Genotoxic Response and Mortality in 3 Marine Copepods Exposed to Waterborne Copper
Abstract
Copper (Cu) is an essential trace metal, but may also be toxic to aquatic organisms. Although many studies have investigated the cytotoxicity of Cu, little is known about the in vivo genotoxic potential of Cu in marine invertebrates. We investigated the genotoxicity of Cu in 2 pelagic calanoid copepods, Acartia tonsa and Temora longicornis, and the intertidal harpacticoid copepod Tigriopus brevicornis by exposing them for 6 and 72 h to waterborne Cu (0, 6, and 60 µg Cu/L). A subsequent 24-h period in filtered seawater was used to investigate delayed effects or recovery. Genotoxicity was evaluated as DNA strand breaks in individual copepods using the comet assay. Copper did not increase DNA strand breaks in any of the species at any concentration or time point. The treatment did, however, cause 100% mortality in A. tonsa following exposure to 60 µg Cu/L. Acartia tonsa and T. longicornis were more susceptible to Cu-induced mortality than the benthic harpacticoid T. brevicornis, which appeared to be unaffected by the treatments. The results show major differences in Cu susceptibility among the 3 copepods and also that acute toxicity of Cu to A. tonsa is not directly associated with genotoxicity. We also show that the comet assay can be used to quantify genotoxicity in individual copepods. Environ Toxicol Chem 2019;38:2224–2232. © 2019 SETAC.
INTRODUCTION
Copper (Cu) is an essential element, but also a pollutant that enters marine ecosystems through leaching from sewage effluents, urban stormwater, riverine inputs, industrial effluents, runoff from agriculture, and use as an antifoulant on ships and in fish farming (Schiff et al. 2004; Hack et al. 2008; Skarbøvik et al. 2010; Brooks et al. 2015; Panagos et al. 2018). Copper concentrations in open ocean and coastal waters range from 0.15 to 10 µg/L, with concentrations up to 100 µg/L found in severely polluted locations (Nriagu and Pacyna 1988; World Health Organization 1998). The maximum acceptable guideline value for Cu in European and Norwegian coastal waters is 2.6 µg/L (Van Sprang et al. 2007; Pettersen 2016).
Copper plays important roles in physiological processes, for example, as a cofactor for enzymes (Bertinato and L'Abbé 2004), as an acute phase reactant (Camakaris et al. 1999), and in the respiratory pigments of molluscs and arthropods (Markl 2013). Elevated Cu concentrations can cause cytotoxicity, neurotoxicity, and metabolic toxicity in marine organisms (Brown et al. 2004; Cunha et al. 2007; Lauer et al. 2012). The toxicity of Cu is commonly linked to cellular redox because the metal can contribute to the generation of reactive oxygen species (ROS) in Haber–Weiss and Fenton reactions (Lloyd and Phillips 1999). Copper-mediated ROS may cause lipid peroxidation (Barata 2005), protein degradation (Allen 2001), and DNA damage (Bolognesi et al. 1999; Bopp et al. 2008). Furthermore, Cu genotoxicity has been observed as increased micronucleus frequency (Barka et al. 2016) and apoptosis (Guo et al. 2017) in the cladoceran Daphnia magma and the white shrimp Litopenaeus vannamei. In addition, exposure to Cu has been shown to induce DNA strand breaks in the freshwater clam Corbicula fluminea as well as in marine bivalves and polychaetes (Bonnail et al. 2016).
Copepods are among the most abundant organisms and marine invertebrates on earth (Huys and Boxshall 1993). Coastal copepods, such as Tigriopus spp., Acartia tonsa, Nitocra spinipes, and Tisbe battaglia, are widely used in ecotoxicological studies because of their wide distribution and high ecological relevance (Sverdrup et al. 2002; Organisation for Economic Co-operation and Development 2006; Kusk and Wollenberger 2007; Raisuddin et al. 2007; Dahms et al. 2016). Copepods are pivotal for carbon transfer in marine food webs through their roles as predator, prey, and flux vehicles (Ruppert et al. 2004). Calanoid copepods (e.g., Calanus, Acartia, and Temora) are important in temperate pelagic zooplankton communities. Both Acartia tonsa and Temora longicornis are common in Atlantic temperate coastal waters. Although both species can tolerate environmental fluctuations in temperature and salinity, A. tonsa is a more estuarine species and has been observed to tolerate lower salinities than T. longicornis (Brylinski 1981; Calliari et al. 2006; Holste et al. 2009). Most harpacticoid copepods belong to benthic communities (Raisuddin et al. 2007). The harpacticoid Tigriopus brevicornis is widely distributed along the European Atlantic coast, commonly as an inhabitant of supratidal rock pools (Denis et al. 2009), a habitat with strong physicochemical variations (Davenport et al. 1997).
