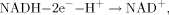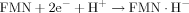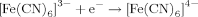Effect of humic substances on toxicity of inorganic oxidizer bioluminescent monitoring
Abstract
The current study deals with the effect of humic substances (HS) on toxicity of solutions of a model inorganic oxidizer, potassium ferricyanide. Chemical reactions responsible for toxicity changes are under consideration. The bioluminescent system of coupled enzymatic reactions catalyzed by bacterial luciferase and oxidoreductase was used as a bioassay. General and oxidative toxicity of ferricyanide solutions were evaluated. Ability of HS to decrease or increase general and oxidative toxicity of the solutions was revealed. Two types of chemical processes are supposed to be responsible for detoxification by HS: ferricyanide–HS complex formation and acceleration of endogenous redox reactions in the bioluminescent assay system. Decrease of oxidative toxicity of ferricyanide solution was observed under incubation with HS at all concentrations of HS used. Conditions for general toxicity decrease were prior incubation of ferricyanide with HS and low HS concentrations (<10−4 g/L). Acceleration of NADH auto-oxidation under higher HS concentrations was supposed to result in a toxicity increase. Environ. Toxicol. Chem. 2011; 30:1013–1017. © 2011 SETAC
INTRODUCTION
Redox processes are a part of the main vital metabolic cycles, such as respiration, photosynthesis, and others, and the excess of exogenous redox compounds, such as quinones, phenols, aliovalent metals, in the environment may disturb the redox equilibrium, resulting in toxic impact on organisms 1. This is why oxidizers, as sewage components, are at the top of the list of toxic pollutants.
Humic substances (HS) are irregular polymers of a complex structure 2. They are ubiquitous and result from the natural transformation of organic matter in soil and sediments. Among the effects of HS in the environment, increasing evidence indicates that they can act as natural attenuators of toxicity of heavy metals, surfactants, hydrocarbons, and organic oxidizers 2-6. Elucidation of the detoxification mechanism by HS is of great interest 3, 7-12. Detoxifying properties of HS are generally attributed to their binding ability and redox properties. Interaction of xenobiotics with HS causes the formation of complexes of lower bioaccumulation and toxicity 13; quinoid, phenolic, sulfhydryl, and other groups are responsible for redox activity of HS-macromolecules. Evidence has accumulated that HS, and particularly their quinoid moieties, can play an important role as electron shuttles in microbial redox reactions involved in biodegradation of pollutants 14.
In a study by Fedorova et al. 15, bioluminescent assays were applied for the first time to monitor changes in the toxicity of organic oxidizers, quinones, under exposure to HS. A series of homologous quinones with different redox characteristics were used as model oxidizers. Two mechanisms of HS effect were investigated in quinone solutions: reduction activity of HS and intensifying of self-protection of bacterial cells on HS addition. In the study by Vetrova et al. 16, a high contribution of quinone hydrophobicity to the toxicity of quinone solutions, along with redox properties, was proven. The data point to the importance of hydrophobic binding of quinone molecules by HS.
Enzymatic redox reactions can be used as bioassays for monitoring toxicity of redox compounds in solutions. Among those is a bacterial bioluminescent enzyme system 17-24.
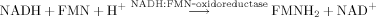 (1)
(1)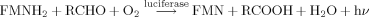 (2)
(2)Luminescent intensity is the main test parameter in this assay system 25.
Oxidizers are capable of competing with FMN in reduction by NADH in the first reaction and, hence, inhibiting the second luminescent reaction. In this case, the delay period appears in bioluminescent kinetics. In Kudryasheva et al. 18 and Vetrova et al. 26, the bioluminescent enzymatic assay system was already used to monitor oxidative toxicity of organic oxidizers, quinones. Figure 1 presents schematically the bioluminescence kinetics in the presence and absence of the organic oxidizers according Kudryasheva et al. 18. The oxidizers were shown to decrease bioluminescent intensity (I) and increase the time of bioluminescent maximum delay (T0.5). The value ΔI was chosen to evaluate the general toxicity, and T0.5 the oxidative toxicity of a sample.
