Investigating the Impact of Manufacturing Processes on the Ecotoxicity of Carbon Nanofibers: A Multi–Aquatic Species Comparison
Abstract
Manufactured nanomaterial production is outpacing the ability to investigate environmental hazard using current regulatory paradigms, causing a backlog of materials requiring testing. To ameliorate this issue, regulatory bodies have proposed integrating safety into the production of novel nanomaterials, allowing for hazards to be identified early in development rather than aftermarket release. In addition, there is a growing interest in short-term ecotoxicity testing to rapidly identify environmental hazards. In this sense, the present study investigated 3 carbon nanofibers (CNFs), created with different production methods, using short-term in vitro and in vivo exposures on fish cell lines, mussel hemocytes, crustacea, and algae. The present study investigated if differences in ecotoxicity hazard between the CNFs could be identified and, if so, which product could be considered less hazardous. A major challenge in assessing the potential hazards posed by manufactured nanomaterials is standardizing the preparation for testing. Standardized operating protocols have been proposed using protein to facilitate the preparation of stable stock suspension, which is not environmentally representative. As such, the study also assessed the potential impacts these standardized protocols (with or without the use of protein) could have on the interpretation of environmental hazard. The results demonstrated that there were clear differences between the 3 CNFs and that the dispersion protocol influenced the interpretation of hazard, demonstrating a need for caution when interpreting ecotoxicity in a regulatory context. Environ Toxicol Chem 2019;38:2314–2325. © 2019 SETAC.
INTRODUCTION
The emergence of manufactured nanomaterials (MNMs) has the potential to completely revolutionize almost every facet of the global economy. Although achieving an accurate definition for MNMs is rather difficult, according to an European Union recommendation, MNMs should have 50% or more of the particles with between 1 and 100 nm in at least one of its dimensions (European Union 2011). It is estimated that the production of MNMs will be close to half a million tons by 2020, which will inevitably be released into the environment, with a final fate in aquatic ecosystems, making it an emerging concern for the environment (Canesi et al. 2015). Manufactured nanomaterials are of unique concern because they display novel properties, mainly attributable to the reduction in size resulting in a consequent increase in the surface area to volume ratio, not observed in the source material of the nanoparticles, making it difficult to quantify potential hazards using conventional approaches. As a result, the ecotoxicity of MNMs cannot be estimated to be comparable to that of bulk materials and can lead to new unforeseen risks and hazards to human health and the environment (Auffan et al. 2009; Singh 2016). This can be a challenge for regulatory programs in which novel paradigms may be necessary to address potential human and environmental impacts of MNMs.
One of the challenges associated with the production, marketing, and use of MNMs is how to develop appropriate regulatory policies without hindering innovation. In this sense, it is essential to identify potential hazards of MNMs at an early stage in the production process to reduce the effort and resources required to demonstrate that the product is safe and obtain approval by regulatory agencies for market use. From an environmental perspective, this can be achieved by investigating the potential ecotoxicity of the initial MNMs prior to their use in products (Baun et al. 2008). There is, however, a lack of knowledge on ecotoxicity data that limits the integration of this kind of information in the development of less environmentally hazardous MNMs (Schwarz-Plaschg et al. 2017). This is most likely attributable to the fact that many authors and manufacturing companies focus research on the possible effects of MNMs on human health with an emphasis on exposure risks toward factory workers or accidental release in confined spaces. When environmental hazards are considered, the possible exposure of biota in the environment is often perceived as a secondary concern. Nevertheless, the high production volume of MNMs will inevitably lead to their release (during the use and disposal of MNM-based products) into the environment and their accumulation in aquatic media (Canesi et al. 2015). Therefore, it is essential to characterize their potential ecotoxicity.
Presently, ecotoxicity test guidelines developed by the Organisation for Economic Co-operation and Development (OECD) have been widely used in the assessment of potential hazards associated with novel materials. It has been recognized that OECD test guidelines are generally applicable to MNMs if particular issues derived from their peculiar physicochemical properties are appropriately addressed (Kühnel and Nickel 2014). These conclusions have been confirmed in a number of studies, some of them referring to specific test guidelines. For instance, Hund-Rinke et al. (2016) suggested that preference be given to particular media preparation protocols and output measurement for OECD test guidelines 201 and 202 that would not require modifications to the initial test guideline. In this context, the OECD published a document giving guidance for sample preparation (Organisation for Economic Co-operation and Development 2012a) without specific changes to the test guidelines, considering that the main limitations were those related to the generation of appropriate test suspensions. These test guidelines were also applied to single- and multiple-wall carbon nanotubes (CNTs) in the sponsorship program carried out at the OECD to study the applicability of OECD test guidelines to carbon-based MNMs (Organisation for Economic Co-operation and Development 2015a, 2015b). Taking all this into account, OECD test guidelines could be applied without major modifications as long as an appropriate test suspension is achieved.
As such, the preparation of test suspensions is a major issue in assessing the potential hazards posed by MNMs because it is well established that changes in the dispersion properties, such as the stability of the size of agglomerates and aggregates, can influence their toxicity (Kim et al. 2011; Hartmann et al. 2015; Tantra et al. 2015; Langevin et al. 2018). Special attention must also be paid to MNMs which have demonstrated difficulty in forming stable dispersions, such as nanocarbons, because of their intrinsic hydrophobicity. Previous research assessing nanocarbon ecotoxicity has also demonstrated a wide range of conflicting results, mainly associated with differences in the production process (Eckelman et al. 2012; Jackson et al. 2013). Considering the enormous variability in sizes, shapes, and other physicochemical properties of carbon-based MNMs on the market, it is necessary to establish consistent preparation techniques for this group of MNMs to avoid possible confounding factors when investigating their ecotoxicity.