Copper exposure has been shown to cause a range of effects in copepods, such as decreased growth and developmental rates (Lee et al. 2008a), embryotoxicity (Mai et al. 2012), and modulation of cellular antioxidant defence mechanisms (Gaetke and Chow 2003; Zeeshan et al. 2016). Earlier observations of Cu genotoxicity in aquatic invertebrates led us to expect that coastal copepods may similarly experience increased DNA strand breaks after exposure to Cu. Previous studies of copepods exposed to persistent organic pollutants (Won and Lee 2014; Han et al. 2015; Shi et al. 2017), crude oil (Han et al. 2014), and cadmium (Pavlaki et al. 2016) suggest susceptibility to pollutant-induced DNA strand breaks, but it is not known whether Cu may have the same potential. The comet assay is a widely used method to quantify DNA strand breaks (de Lapuente et al. 2015; Martins and Costa 2015) and has previously been applied to copepods, but only for pooled samples of 100 to 120 individuals (Won and Lee, 2014; Han et al. 2015; Pavlaki et al. 2016; Shi et al. 2017).
The aim of the present study was to assess the genotoxicity of Cu to 3 marine copepods: the pelagic calanoid copepods A. tonsa and T. longicornis and the benthic harpacticoid copepod T. brevicornis. Following an in vivo short-term exposure, genotoxicity was quantified as DNA strand breaks using the comet assay for individual copepods.
MATERIALS AND METHODS
Animal collection and husbandry
All 3 copepod species (A. tonsa, T. longicornis, and T. brevicornis) were from long-standing stock cultures (>9 mo), originally sampled in the outer Oslofjord, Norway. The A. tonsa and T. longicornis cultures were kept at 8 °C, whereas T. brevicornis cultures were kept at 18 °C. All cultures were kept in filtered ambient seawater under a 12:12-h light:dark photoperiod, and fed ad libitum with a 1:1:1 mixture of Dunaliella tertiolecta, Isochrysis galbana, and Rhodomonas salina. Copepods were acclimated to 12 °C before the experiment.
Experimental setup and exposure conditions
We incubated copepods individually in 70-mL cell culture flasks (TC Flask T25; Sarstedt). To best simulate their natural habitats, the 2 pelagic species, A. tonsa and T. longicornis, were incubated on a slowly rotating plankton wheel (0.3 rpm) keeping them in suspension. The semibenthic copepod T. brevicornis, generally found in tidal pools, was incubated stationary. Copepods were not fed during incubation. The Cu treatments were prepared using CuSO4, with pH 8.2 and salinity 29. The control treatment was prepared using the same procedures, but distilled water was added instead of CuSO4. Before the experiment, we filled all tissue flasks with the respective treatment solutions for 12 h to minimize potential differences between nominal and true Cu concentration during exposure due to absorption to the flask walls. Treatment solutions were renewed at the start of exposure and kept static throughout the experiment.
To examine baseline levels of DNA strand breaks for each species, individuals of both sex were sorted under a stereomicroscope and transferred directly from the cultures into 6-well plates filled with filtered seawater, 1 individual/well. Baseline levels of DNA strand breaks were measured using 24 replicates/species. Individuals for incubation were transferred into their respective treatment tissue flasks. All 3 species were exposed as individuals in vivo to 0, 6, and 60 µg Cu/L for each of the 2 incubation periods, 6 and 72 h. In addition, there was a 24-h post exposure recovery period in clean seawater following each incubation. Each species × treatment × incubation period combination consisted of 8 replicates. For the 24-h postexposure recovery period (referred to as 24 h post exposure), copepods were transferred to bottles with filtered seawater immediately following 72 h of exposure.
At the end of each incubation period, individuals were sampled and prepared for comet analysis. To avoid systematic differences among species and treatments, we divided the copepods into groups of 18 individuals for the comet procedure, across species and treatments. The sampling of each group was synchronized to its own incubation time. Mortality was quantified at each sampling time point and following 24 and 48 h of exposure. Only live copepods were used for the comet assay.