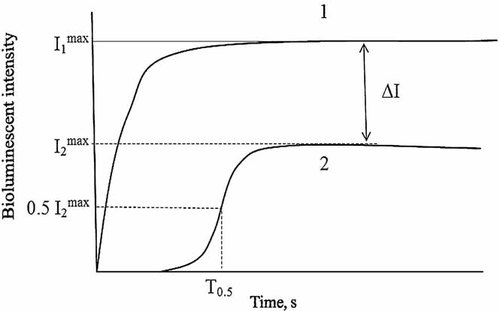
Bioluminescence kinetics scheme for a coupled system of enzymatic reactions in the absence (1) and presence (2) of an oxidizer 18.
This work studies the oxidative and general toxicity of potassium ferricyanide (K3[Fe(CN)6]) as a model inorganic oxidizer, in the absence or presence of HS in solution. The K3[Fe(CN)6] was chosen because of its stability in water solution (in contrast to unclustered iron salts) and mono-electron oxidative transition Fe3+/Fe2+ 27, 28. We studied the detailed chemical processes taking place in the enzyme system sensitive to oxidizers.
MATERIALS AND METHODS
Reagents
The Gumat-80 preparation (Gumat) was used as a source of HS. It was produced by non-extracting treatment of coal with alkali 29. The amount of potassium humate in preparation exceeded 70%.
Chemicals used were NADH (ICN); FMN, and tetradecanal (SERVA), K3[Fe(CN)6] of analytical grade.
The toxicity of water solutions was assessed using the bioluminescent assay preparation based on a coupled enzyme system: NADH:FMN–oxidoreductase from Vibrio fischeri (0.15 activity units) and luciferase from Photobacterium leiognathi, 0.5 mg/ml 30. The preparation was produced at the Institute of Biophysics, Krasnoyarsk, Russia. To construct the assay system, 0.1 mg/ml enzyme preparation, 5.4 × 10−4 M FMN, 4 × 10−4 M NADH, and 0.002% tetradecanal solutions were used. The assay was performed in 0.05 M phosphate buffer (pH 6.8) at room temperature.
Concentrations of HS (2 × 10−6/10−3 g/L) that inhibit bioluminescence by less than 10% were applied. Higher concentrations inhibited bioluminescent intensity because of the effect of optic filter 15; this is why those were not used in the experiments.
The time of incubation of K3[Fe(CN)6] with HS varied from 0 to 50 min.
The measurements of bioluminescent intensity were carried out with bioluminometers BLM-3606 (Nauka Special Design Bureau) and TriStar LB 941 (Berthold Technologies). The results were processed statistically by the least-squares method. Values of standard deviations did not exceed 10%.
To study the rates of NADH oxidation, optical density, D, of solutions was registered by double-beam spectrophotometer UVIKON-943 (Kontron Instruments, Italy).
Processing of experimental data
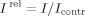 (3)
(3)Here, Icontr and I are maximal bioluminescent intensities in the absence and presence of potassium ferricyanide, respectively.
Concentrations of toxic molecules decreasing bioluminescent intensity by 50% (I rel = 0.5), C50, were used to compare general toxicities of individual molecules.
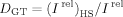 (4)
(4)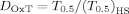 (5)
(5)The DOxT > 1 and DOxT < 1 indicated decrease and increase of oxidative toxicity of a solution, respectively.
Values of standard deviations for DGT and DOxT did not exceed 0.04.
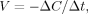 (6)
(6)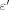 L and Δt = 10 min. Here, ΔC is a change of NADH concentration;
L and Δt = 10 min. Here, ΔC is a change of NADH concentration; 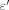 = 8,250 L/(M cm), a molar extinction coefficient at excitation wavelength λ = 340 nm; l = 1 cm, an optical pathway of light in the solution. Absorption of the corresponding oxidized form at λ = 340 nm was negligible: εNAD+ = 0.
= 8,250 L/(M cm), a molar extinction coefficient at excitation wavelength λ = 340 nm; l = 1 cm, an optical pathway of light in the solution. Absorption of the corresponding oxidized form at λ = 340 nm was negligible: εNAD+ = 0.RESULTS AND DISCUSSION
Effect of potassium ferricyanide on bioluminescent enzyme system
The kinetics of bioluminescent enzyme system under various concentrations of potassium ferricyanide is reported in Figure 2.