A strict standardization is thus essential for the preparation of test suspensions to maintain comparability among MNMs when evaluating ecotoxicity (Laux et al. 2017). The development of standardized operating procedures (SOPs) for the preparation is also of interest in the context of regulation, to guarantee intra- and interlaboratory repeatability of results. Considering this, a dispersion SOP was defined in the European project NANOGENOTOX for the preparation of MNM suspensions and improved on in the project NANoREG (NANOGENOTOX 2011). In a first step, a stable stock suspension is established by adding bovine serum albumin (BSA), which has been demonstrated to promote stable suspensions for hydrophobic MNMs (such as single- and multiwalled CNTs) in a wide array of different test media (Wang et al. 2010; Vietti et al. 2013). This SOP was designed in the context of human toxicology however, and its use in environmental studies is questionable.
Overall the objective of the present study was to assess the applicability of short-term ecotoxicity testing (based on in vitro and in vivo approaches) for a poorly studied group of MNMs, carbon nanofibers (CNFs), in a regulatory context. Under the scope of the European Union's Horizon2020 project, one of the industrial partners increased the production volume of CNFs using a scaled-up production process. The objectives of the study were to determine 1) if there were differences in ecotoxicological hazards between 3 industrial products from different production processes using short-term testing based on in vitro and in vivo approaches, 2) which product might be considered less hazardous for the aquatic environment, and 3) whether or not the use of a dispersion agent such as BSA in the dispersion SOP influenced the interpretation of the results.
MATERIALS AND METHODS
Products
Chemicals for ecotoxicological testing on in vitro cell cultures were purchased from Sigma-Aldrich unless otherwise stated. Eagle's minimum essential medium with nonessential amino acids and Na pyruvate without L-glutamine, Eagle's minimum essential medium with Earle's Balanced Salt Solution without L-glutamine, penicillin/streptomycin (10 000 units penicillin and streptomycin/mL), L-glutamine solution (200 mM), and 100× nonessential amino acid were obtained from Lonza. Serum-free/phenol red–free minimal essential medium (MEM) was purchased from PAN Biotech. Alamar blue reagent and 5-carboxyfluorescein diacetate-acetoxymethyl ester (CFDA-AM) were from Life Technologies. Bovine serum albumin was from Merck.
Materials necessary for preparing culture media for Mytilus edulis hemocyte BSA, Leibovitz L-15 medium (L-15), sodium chloride (NaCl), potassium chloride (KCl), calcium chloride dihydrate (CaCl2-2H2O), magnesium sulfate heptahydrate (MgSO4-7H2O), magnesium chloride hexahydrate (MgCl2-6H2O), glucose, sodium citrate, ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA), fetal bovine serum (FBS), penicillin G, streptomycin sulfate, and gentamycin sulfate were purchased from Sigma-Aldrich.
Products for testing on Daphnia magna immobilization and reproduction tests as well as algae growth inhibition test were purchased from Fisher Scientific.
Nanomaterials
Grupo Antolin, one of the industrial partners in the NanoReg2 project, provided 3 CNFs: GANF, GANFg, and GATam. The initial product manufactured is GANF, which is a CNF created through catalytic vapor deposition using a natural gas and sulfur feed stock at temperatures >1100 °C in a floating catalyst reactor (Vera-Agullo et al. 2007; Weisenberger et al. 2009). Deposition of graphene layers is promoted by metallic Ni, whereas catalytically inactive NiS allows for the formation of helical ribbons with a stacked-cup structure. This could be considered the base product design, with the other 2 CNFs being derivatives of the initial one. The second type of CNF, GANFg, is produced by superheating GANF at 2500 °C, which decreases the interlayer spacing in the CNF and increases the purity, in terms of C content, by decreasing the concentration of O and H (Weisenberger et al. 2009). Finally, GATam is generated using a scaled-up version of the production process for GANF. Differences in materials provided by Grupo Antolin are summarized in Table 1.
| Measured property | Unit | GANF | GANFg | GATam |
|---|---|---|---|---|
| Fiber diameter (TEM) | nm | 20–80 | 20–80 | 20–80 |
| Carbon purity (TGA) | % | >85 | >99 | >80 |
| Apparent density | g/cc | ~0.06 | ~0.08 | ~0.08 |
| Specific surface area (BET N2) | m2/g | 100–170 | 70–90 | 70–140 |
| Graphitization degree (XRD) | % | ≈70 | ≈90 | ≈60 |
| Electrical resistivity | Ω- × m | 1 × 10–3 | 1 × 10–4 | 1 × 10–3 |
- BET = Brunauer-Emmett-Teller; TEM = transmission electron microscopy; TGA = thermogravimetric analysis; XRD = X-ray diffraction.
Preparation of stock suspensions
Suspensions were prepared following the SOP with and without BSA. For both dispersion methods, 15.36 mg of the corresponding CNF were prewet with 30 µL of absolute ethanol in glass vials. Stock suspensions were prepared using 0.05% BSA–water (w/v) to achieve a final concentration of 2.56 mg/mL of CNF. For the dispersion without BSA, Milli-Q water was used instead of the BSA solution. The stock suspensions were then placed in an ice-water bath solution and sonicated using a probe sonicator for 16 min. The sonicators used were Vibra-Cell™ VCX 130 (Sonics, Newton, CT, USA) in the laboratory working with fish cells, Branson-S450 (FisherScientific) in the laboratory working with mussel cells, and Vibra-Cell™ VCX 750 (Sonics) in the laboratory performing the algae and Daphnia tests. To standardize the amount of energy provided to the suspension among laboratories, the NANoREG probe sonicator calibration SOP was used to identify the appropriate settings to achieve 7056 J of delivered acoustic energy.