Comet assay
The comet assay protocol we used is based on that of Gutzkow et al. (2013) with the following modifications for the use with copepods: Cells of unexposed individuals (n = 24) of each species were used to establish a baseline for each species. A subsample of cells of the same unexposed individuals were exposed to hydrogen peroxide (10 µM for 15 min), used as a positive control. Baseline individuals were gently removed from the 6-well plate with a plastic pipette with enlarged opening (cut tip). Copper-exposed individuals were retrieved by sieving the contents of each tissue flask on a 100-µm mesh. The cell suspension was prepared by crushing single copepods with plastic hand-held pestles in 30 µL of ice-cold phosphate-buffered saline (PBS)/ethylenediamine tetraacetic acid (EDTA; 14.5 mM NaCl, 0.6 mM Na2HPO4, 0.4 mM KH2HPO4, 10 mM EDTA, pH 7.7, adjusted to 1000 mOsm) in 1.5-mL Eppendorf tubes. A 10-µL cell suspension was mixed with 90 µL of 0.75 wt-% low-melting-point agarose before it was added to a hydrophilic GelBond® film (Lonza). Each individual was represented by 1 gel (total of 18 gels on each film; Supplemental Data 1). Drops of PBS/EDTA buffer were applied as required to keep the gels moist during the process. Comet films were immediately placed in cold lysis buffer for a 24-h lysis period after embedding. Comets were scored with a Nikon Eclipse LV100ND microscope with an LED pE-300 light source and a Nikon Plan Fluor 20 objective using Comet Assay IV software (Ver 4.3; Perceptive Instruments). As a consequence of using single individuals, 50 cells could not be obtained for all gels. Varying cell counts were accounted for in the statistical analysis (see Statistical analysis section). The scoring was performed blind. Percentage of tail intensity was used to express DNA strand breaks.
DNA strand break normalization
The DNA strand break of each treatment and incubation period combination was normalized as follows against the body mass of the respective species to test whether biomass affected the level of strand breaks: A. tonsa 6.7 µg dry weight, T. longicornis 33.5 µg dry weight, and T. brevicornis 7.4 µg dry weight. Body masses for A. tonsa and T. longicornis were derived from Brun et al. (2017); body mass for T. brevicornis was calculated based on a weight–length relationship for harpacticoid copepods (log W 1⁄4 2.74 ln L–16.41) presented in Hopcroft et al. (1998). Using the conversion factor for adult copepods (0.37) as given by Harris and Paffenhöfer (1976), ash-free dry weight was converted to dry weight.
Statistical analysis
All statistical analyses were performed using statistical software (R Ver 3.4.1; R Development Core Team 2018) and its add-on packages nlme (Ver 3.1-137; Pinheiro et al. 2017) and emmeans (Lenth et al. 2019).
All comet data were analyzed by linear mixed effects models (LME) with DNA strand breaks as the response variable. We determined interspecies differences based on least-square means (Mangiafico 2016). To avoid replicate loss due to lower count numbers, we included squared counts of each individual as a weighted random effect in all models. Model selection was based on stepwise backward deletion selection on the initial model that allowed for interaction between all independent factors. Sex did not have an effect in any analysis. Untransformed data were used in all analyses, if not stated otherwise.
The DNA strand break differences in baseline and responses to H2O2 treatment were analyzed with species and treatment as fixed effects. Because the same individual was sampled for baseline and H2O2 treatment, each treatment had its respective comet film, and we accounted for film and individual variability by including the individual nested in film number as a random factor. Welch's t test was used to reveal treatment differences within each species.
We tested the effect of Cu on DNA strand breaks with species, treatment, and incubation period as factorial fixed effects, and film number as a random factor. The same model definition was used to test the effect of biomass on DNA strand breaks using biomass-normalized strand breaks. Differences in mortality were tested by Pearson's chi-squared test (McHugh 2013). A level of significance of p = 0.05 was set for the rejection of the null hypothesis in all models. Model estimates and statistics are presented in Supplemental Data 2 to 4.
RESULTS
All 3 species had similar baseline levels of DNA strand breaks, with median values of 32% in A. tonsa (n = 22), 35% in T. longicornis (n = 24), and 31% T. brevicornis (n = 23).