Bioluminescent intensity (Irel) of the coupled system of enzymatic reactions at different concentrations of potassium ferricyanide: 1, control; 2, 3 × 10−6 M; 3, 3 × 10−5 M; 4, 6 × 10−5 M; 5, 8 × 10−5 M; 6, 10−4 M; 7, 2 × 10−4 M.
Low concentrations of ferricyanide (≤3 × 10−5 M) did not affect bioluminescence, whereas higher concentrations increased the delay period and decreased the maximum bioluminescent intensity (Fig. 2).
The value of C50 in ferricyanide solutions was 2 × 10−4 M (Fig. 2, curve 7), this being close to those reported for solutions of organic oxidizers, quinones 15.
The delay effect was studied previously in quinone solutions 15, 18, 26, 31 and was found to depend on concentration and standard redox potential of quinones. The bioluminescence delay was explained in terms of hydrogen (e− + H+) exchange in the process of quinone competition with FMN for NADH in reaction (1).
In ferricyanide solution, T0,5 equaled 850 s (Fig. 2, curve 7), whereas in quinone solutions it varied from 200 to 400 s 18, 26. This difference is supported by the higher standard redox potential of ferricyanide (0.77 V) relative to those of quinones (<0.71 V) (http://employees.csbsju.edu/hjakubowski/classes/ch331/oxphos/standredpotentialtab.htm). Hence, oxidative contribution to general toxicity is higher for ferricyanide than for quinones.
To demonstrate the effects of HS on oxidative and general toxicities, the concentration of ferricyanide solution was taken as 8 × 10−5 M (Fig. 2, curve 5).
Ferricyanide toxicity in the presence of humic substances
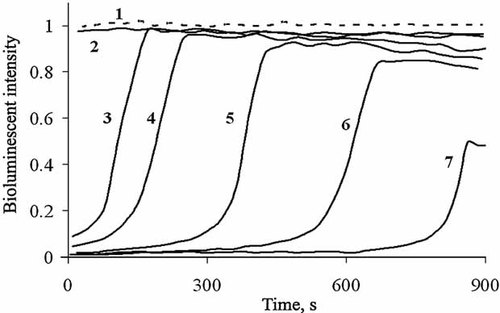
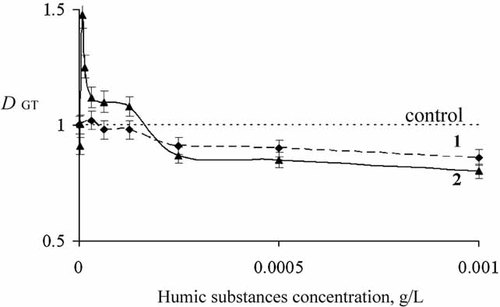
To study general and oxidative toxicity of ferricyanide solution (C = 8 × 10−5 M), the DGT and DOxT were calculated according to Equations (1) and (2). Figures 3 and 4 show dependences of DGT and DOxT on concentration of HS. Curves 2 in Figures 3 and 4 present the data under incubation of ferricyanide with HS for 50 min.
Detoxification coefficient (DGT) for potassium ferricyanide solution, C = 8 × 10−5 M, versus concentration of humic substances (HS), CHS. 1, without incubation; 2, 50-min incubation time.
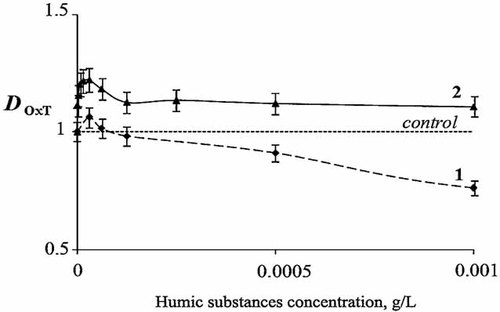
Detoxification coefficient (DOxT) for potassium ferricyanide solution, C = 8 × 10−5 M, versus HS concentration, CHS. 1, without incubation; 2, 50-min incubation time.