Characterization of MNM suspensions
Dynamic light scattering (DLS) was used to determine the hydrodynamic size of CNFs in the stock and test suspensions at the start and at the end of the experiment. Transmission electron microscopy (TEM) was also used to observe the morphology of the CNFs in ultrapure water as well as test media. For the preparation of TEM images of the CNF stock and test suspensions, carbon-coated grids were hydrophilized using a glow discharge apparatus (K100X; Emitech). The glow discharge was performed for 180 s at an air pressure of 10–1 mbar and an electric current of 40 mA. This treatment was applied to TEM grids prior to the suspension deposition because it prevents most of the artifactual agglomeration phenomena during the drying of the suspensions on the TEM grids (Dubochet et al. 1982). In stock suspensions and fish cell culture media CNFs were observed by means of a 1400 PLUS (JEOL) microscope, whereas CNFs in M. edulis were analyzed using a FEI Philips CM12® microscope.
The CNF suspensions were also analyzed through colorimetric analysis to characterize the stability of the suspensions. Each suspension was measured across the 340- to 700-nm light wavelength at 10-nm intervals to determine the wavelength yielding the highest value, which was 340 nm for all 3 CNFs. Samples were taken from the top of the media at 0, 2, 4, 6, and every 24 h until the end of the experiment. The results were then normalized using a blank for each medium suspension, and values at each time point were adjusted relative to the starting absorbance.
In vitro testing
Fish cell line preparation and exposure
Two fish cell lines, Poecilipis lucida liver cells (PLHC-1) and Cyprinus carpio leukocytes (CLC), were obtained from the American Type Culture Collection. The PLHC-1 line was maintained in Eagle's minimum essential medium (with nonessential amino acid and Na pyruvate without L-glutamine) supplemented with 1% L-glutamine, 1% penicillin/streptomycin, and 5% FBS at 30 °C, in an atmosphere of 5% CO2. For cell treatments, medium was supplemented with 10% FBS. The CLC line was cultured in Eagle's minimum essential medium (Earle's Balanced Salt Solution without L-glutamine) supplemented with 1% L-glutamine, 1% penicillin/streptomycin, 1% nonessential amino acid, and 10% FBS at 28 °C, 5% CO2. Both cell lines were subcultured twice a week using trypsin EDTA in phosphate-buffered saline (PBS).
The PLHC-1 cells were seeded in 96-well plates (Greiner Bio-One) at a density of 5 × 104 cells/mL, whereas CLC cells were plated on poly-L-lysine-coated 96-well plates (Greiner Bio-One) at a density of 7.5 × 104 cells/mL. Following a 24-h incubation, culture medium was refreshed with medium containing MNMs for 8 serial concentrations ranging from 0 to 256 mg/L in a 100-µL volume for 72 h. We selected 256 mg/L because it is the highest possible concentration (one-tenth of the medium) able to be tested. Culture media for test concentrations <256 mg/L were supplemented with stock medium, BSA, or ultrapure water, prior to the addition of medium containing MNMs to guarantee that concentrations of stock medium were consistent across test concentrations.
Fish cell line cytotoxicity measurements
Cell viability was measured on the same set of cells according to a modified version (Lammel et al. 2013; Lammel and Navas 2014) of a protocol described by Dayeh et al. (2013). After 72 h of exposure to MNMs, medium was removed and cells were washed twice with PBS and incubated with 1.25% (v/v) Alamar blue and 4 µM CFDA-AM in serum-free/phenol red-free MEM (containing 1% nonessential amino acid). Fluorescence was measured on a microplate reader (Tecan GENios) at a wavelenght of 535/590 nm (excitation/emission) for Alamar blue and 485/535 nm for CFDA-AM after 30 min of incubation in the dark. Cells were washed with PBS and incubated with 100 µL of neutral red solution (33 µg/mL in serum-free/phenol red-free MEM containing 1% nonessential amino acid) for 1 h in the dark. Following incubation, cells were rinsed with PBS, and the retained dye was extracted with 100 µL of an acidified (1% glacial acetic acid) 50% ethanol/49% Milli-Q water solution.
Potential interference by the CNF dispersions with the cytotoxicity assays were tested in the presence of cells to simulate a more realistic assay scenario. Cells were seeded and exposed to CNFs in the same way as for the toxicity tests. Fluorescence readings of exposed cells were taken at the same wavelengths used for the 3 cyotoxicity assays before and after washing the cells twice with PBS. Measurements were repeated following the addition of serum-free/phenol red-free MEM (containing 1% nonessential amino acid) to the cells. Next, cells were incubated in the dark under exposure conditions with the conversion products of Alamar blue (0.1 and 1 µM of resorufin) and CFDA-AM (0.4 and 4 µM of 5-carboxyfluorescein) and fluorescence was read at time 0 and after 30 min. After the washing step, neutral red (33 µg/mL) prepared in extraction solution was added, and fluorescence was determined at time 0 and after 1 h at the corresponding wavelengths.