According to the significant treatment effect in the LME model (F1,117 = 7.4; p = 0.008), hydrogen peroxide significantly induced DNA strand breaks in A. tonsa (t(39) = –2.9, p = 0.005) but not in T. longicornis and T. brevicornis (Figure 1). Median strand break levels following hydrogen peroxide exposure were 49% in A. tonsa (n = 23), 45% in T. longicornis (n = 18), and 45% in T. brevicornis (n = 24). There was no difference between male and female copepods for either of the treatments (F1,116 < 0.001; p = 0.99).
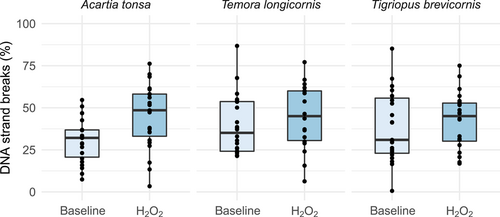
Baseline and hydrogen peroxide–induced DNA strand breaks in the copepods Acartia tonsa, Temora longicornis, and Tigriopus brevicornis; median, 25 and 75 quartiles, median; values for each individual shown as dots.
An interaction between species and time explained the observed pattern of DNA strand breaks in the copepods (F1,151 = 7.4, p < 0.0001), with a marginally nonsignificant concentration-dependent effect (F1,150 = 3.5, p = 0.06; Figure 2). Following Cu exposure, A. tonsa and T. longicornis maintained similar levels of DNA strand breaks up to 72 h. The Cu-induced mortality in A. tonsa affected the determination of concentration effects in DNA strand breaks, because only live individuals could be included in the analysis. Copper exposure did not affect DNA strand break levels in T. brevicornis at any time point or concentration (t ratiodf=8 = 0.6, p = 0.9). All A. tonsa died when kept in filtered seawater following exposure to 60 µg Cu/L (chi-squared test2,7 = 7, p = 0.03; pie charts in Figure 2).
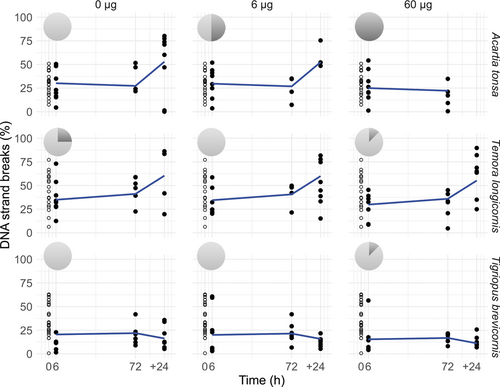
DNA strand breaks in Acartia tonsa, Temora longicornis, and Tigriopus brevicornis following exposure to 0, 6, and 60 µg Cu/L for 6 h, 72 h, and subsequently 24 h post exposure (+24 h). Baseline levels (open circles) are included graphically at time 0 h. Pie charts of % survival (light gray)/mortality (dark gray) for 24 h post exposure (+24 h). The 100% mortality at 60 µg Cu/L post exposure was significant in Acartia tonsa (chi squared test, p = 0.03). Predictions from the linear mixed effect model (blue lines) show an interaction between species and time (F1,151 = 7.4, p < 0.0001).
An interaction between species and time explained the observed pattern in DNA strand breaks/dry weight (F1,148 = 7.9, p < 0.001; Figure 3). Acartia tonsa had higher levels that increased over time, whereas levels of the other 2 species did not change or decrease. An interaction between species and treatment further described DNA strand break levels/dry weight with slightly decreasing levels with concentration for A. tonsa, and little change observed in T. longicornis and T. brevicornis (F1,148 = 3.5, p = 0.3; Figure 3).
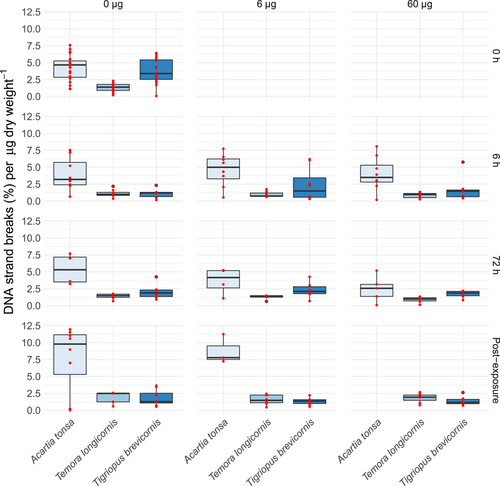
The DNA strand breaks normalized to body mass (µg dry wt) for Acartia tonsa (6.7 µg dry wt), Temora longicornis (33.5 µg dry wt), and Tigriopus brevicornis (7.4 µg dry wt). Red dots represent the underlying raw data. In this graph baseline levels are included graphically and referred to as time 0 h.