Curve 1 in Figure 3 shows a slight increase of general toxicity (DGT < 1) without incubation with HS. The incubation (Fig. 3, curve 2) provokes detoxification (DGT > 1) at CHS < 0.0002 g/L and increase of general toxicity (DGT < 1) at higher HS concentrations.
As follows from Figure 4, curve 1, addition of HS increases oxidative toxicity of ferricyanide solution (DOxT < 1) at CHS > 10−4 g/L. Incubation of ferricyanide with HS decreases oxidative toxicity (DOxT > 1) at all concentrations of HS (Fig. 4, curve 2).
Variation of incubation time from 7 to 50 min had no substantial effect on both toxicities in Figures 3 and 4, curves 2. Table 1 presents detoxification constants DGT and DOxT for CHS = 8 × 10−5 g/L at different incubation times.
| Incubation time, min | 0 | 7 | 10 | 15 | 20 | 25 | 50 |
|---|---|---|---|---|---|---|---|
| DGT | 1.00 | 1.15 | 1.14 | 1.13 | 1.14 | 1.14 | 1.14 |
| DOxT | 1.01 | 1.27 | 1.26 | 1.27 | 1.27 | 1.27 | 1.26 |
Similar results were obtained under variation of HS concentration from 2 × 10−6 to 10−4 g/L.
Hence, detoxification ability of HS does not depend on incubation time, and it changes abruptly during the first 7 min of incubation. It means that all interactions of HS with components of the solutions occur during this period.
Mechanism of HS influence on toxicity of ferricyanide solutions should be analyzed, and the next subsections of this report consider chemical processes accounting for toxicity changes in the bioluminescent system.
Effect of humic substances on components of solutions
To identify the components of the bioluminescent enzymatic system sensitive to HS, we studied the rates of chemical reactions taking place in the bioluminescent system in the presence of HS.
Concentrations of oxidizers (FMN and K3[Fe(CN)6]) did not depend on HS. In the case of NADH, organic reducer, the action of HS was obvious.
The rates of NADH decomposition were analyzed without and under incubation with HS. Because ferricyanide can be reduced nonenzymatically in a bioluminescent enzyme system 26, the rates of nonenzymatic reactions along with those of enzymatic reactions were analyzed.
Rates of NADH oxidation without incubation
The range of HS concentrations applied was 6 × 10−5 to 10−3 g/L. All HS concentrations produce similar effects on the components of bioluminescent system.
The rates of nonenzymatic and enzymatic decomposition of NADH in the absence (V) and presence (VHS) of HS are presented in Table 2, with CHS = 2 × 10−4 g/L as an example.
| N | Components of solution | V × 107, M/min | ||
|---|---|---|---|---|
| V | VHS | ΔV = VHS – V | ||
| Nonenzymatic processes | ||||
| 1 | NADH | 1.2 | 2.3 | 1.1 |
| 2 | NADH + FMN | 3.9 | 5.1 | 1.2 |
| 3 | NADH + K3[Fe(CN)6] | 7.8 | 7.6 | −0.2 |
| Enzymatic processes | ||||
| 2E | NADH + FMN + E | 7.0 | 8.0 | 1.0 |
| 3E | NADH + K3[Fe(CN)6] + E | 1.9 | 2.1 | 0.2 |
- FMN = flavin mononucleotide; K3[Fe(CN)6] = potassium ferricyanide; E = enzyme preparation.
As follows from Table 2, the addition of HS to NADH solution increases the NADH autooxidation rate (Table 2, solution 1, ΔV = 1.1 × 10−7 M/min). These data show the ability of HS to decrease the reductive activity of media. Carbonyl, carboxyl, and quinoid groups of HS may account for this process 2.
The decrease of NADH concentration under the HS effect is a probable reason of enhancement of oxidative and general toxicities (Figs. 3, 4; curve 1).