M. edulis cell culture preparation and exposure
Mytilus edulis individuals were collected from a relatively clean site, Saint-Cast-le-Guildo (48°37′48″N, 2°15′24″W), previously identified as suitable for experimental research (Chevé et al. 2015). Mussels were placed in artificial seawater (30 psu, at 15 °C with a 12:12-h light:dark cycle) for a 2-d acclimation period (unfed) prior to testing. A primary cell culture on M. edulis hemocytes was established following the methodology described in Barrick et al. (2018) with minor adjustments. Briefly, hemolymph was extracted from 50 mussels using a 23-gauge, 2-mL syringe containing 0.1 mL of Alseve buffer (20.8 g/L glucose, 8 g/L sodium citrate, 3.36 g/L EDTA, 22.5 g/L NaCl, pH 7.0; Cao et al. 2003). The total volume of the hemolymph was then stored, and cell viability and cell concentration were determined through the trypan blue exclusion method. Hemocyte was diluted to reach a concentration of 1 × 106 cells/mL using the Alseve solution. Hemolymph (200 µL/well) was then seeded into a tissue culture–treated 96-well microplate (Corning) to reach a concentration of 2 × 105 cells/well. The plate was then placed into an incubator at 18 °C (3.5% CO2) for 30 min. After 30 min, hemolymph was aspirated and replaced with adjusted L-15 medium (20.2 g/L NaCl, 0.54 g/L KCl, 0.6 g/L CaCl2, 1g/L MgSO4, 3.9 g/L MgCl2) containing 100 units/mL penicillin G, 100 µg/mL streptomycin, 40 µg/mL gentamycin, 10% glucose, and 10% FBS (pH 7.0). Cells were left to adhere overnight prior to exposure. After 24 h, cell culture medium was refreshed with cell culture medium containing MNMs in suspension (0–256 mg/L). Culture media for the 10 exposure conditions were prepared in Eppendorf tubes and vortexed for 10 s prior to refreshing media. Exposure conditions <256 mg/L were supplemented with stock medium, BSA, or ultrapure water depending on which SOP was being tested, prior to preparing the serial dilutions to ensure consistency across test conditions. Both dispersion techniques were tested in parallel and on the same microplate to reduce the risk of interplate variation influencing the interpretation of results. Wells containing no cells were also prepared for all test concentrations to account for potential interference as well as to limit the risk of false-positive results (Drasler et al. 2017). The cell culture was then returned to the incubator for 24 h.
M. edulis cytotoxicity measurements
The previously described method was adapted to M. edulis hemocytes to assess cell viability. Briefly, cell culture medium was removed, and the cells were washed twice with PBS adjusted for more marine organisms (Le Marrec-Croq et al. 1999) and incubated with 10% (v/v) of Alamar blue and 2 µM of CFDA-AM prepared in PBS for 30 min at 18 °C (3.5% CO2). Alamar blue was measured using the colorimetric method described by the manufacturer using a spectrophotometer at light wavelengths of 570 and 600 nm for absorbance. Readouts were measured at the start and end of the assay (Rampersad 2012). To account for coloration and physical obstruction of light by CNFs remaining in the wells, the rate of change in dye reduction was used to measure cellular metabolism. The rate of change in the wells containing only CNFs was also measured to determine if the presence of the CNFs interfered with dye reduction. Also, CFDA-AM was measured as previously described with wells containing only CNFs at each respective concentration being used to account for interference.
In vivo testing
Algal growth inhibition tests
Growth inhibition testing was conducted following OECD test guideline 201 (Organisation for Economic Co-operation and Development 2011). Prior to exposure, Pseudokirchneriella subcapitata was cultured for 3 d at 22 °C and constant illumination to ensure that the algae were in the exponential growth phase. For the exposure assay algal densities were prepared at 8 × 103 cells/mL and exposed by adding medium prepared with CNFs using 8 concentrations ranging from 0.78 to 50 mg/L for 72 h. Uniform exposure was maintained through magnetic stirring using a previously defined protocol (Manier et al. 2016). Algal growth was measured using a fluorescence microplate reader (Tecan Safire 2) at a wavelength of 438/685 nm (excitation/emission). Negative controls containing only CNF suspensions were performed in parallel to identify potential interference in the measurement by CNFs. Growth rates were determined as relative to the control. The use of BSA in the dispersion SOP was determined to interfere with algal growth. As a result, the dispersion protocol without BSA was the only protocol tested.
D. magna testing
Acute toxicity testing was conducted following OECD test guideline 202 (Organisation for Economic Co-operation and Development 1984), and chronic toxicity testing was conducted following OECD test guideline 211 (Organisation for Economic Co-operation and Development 2012b). For each test, CNF suspensions were prepared by a dilution of the stock medium in test medium (ISO or M4 medium) and stirred continuously prior to conducting the assays. Young daphnids, aged less than 24 h at the start of the test, were exposed to 6 different concentrations of CNFs (3.125–100 mg/L) for a period of 48 h. After 24 and 48 h, immobilization of the young daphnids exposed to CNFs was recorded and compared with control values.
For the chronic reproduction test, young female D. magna (10 animals at each test condition) were exposed to the CNF suspensions at 8 concentrations (0.19–25 mg/L). The total number of living offspring produced per parent animal was recorded after a 21-d exposure period and compared with control values. To maintain a uniform exposure in the water column concentrations, a complete renewal of the test medium with new CNF suspensions was performed after 24 h during the acute test and every working day for the chronic reproduction test.
Statistical analysis
Effective concentration values of toxicity were calculated by fitting to dose–response curves using the Hill equation with the REGTOX_software, Ver 7.0.4 macro from Microsoft Excel.