DISCUSSION
There was no indication of Cu-dependent genotoxicity in the copepods, but mortality was high in A. tonsa following exposure to the highest Cu concentration. Acartia tonsa appeared to be more susceptible to Cu exposure than T. longicornis and T. brevicornis.
Baseline DNA damage has previously been shown to vary between and within species (Sahlmann et al. 2017). The 3 copepods in our study had similar baseline levels that agreed well with published data for copepods and other zooplankton species (Table 1). The within-treatment variability observed in the present study of single copepods was also within the range observed earlier in studies using pools of up to 120 individuals (Ternjej et al. 2009; Charry et al. 2018). An advantage of studying single copepods is that individual variability in responses to genotoxic insult can be determined. There was substantial variability in DNA strand breaks within a species and treatment, particularly following the postexposure period. Knowledge about this variation will improve the ability to predict a response and the potential adaptability of natural populations.
| Species | % DNA strand breaks, mean | Standard error (%) | Range (%) | Sample size | Reference | |
|---|---|---|---|---|---|---|
| Marine copepod | Acartia tonsa | 31 (26)a | 3 (6)a | 7–55 | Single ind. | Present study |
| Temora longicornis | 39 (38)a | 4 (7)a | 21–86 | Single ind. | Present study | |
| Tigriopus brevicornis | 39 (11)a | 4 (3)a | 1–85 | Single ind. | Present study | |
| Acartia tonsa | ∼24 | ∼0.9 | n.a | Pooled, ∼100 ind. | Pavlaki et al. (2016) | |
| Gladioferense pectinatus | ∼8 | n.a. | 0–60–(98) | Pooled, 120 ind. | Charry et al. (2018) | |
| Paracalanus parvus | <6 | n.a. | n.a. | Pooled, 100 ind. | Goswami et al. (2014) | |
| Oithona rigida | <6 | n.a. | n.a. | Pooled, 100 ind. | Goswami et al. (2014) | |
| Euterpina acutifrons | <6 | n.a. | n.a. | Pooled, 100 ind. | Goswami et al. (2014) | |
| Freshwater copepod | Cyclops abyssorum tatricus | 14–23 | ∼1–3 | n.a | Pooled, ∼100 ind. | Tartarotti et al. (2014) |
| Eudiaptomus padanus | 1.9 | 0.3 | 0–16–(62) | Pooled, 100 ind. | Ternjej et al. (2009) | |
| Cyclops etruscus | 1.6 | 0.2 | 0–11 | Pooled, 100 ind. | Ternjej et al. (2009) | |
| Freshwater planarian | Dugesia schubartii | ∼11–17 | ∼1–2 | n.a | Pooled, 4 ind. | Guecheva et al. (2001) |
| Freshwater cladoceran | Daphnia magna | 3.6 | 0.5 | 0–31 | Pooled, 100 ind. | Ternjej et al. (2009) |
| Freshwater hydrozoa | Hydra magnipapillata | ∼22b | ∼3b | ∼0–68b | Pooled, 30 ind. | Zeeshan et al. (2016) |
| Freshwater ciliate | Paramecium | 13–22 | 2–5 | n.a | Pooled, ∼800–1200 indv. | Kammerlander et al. (2017) |
- a Baseline values used as time 0 h, in parentheses values from 6 h control treatment.
- b We used the results from % cells and DNA damage categories provided by the referenced study to convert into % DNA strand breaks, standard error, and the % average of the respective categories for range approximation.
- ind. = individual(s); n.a. = not available.
Hydrogen peroxide exposure caused increased strand break levels in A. tonsa, but not in the other 2 species. High variability in baseline levels in T. longicornis and T. brevicornis caused low test power and decreased the ability to detect a hydrogen peroxide effect, but there were no obvious changes in the median. Most earlier studies using the comet assay with copepods (Table 1) did not use positive controls (Pavlaki et al. 2016). Earlier studies in our laboratory have found invertebrate circulating cells to be very sensitive compared with vertebrate cells, and increased strand breaks were seen at 5 µM (Sahlmann et al. 2017). Hydrogen peroxide at 10 µM caused a significant increase in DNA strand breaks in Scrobicularia plana hemocytes (Petridis et al. 2009). More studies are clearly needed to clarify why copepod cells are so robust to oxidative stress.