In nonenzymatic processes, the addition of FMN to NADH increases V and VHS (compare solutions 1 and 2, Table 2), whereas the addition of K3[Fe(CN)6] to NADH increases these rates to a higher extent (compare solutions 1 and 3; Table 2). This difference deals with redox potentials of FMN and K3[Fe(CN)6]: these for semireactions (4) and (5) were calculated as 0.13 and 0.53, respectively, using the Nernst equation.
Addition of HS to solution 2 enhances the reaction rate (ΔV = 1.2 × 10−7 M/min; Table 2), but in the case of solution 3 it does not (Table 2). Similar tendencies were observed in enzymatic solutions 2E and 3E, Table 2: ΔV-values were 10−7 and 0.2 × 10−7 M/min, respectively.
Hence, the HS make FMN more competitive than ferricyanide (Eqns. 8 and 9, respectively) in reducing by NADH in enzymatic and nonenzymatic reactions. This fact may account for a decrease of oxidative toxicity (increase of DOxT) of ferricyanide solution in the presence of HS in a bioluminescent system.
Acceleration of FMN reduction process is an internal bioassay property responsible for detoxification. Extrapolation of this fact onto higher organisms provides understanding as to why the sensitivities of organisms to toxic substances, as well as to detoxifying agents, are so different.
Differences in sensitivities of different bacterial bioassays have been reported by Kudryasheva et al. 32. An application of a set of bioassays was suggested in this report for complex toxicity monitoring of wastewaters.
Rates of NADH oxidation under incubation with HS
We found that VHS in solutions 3 and 3E (Table 2) change on incubation of ferricyanide with HS. The VHS values in solution 3 were 7.8 × 10−7, 7.1 × 10−7, 7.0 × 10−7, 7.0 × 10−7, 7.2 × 10−7 M/min for incubation times 0, 7, 10, 25, and 50 min, respectively. (standard deviation = 0.1 × 10−7 M/min). Similar effects were found in solution 3E.
Hence, during the first seven incubation minutes the reaction rate drops by approximately 10%, and the following incubation does not affect the rate. The time dependence is similar to those presented in Table 1. Probably, this dependence is a basis for toxicity decrease (the increase of DGT and DOxT) under incubation.
Detoxification under incubation seems to result from complexation of ferricyanide by HS macromolecules.
CONCLUSION
This work studied the effect of HS on solutions of potassium ferricyanide as a model inorganic oxidizer. The bioluminescent system of coupled enzymatic reactions was used as a bioassay. General and oxidative toxicities of ferricyanide solutions were evaluated. The ability of HS to decrease or increase general and oxidative toxicity of the solutions was revealed. The conditions for toxicity decrease and increase were determined. To find the reasons of toxicity change, the rates of redox reaction in a bioluminescent assay system were analyzed in the absence and presence of HS. Acceleration of NADH auto-oxidation by HS resulted in an increase in toxicity, whereas a decrease in toxicity was caused by acceleration of endogenous NADH-dependent redox reactions in the bioluminescent assay system.
We focused on the conditions required to detoxify solutions of the model inorganic oxidizer. Concentrations of HS lower than 10−4g/L and incubation of HS with ferricyanide enhanced the detoxification effect. The time of detoxification of ferricyanide by HS did not exceed 7 min. Detoxification under incubation seems to result from complexation of ferricyanide by HS macromolecules.
Hence, two types of processes are supposed to be responsible for detoxification of the oxidizer solutions by HS: acceleration of endogenous NADH-dependent redox reactions in a bioluminescent assay system and complexation of ferricyanide by HS.
From the standpoint of environmental implications, the present study promotes application of a luminescent bioassay to monitor toxicity of environmental waters in the remediation processes. Insufficient time of exposure to HS decreases their detoxification efficiency in oxidizer solutions, and high HS concentrations can cause an undesirable increase in toxicity.
Acknowledgements
Funding for this project was provided by the Molecular and Cellular Biology Program of the Russian Academy of Science; grants from the Russian Ministry of Education and Science, Leading Scientific School N 1211.2008.4 and N 2.2.2.2/5309; Federal Target Program Research and scientific-pedagogical personnel of innovation in Russia for 2009–2013 years, contract N 02.740.11.0766; and a grant from the Russian Foundation for Basic Research N 10-05-01059-a.