RESULTS
Physicochemical characterization of the CNFs
The TEM results indicated that aggregates and agglomerates of approximately 1 µm in size formed during the preparation of the stock suspension (Figure 1). No differences among CNFs as well as test media could be easily identified. The DLS results indicated that frequency size distribution of CNF agglomerations could be measured reliably for the dispersions generated with the SOP when BSA was used (Table 2). Typically, DLS is considered unsuitable for carbon-based products because it assumes that particles in suspension are spherical. However, CNF agglomerates can be approximated as spherical and analyzed as long as the polydispersion index (PdI) is low (Reinert et al. 2015). In general, the Z-average was <500 nm with a low PdI of approximately 0.1 to 0.3, suggesting a low variation in the size distribution of the agglomerates in the stock suspensions. The stock suspensions, as approximated by DLS, of all 3 CNFs were comparable in aggregate and agglomerate size for GANF (489.3 d-nm), GATam (414.3 d-nm), and GANFg (479.8 d-nm). In PLHC-1 medium, aggregates for GANF (471 d-nm), GATam (462.1 d-nm), and GANFg (393.1 d-nm) exhibited similar z-average values. In CLC medium, GANF (460.1 d-nm), GATam (426.6 d-nm), and GANFg (344 d-nm) z-averages were also similar to those detected in PLCH-1 medium. In M. edulis hemocyte culture medium, there was a decrease in the size of the agglomerates by approximately half for GANF (260.1 d-nm), GATam (197.8 d-nm), and GANFg (244.1 d-nm). The DLS results could not be accurately analyzed in media for D. magna and P. subcapitata because of high polydispersion. In media where DLS could be measured, the approximated agglomerate sizes suggested a stable suspension throughout the duration of the experiment.
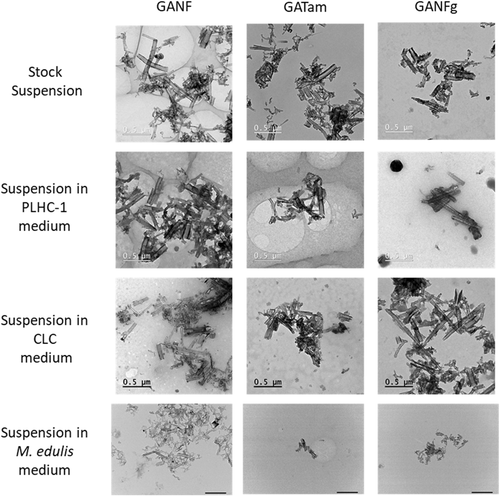
Transmission electron microscopic images of GANF, GATam, and GANFg stock suspensions and of suspensions in the different media. Scale bars in stock suspension images, Poecilipis lucida liver cells (PLHC-1) and Cyprinus carpio leukocytes (CLC) medium suspension images indicate 0.5 µm. Scale bars in Mytilus edulis medium suspension images indicate 1 µm.
| GANF | GANFg | GATam | ||||
|---|---|---|---|---|---|---|
| Start of experiment (dispersion SOP with BSA) | ||||||
| Test media | Z-average (d-nm) | PdI | Z-average (d-nm) | PdI | Z-average (d-nm) | PdI |
| Stock suspension | 489.3 | 0.13 | 479.8 | 0.18 | 414.3 | 0.23 |
| Culture media (PLHC-1) | 471 | 0.4 | 393.1 | 0.4 | 462.1 | 0.4 |
| Culture media (CLC) | 460.1 | 0.5 | 344.0 | 0.4 | 426.6 | 0.4 |
| Culture media (Mytilus edulis) | 260.1 | 0.36 | 479.8 | 0.18 | 414.3 | 0.23 |
| Daphnia magna (OECD 202) | N/A | N/A | N/A | N/A | N/A | N/A |
| D. magna (OECD 211) | N/A | N/A | N/A | N/A | N/A | N/A |
| Pseudokirchneriella subcapitata (OECD 201) | N/A | N/A | N/A | N/A | N/A | N/A |
| End of experiment (dispersion SOP with BSA) | ||||||
| Test media | Z-average (d-nm) | PdI | Z-average (d-nm) | PdI | Z-average (d-nm) | PdI |
| Stock suspension | 527.5 | 0.12 | 441.2 | 0.29 | 389.4 | 0.16 |
| Culture media (PLHC-1) | 403.4 | 0.4 | 370.6 | 0.4 | 356.6 | 0.4 |
| Culture media (CLC) | 526.8 | 0.5 | 340.8 | 0.4 | 327.5 | 0.4 |
| Culture media (M. edulis) | 527.5 | 0.12 | 441.2 | 0.29 | 389.4 | 0.16 |
| D. magna (OECD 202) | N/A | N/A | N/A | N/A | N/A | N/A |
| D. magna (OECD 211) | N/A | N/A | N/A | N/A | N/A | N/A |
| P. subcapitata (OECD 201) | N/A | N/A | N/A | N/A | N/A | N/A |
- a Size distribution graphs are provided in the Supplemental Data.
- BSA = bovine serum albumin; CLC = Cyprinus carpio leukocytes; N/A = not applicable (results were not suitable for reporting); OECD = Organisation for Economic Co-operation and Development; PdI = polydispersion index; PLHC-1 = Poecilipis lucida liver cells; SOP = standard operating procedure.
Most of the dispersions prepared without BSA could not be accurately analyzed through DLS, indicating large agglomerate sizes and poor colloidal stability. Only in the case of GANFg suspension obtained in M. edulis culture medium could the z-average (174.9 d-nm) be reliably measured with an associated PdI of 0.22.
Colorimetric results were analyzed in relation to the initial concentration of CNF in suspension approximated through absorbance. The results demonstrated that the stability of the stock suspensions was improved when using BSA with more of the CNF remaining in suspension for the duration of the experiment, as summarized in Table 3. When testing the stability of the preparation using BSA in the cell culture media, both fish cell lines and mussels appeared to promote stability with all 3 CNFs. When using the suspensions prepared without BSA, GANF and GATam displayed rapid sedimentation whereas GANFg displayed higher stability for the duration of the experiment. With respect to test media for D. magna, GANF and GATam quickly fell out of suspensions when prepared without BSA; GANFg was still able to be measured in suspension at the end of the experiment in OECD 201, 202, and 211 test media and when not using BSA. Again, BSA notably improved stability for the CNFs in OECD 201 and OECD 211 media.