Earlier studies have addressed genotoxicity in copepods following exposure to cadmium (Pavlaki et al. 2016), polluted sediments (Charry et al. 2018), and ultraviolet (UV) light (Tartarotti et al. 2014). To our knowledge, the present study is the first in which DNA strand breaks in copepods have been quantified following Cu exposure. In contrast to other studies on DNA strand breaks in marine organisms following Cu exposure (Gabbianelli et al. 2003 and references in Table 2 of the present study), there were no clear genotoxic effects in A. tonsa, T. longicornis, or T. brevicornis following 6- and 72-h exposure to 6 and 60 µg Cu/L in the present study.
| Species | Concentration (µg Cu L–1) | % DNA strand breaks, mean | Exposure time | Tissue | Reference | |
|---|---|---|---|---|---|---|
| Planarian | Dugesia schubartii | 0, 600, 1200, 1900, 2500, 3100 | 10–28 | 2 h | Whole animal | Guecheva et al. (2001) |
| Polychaete | Nereis virens | 0, 13, 130, 1300, 13 000 | 8–17 | 0.5 h | Sperm | Caldwell et al. (2011) |
| Clam | Corbicula flumineab | 0, 500, 1000, 2000 | 5–8 µg DNA mg–1 protein | 168 h (7 d) | Whole soft body | Bonnail et al. (2016) |
| Mussel | Mytilus edulis | 0, 20, 30, 50 | 10–58 | 120 h (5 d) | Hemocytes | Al-Subiai et al. (2011) |
| Mytilus edulis | 56 | ∼34 | 144 h (6 d) | Hemocytes | Trevisan et al. (2011) | |
| Oyster | Crassosteras gigas | 0, 0.1, 1, 10 | 9–19.5 | 16 h | Embryos | Mai et al. (2012) |
| Copepod | Acartia tonsa | 0, 0.6, 6, 60 | 21–66 | 72 + 24 h post exposure | Whole animal | Present study |
| Copepod | Temora longicornis | 0, 0.6, 6, 60 | 33–83 | 72 + 24 h post exposure | Whole animal | Present study |
| Copepod | Tigriopus brevicornis | 0, 0.6, 6, 60 | 12–21 | 72 + 24 h post exposure | Whole animal | Present study |
| Fish | Euterpina acutifrons | 0, 3.2, 10, 32, 64, 128 | 17–37 | 96 h | Blood | Santos et al. (2010) |
- a All exposure concentrations in the respective studies were converted to µg L–1, with effect concentrations in bold.
- b Alkaline unwinding precipitation (Olive 1988).
The lack of DNA strand break induction in the 3 copepod species following Cu exposure could be due to processes that limit the uptake of Cu, internal redistribution, or cellular mechanisms. Copper is an essential element and will be taken up from water by marine invertebrates, but tissue distribution, storage, and excretion are highly species specific (Rainbow and White 1989; Barka 2007; Rainbow 2007). Cellular defence mechanisms include the activity of antioxidant enzymes and concentrations of cellular antioxidants such as glutathione (cf. Ellesat et al. 2011). It is generally assumed that Cu genotoxicity is predominantly due to ROS formation and subsequent interaction of these radicals with DNA (Barata et al. 2005). A Cu concentration similar to that used in the present study did increase expression of antioxidant- and detoxification-associated genes in the copepod Tigriopus japonicus (Ki et al. 2009). Higher exposure concentrations (>1 and 2 mg Cu/L) were, however, needed to affect antioxidant enzyme activity in the same species (Lee et al. 2008b; Rhee et al. 2013; Kim et al. 2014), which suggests that the concentrations used in our study were within a range that could be compensated for by antioxidant defence mechanisms in the copepods. Copper is known to affect Na,K-adenosine triphosphatases (ATPases) in marine organisms (Canli and Stagg 1996). Acute toxicity such as that observed for Acartia exposed to 60 µg/L Cu is generally caused by an interaction with fundamental processes. Ion regulation is critical for the survival of the copepod, and any inhibition of the ATPases could be deleterious.
