed in the present study 3, 21, 70, 71. For mean concentrations of MeHg ranging from approximately 125 to 325 ng/g in liver, Larose et al. 70 observed adverse effects including reduced hepatosomatic indices and lower glutathione-S-transferase, glutathione reductase, and glutathione peroxidase selenium-dependent activity compared with fish with lower concentrations of MeHg in their livers. Drevnick et al. 21 reported poor condition and an increase in hepatic lipofuscin, a pigment that results from lipid peroxidation of membranous organelles, in fish with total Hg concentrations in muscle ranging from 69 to 622 ng/g wet weight (∼350–3,100 ng/g dry wt). In two recent reviews, Crump and Trudeau 71 and Sandheinrich and Wiener 3 concluded that there is sufficient evidence to link exposure of Hg to reproductive impairment in fish species. Several of the studies reviewed by these authors involved fish with concentrations of Hg in their tissues similar to those observed in the present study. Collectively, these studies suggest that the Hg concentrations observed in Caddo Lake fish may be high enough to impact their health negatively.
Acknowledgements
Support for this project was provided by the Caddo Lake Institute, the Coypu Foundation, Texas Christian University, and Texas Tech University. We thank Dale Renkenberger for his expert boatmanship and invaluable assistance with sample collection on Caddo Lake. We also thank the following for assistance in the field or laboratory: Sam and Randi Canup, Billy Carter, Emily Chumchal, Terry Coleman, Yanci Deng, Ray Drenner, Gary Endsley, Charles Hardin, Jaron Hill, Brianne Kiester, Niki Long, Alden Park, Sean Richards, and Bob, Kimmie, and Dustin Sanders. Mark Williams provided access to the Caddo Lake National Wildlife Refuge. Vanessa Adams of the Texas Parks and Wildlife Department provided permits for sample collection within the Caddo Lake Wildlife Management Area and donated white-tailed deer samples. Figure 1 was drawn by Katherine Burgess.