| Colorimetric stability of test suspensions at end of the experiment | ||||||
|---|---|---|---|---|---|---|
| GANF | GANFg | GATam | ||||
| Test media | BSA | No BSA | BSA | No BSA | BSA | No BSA |
| Stock suspension | 11.5 | 0 | 47.72 | 48.26 | 17.62 | 0 |
| Culture media (PLHC-1) | 72 | 27 | 95 | 71 | 97 | 30 |
| Culture media (CLC) | 69 | 28 | 92 | 95 | 103 | 37 |
| Culture media (Mytilus edulis) | 47.88 | 0 | 75.19 | 89.8 | 86.34 | 0 |
| Daphnia magna (OECD 202) | 20.6 | 0 | 8.79 | 16.73 | 37.28 | 0 |
| D. magna (OECD 211) | 81.55 | 0 | 87.35 | 6.39 | 66.86 | 0 |
| Pseudokirchneriella subcapitata (OECD 201) | 91.67 | 0 | 87.98 | 94.31 | 68.11 | 0 |
- BSA = bovine serum albumin; CLC = Cyprinus carpio leukocytes; OECD = Organisation for Economic Co-operation and Development; PLHC-1 = Poecilipis lucida liver cells.
Fish cell lines
Results of cytotoxicity in fish cell lines appear in Table 4 (initial dispersion with BSA) and in Table 5 (initial dispersion without BSA). The use of BSA did not appear to have a deep influence on the cytotoxicity of CNFs when tested on fish cell lines. In PLHC-1 cells, CNF cytotoxicity was not detectable for some of the used assays at the concentrations tested (25% effect concentration [EC25] or EC50 > 256 mg/L). In a number of cases, CNFs provoked some kind of interference, with the fluorescent readouts at the higher concentrations tested preventing the calculation of accurate EC25 or EC50. However, for all 3 CNFs the EC25 for the CFDA-AM assay could be calculated (ranging 10.5–58.0 mg/L). In addition, for some of the CNFs and assays used, the calculated EC25 and EC50 values were close to the maximal concentrations applied (Tables 4 and 5).
| In vitro testing | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GANF | GANFg | GATam | ||||||||||||||||
| Alamar blue | CFDA-AM | Neutral red | Alamar blue | CFDA-AM | Neutral red | Alamar blue | CFDA-AM | Neutral red | ||||||||||
| Cell type | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 |
| PLHC-1 | >64 | >64 | 10.5 | >256 | >256 | >256 | >32 | >32 | 28.2 | >256 | >256 | >256 | >128 | >128 | 32.2 | >256 | 147 | 234.4 |
| CLC | 3.9 | 18.9 | 4.3 | 37.6 | 116 | >256 | >32 | >32 | 114.7 | 252 | >256 | >256 | 15 | 46.7 | 14.7 | 89.9 | 113.4 | >256 |
| Mytilus edulis Hemocytes | 112.8 | 219.8 | 160.8 | 219.8 | — | — | 10.5 | 34.4 | 56.9 | 191.1 | — | — | 120.8 | 178.6 | 53.9 | 191.1 | — | — |
| In vivo testing | ||||||||||||||||||
| GANF | GANFg | GATam | ||||||||||||||||
| OECD test | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | ||||||||||||
| Daphnia magna (OECD 202) | >100 | >100 | >100 | >100 | 58.81 | 62.8 | ||||||||||||
| D. magna (OECD 211) | 1.24 | 1.58 | 1.61 | 6.18 | 0.28 | 0.32 | ||||||||||||
| Pseudokirchneriella subcapitata (OECD 201) | 1.87 | 3.09 | 5.12 | 8.48 | 1.04 | 2.12 | ||||||||||||
- a EC25 and EC50 values (dispersion SOP with BSA).
- >indicates EC values exceeded concentrations where results could reliably be analyzed.
- BSA = bovine serum albumin; CFDA-AM = 5-carboxyfluorescein diacetate-acetoxymethyl ester; CLC = Cyprinus carpio leukocytes; EC25/EC50 = 25% and 50% effect concentrations, respectively; OECD = Organisation for Economic Co-operation and Development; PLHC-1 = Poecilipis lucida liver cells; SOP = standard operating procedure.
| In vitro testing | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GANF | GANFg | GATam | ||||||||||||||||
| Alamar blue | CFDA-AM | Neutral red | Alamar blue | CFDA-AM | Neutral red | Alamar blue | CFDA-AM | Neutral red | ||||||||||
| Cell type | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 |
| PLHC-1 | 61.8 | 175.8 | 34.7 | >256 | >256 | >256 | 201.7 | >256 | 58.0 | >256 | >256 | >256 | 69.3 | 160.0 | 50.8 | >256 | >256 | >256 |
| CLC | 12.8 | 36.9 | 10.2 | 51.4 | 184.8 | >256 | 35.3 | 101.2 | 32.2 | 220.9 | >256 | >256 | 8.1 | 26.1 | 11.2 | 49.7 | 65.8 | 215.6 |
| Mytilus edulis Hemocytes | 8.93 | 36.9 | 27.8 | 159.5 | — | — | 42.8 | 83.4 | 44.4 | >256 | — | — | 67.2 | 120.3 | 60.8 | >256 | — | — |
| In vivo testing | ||||||||||||||||||
| GANF | GANFg | GATam | ||||||||||||||||
| OECD test | EC25 | EC50 | EC25 | EC50 | EC25 | EC50 | ||||||||||||
| Daphnia magna (OECD 202) | 6.81 | 9.99 | 3.94 | 5.83 | 5.5 | 8.88 | ||||||||||||
- a EC25 and EC50 values (dispersion SOP with BSA).
- >indicates EC values exceeded concentrations where results could reliably be analyzed;—indicates that analysis was not conducted.