The Cu-dependent inhibition of DNA repair gene expression and corresponding enzyme activity (Predki and Sarkar 1992; Hartwig 1995) could conceivably contribute to lower levels of repair-related intermediate strand breaks (Cadet et al. 2017), as observed for T. japonicus (Kim et al. 2011) and Dugesia schubartii (Guecheva et al. 2001). In contrast to UV-induced strand breaks in the freshwater copepod Cyclops abyssorum (Tartarotti et al. 2014), the same recovery period was not sufficient to reduce strand breaks in our study. Instead, DNA strand breaks increased during the 24 h post exposure in A. tonsa and T. longicornis. There is no obvious explanation for this increase, but it is possible that other stress factors such as handling or food deprivation may contribute to oxidative stress (Guecheva et al. 2001). However, A. tonsa can withstand longer starvation periods (6–10 d) than the experimental period in the present study (Dagg 1977). The increase of DNA strand breaks in the control treatment suggests that standard protocols for toxicological testing of copepods might fail to detect hidden physiological impacts.
Although there was no difference between groups in the level of DNA strand breaks, we observed 50 and 100% mortality post exposure in Cu-treated A. tonsa, and no mortality in the control group. Copper interferes with enzyme activity, acid–base homeostasis (Truchot 1993; Pourahmad and O'Brien 2000), and osmoregulation (Bambang et al. 1995; Grosell et al. 2007; Lee et al. 2010). This has also been observed for A. tonsa (Pinho et al. 2007). We cannot rule out a genotoxic role of Cu in the observed mortality in A. tonsa, but the Cu-induced mortality could also be a consequence of other mechanisms of cellular toxicity. The 50% mortality at 6 µg Cu/L that we observed in A. tonsa is 5 to 14 times, and up to 50 times, more sensitive than previously reported 96-h median lethal concentrations for A. tonsa (Reeve et al. 1977; Sosnowski and Gentile 1978).
The distribution of DNA strand breaks was similar for A. tonsa and T. longicornis surviving after exposure to 6 µg Cu/L, with no significant mortality (Supplemental Data 5). The distinct susceptibility of individual A. tonsa may reflect innate tolerance or sex-related susceptibility in some individuals (Cervetto et al. 1999; Medina et al. 2008; Stringer et al. 2012). Our data did not indicate a difference in susceptibility between males and females.
The species we examined differ in size, with the body mass of T. longicornis being almost 4 times more than that of A. tonsa and T. brevicornis. A larger body mass could be advantageous for coping with metal exposure (Grosell et al. 2007). Also, metabolic rate scales with size along with requirements for gas exchange; acid–base balance regulation also scales with size (Grosell et al. 2007). Thus, higher DNA strand breaks/µg/dry weight could reflect size-related Cu sensitivity (in terms of mass [g]) and could have contributed to the differential Cu susceptibility of A. tonsa and T. longicornis (Grosell et al. 2007), but did not appear to account for differences between T. longicornis and T. brevicornis.
The resistance of T. brevicornis in our study corresponds well with its previously reported tolerance to pollution stress and harsh environmental conditions (Seo et al. 2006; Medina et al. 2008). Results from a pilot study showed similar resistance of adult T. brevicornis brought up under Cu exposure (20 µg Cu/L) to DNA strand break formation (Supplemental Data 6). In addition to antioxidants, metal complexation by metallothionein and in granules (Barka 2007; Barka et al. 2010) may have contributed to the tolerance differences observed between T. brevicornis and the other 2 copepods (Barka et al. 2001).
We show that the comet assay can be performed using individual copepods. We could not identify direct Cu-related genotoxicity in any of the 3 species examined. The species differed strongly in their responses, with T. brevicornis being particularly robust to Cu exposure. The results of the present study provide a good basis for further study of the variability and sensitivity to genotoxicants within a population. We found clear effects on mortality in A. tonsa exposed to Cu, but following 24-h post-exposure. Although we cannot rule out effects of genotoxicity on induced mortality rates, our results suggest that other toxic effect(s) caused the observed mortality. The DNA strand break differences among species could be related to biomass differences between A. tonsa and T. longicornis, but not between T. brevicornis and T. longicornis. A number of issues warrant further research: relationships between oxidative stress and strand breaks, the mechanism of toxicity for Acartia, and finally the reasons for the apparent stability of DNA in Tigriopus. Copepods are ecologically vital in the oceans, and we need to understand how they are affected by toxicants.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4541.
Acknowledgment
We thank B. Kaasa for assistance during comet scoring. The present study was funded by the University of Oslo.
Open Research
Data Accessibility
Research data pertaining to this article are available at FigShare.