- BSA = bovine serum albumin; CFDA-AM = 5-carboxyfluorescein diacetate-acetoxymethyl ester; CLC = Cyprinus carpio leukocytes; EC25/EC50 = 25% and 50% effect concentrations, respectively; OECD = Organisation for Economic Co-operation and Development; PLHC-1 = Poecilipis lucida liver cells.
In the CLC cells, as in the PLHC-1 cells, no strong differences in cytotoxicity were observed between the 2 dispersion SOPs. The GANF CNF provoked a decrease in cell viability detected by means of the Alamar blue and CFDA-AM assays, allowing the calculation of EC25 (ranging 3.9–12.8 mg/L) and of EC50 (ranging 18.9–51.4 mg/L). However, the neutral red assay did not allow the calculation of the EC50 (>256 mg/L). In the case of GANFg, all EC25 and EC50 values were >32 mg/L, and in some cases it could only be determined that they were above the highest concentration used (>256 mg/L). The cytotoxicity caused by GATam could be detected through the Alamar blue (EC50 of 46.7 mg/L in the dispersion with BSA and 26.1 mg/L in the dispersion without BSA) and the CFDA-AM (EC50 of 89.9 mg/L in the dispersion with BSA and 49.7 mg/L in the dispersion without BSA) assays. The neutral red assay led to EC50 values higher than or close to the highest concentration used, depending on the use of BSA or not in the initial dispersion.
M. edulis hemocytes
Results indicated that the effects (EC50 values) on cellular metabolism (Alamar blue) were observed at lower concentrations for GANF (36.9 mg/L) and GATam (120.3 mg/L) when the dispersion was without BSA compared to dispersions with BSA for GANF (219.8 mg/L) and GATam (178.6 mg/L). The inverse was observed for GANFg, which showed higher EC50 values in the Alamar blue assay when no BSA was applied (83.4 mg/L) compared to the assays with the dispersion with BSA (34.4 mg/L). Higher concentrations were necessary to observe effects on cell membrane integrity (CFDA-AM), but a similar pattern for GANF could be seen in that effects were observed at lower concentrations without BSA (159.5 mg/L) than in tests with BSA (219.8 mg/L). For GATam (>256 mg/L) and GANFg (191.1 mg/L) EC50 values were similar in the CFDA-AM assay with or without BSA being used (ranging 191.1–>256 mg/L for both CNFs).
OECD test guideline 201
Interferences with the fluorescence analyses occurred, attributable to a shading effect of the CNFs potentially limiting algal growth, at concentrations >12.5 mg/L in the algal growth inhibition test; and consequently, these conditions were removed from the analysis. When comparing EC50 values with those generated when no BSA was used, the lowest values were found with GATam (2.12 mg/L), with similar values for GANF (3.09 mg/L) and higher values for GANFg (8.48 mg/L).
OECD 202 and 211
A clear effect of the 3 CNFs on the mobility of D. magna was observed when the test suspension was prepared without BSA. The EC50 values for GANFg (5.83 mg/L) were lower than those for GANF (9.99 mg/L) and GATam (8.88 mg/L). No effects were observed for GANF (>100 mg/L) and GANFg (>100 mg/L) when BSA was used. The EC50 value for GATam (58.81 mg/L) was much higher than that observed when the stock suspension was prepared without BSA.
The use of BSA in the dispersion SOP established a stable stock suspension that could easily be reestablished through vortex, allowing for chronic toxicity testing to be conducted. As a result, chronic testing was only conducted when BSA was added in stock suspension. A clear inhibition of D. magna reproduction was observed whatever the CNF tested. In addition, the GATam CNF showed a lower EC50 (0.32 mg/L) than GANFg (6.18 mg/L) and GANF (1.58 mg/L).
DISCUSSION
In the assessment of MNM hazards, it is essential to use multiple endpoints, generated in vitro and in vivo, with several species because there may be different potential mechanisms of toxicity (Oleszczuk et al. 2015). The present study followed a tiered approach, applying several in vitro assays on fish and mussel hemocyte cells to elucidate different mechanisms of toxicity to help in the design of higher-tier in vivo ecotoxicity testing. Thereafter, 2 in vivo assays, which are used in several regulatory paradigms (e.g., Harmonised Classification and Labelling [CLH], Registration, Evaluation, Authorisation and Restriction of Chemicals [REACH]), were used to gain essential information about the possible environmental hazards of the tested substances. In addition, these short-term assays were accompanied by the reproduction toxicity test in Daphnia to determine if long-term ecotoxicity could discriminate between the 3 CNFs.
Unexpectedly, these minute changes in the production process do lead to differences in the ecotoxicological responses among endpoints investigated. One potential explanation could be the ease with which the CNFs dispersed, with GANFg displaying more stability compared to GANF and GATam regardless of dispersion SOP or medium. This could be linked to the fact that GANFg was reported to have a higher purity (structural and chemical) than GANF and GATam, which could limit its ability to interact with the test media, effectively reducing the probability of aggregate formation. Differences in CNF stability attributable to changes in production method could have potential implications on the assessment of ecosystem hazards because a prolongation in the water column could promote environmental transportation and impact organisms with life cycles within the water column more so than benthic organisms (Jackson et al. 2013). Consequently, a less stable CNF would also sediment rapidly and could be sequestered in an ecosystem, posing a long-term risk to the environment and a strong impact on sediment-dwelling organisms. The present study highlights this challenge in assessing MNM hazards because 3 nearly identical CNFs, produced by the same industrial partner, can have divergent, species-specific impacts, highlighting the need for multispecies assessment of MNM ecotoxicity.
When conducting a literature review, one finds that there is a lack of information regarding the ecotoxicity of CNFs. There are, however, a number of studies conducted on multiwalled and single-walled CNTs. Although CNTs are not directly comparable to CNFs, they do provide a framework for understanding the toxicity of other nanocarbons of fibril nature. Carbon nanotubes have been shown to have EC50 values for P. subcapitata ranging from 1.8 to 24 mg/L and to cause immobilization in D. magna at concentrations ranging from 9 to 25 mg/L (Zhu et al. 2009; Schwab et al. 2011; Jackson et al. 2013). In the context of ecotoxicological hazards and thresholds of toxicity (European Chemicals Agency 2017), these concentrations of CNTs would be considered to result in low (>100 mg/L) to high (<10 mg/L) toxicity toward D. magna. As a result, it is difficult to conclude where or not carbon-based nanomaterials are inherently a risk toward the environment.
This highlights an essential issue in hazard assessment of MNMs in that the generation of stable suspensions, and the respective influence of the protocol used, alters the final determination of ecotoxicity. In the present study, an SOP previously established and applied in several large European Union research projects, involving a number of laboratories, was used to investigate the hazards of the CNFs. This SOP uses BSA as a dispersant to increase the stability of the stock suspension, which facilitates an appropriate exposure of the MNMs within biological systems, ensuring a constant concentration throughout the exposure period (limiting the sedimentation attributable to the formation of big aggregates or agglomerates), which is a prerequisite to calculate an accurate assessment of ecotoxicity. When colloidal suspension stability is poor, it is difficult to maintain uniform exposure conditions throughout the experiment and makes the detected toxicity unclear as to whether it is attributable to the MNMs or to a decrease in the probability of physical contact with big aggregates or agglomerates. At the same time, the use of a dispersant allows a better comparability of results between MNMs. One of the key questions that the present study investigated was the influence of BSA as a dispersant on the interpretation of CNF ecotoxicity. One potential explanation for the results in the present study is that the use of BSA coats the CNFs and effectively reduces the toxicity, as evidenced by testing with D. magna. This could potentially be attributable to the reduction of the release of impurities (nickel, sulfur, hydrocarbons) from the CNFs (GANF and GATam) or a decreased physical interaction between D. magna and the CNFs (Oleszczuk et al. 2015). Previous research also suggested that proteins in test media may alter the organism's ability to interact with carbon-based MNMs (Lukhele et al. 2015). The present study appears to support this idea because BSA visually reduced the interaction between CNFs and D. magna (adsorption onto shell, antenna, and other parts of the organisms; Figure 2).
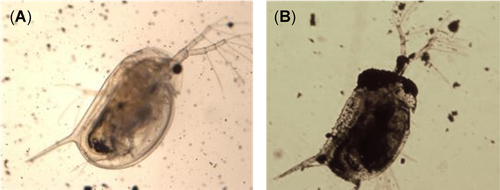
Images of Daphnia magna exposed to GANFg (25 mg/L) when testing was performed with (A) or without (B) bovine serum albumin in the preparation of the stock dispersion.
In vitro testing also elucidated unexpected information regarding the 3 CNFs. Results for the fish cell lines demonstrated a notable difference between the CLC and PLCH-1 cells. Leukocytes from C. carpio were affected by the exposure to the CNFs, with some clear adverse effects. Leukocytes play a role in the clearance of pollutants and are most likely one of the first cell types to respond to CNF exposure. Based on the EC50 values, GANFg displayed the lowest toxicity toward this cell line. This, however, was not in agreement with the results for M. edulis hemocytes, which suggested that GANFg may be more hazardous. One potential explanation for this is that the culture medium for M. edulis has a higher ionic concentration, which can lead to alterations in the CNFs' behavior. In the case of the PLHC-1 cells, the differences in toxicity among CNFs were smaller, but again GANFg showed a slightly lower toxicity than the other CNFs.
Previous work on carbon-based MNMs demonstrated a need for a surfactant to establish a stable suspension. Many of the common surfactants (Tween 20 and isopropanol), however, have been demonstrated to be toxic toward sensitive aquatic organisms including D. magna (Olasagasti et al. 2009). Bovine serum albumin has been proposed as a suitable dispersant for toxicity testing of MNMs because it has been demonstrated to have little to no adverse effects on test organisms. In the present study the results demonstrate that the use of BSA can influence the interpretation of ecotoxicity in some of the assays, which can influence its efficacy in identifying environmental hazards. Although proposing a solution or an appropriate protocol for the dispersion of nanocarbons (with or without BSA) is out of the scope of the present study, it is evident that this must be seriously considered when proposing dispersion protocols to be applied in internationally accepted regulatory testing.
CONCLUSION
To apply environmental hazard assessments in an industrial setting, ecotoxicity testing needs to be able to discriminate between products that are very similar in design. In this sense, Grupo Antolin provided 3 CNFs produced using different methods with the aim of determining if “safety” could be integrated in the development of their product. Unexpectedly, the 3 CNFs did demonstrate some differences in their ecotoxicity hazards toward the test organisms, which suggests that differences in purity (chemical or structural) could determine ecotoxicity. Representative organisms from multiple trophic levels and ecosystems were selected in the present study to better establish a holistic environmental hazard assessment for CNFs. In this sense, it becomes possible to make a prediction as to whether or not these MNMs pose a significant environmental risk. The present study highlights key challenges associated with environmental risk assessment because there is variation in interspecies responses when exposed to the CNFs. Despite this, there does seem to be evidence suggesting that GANFg may be less hazardous for the environment (by comparison with the other CNFs) because of the observation of improved stability, which may alter which environmental compartment is impacted, and that chemical purity may play a role in the hazards associated with the CNFs, as evidenced by the OECD test guidelines. To verify this, however, an analysis of environmental fate needs to be conducted to determine whether or not these CNFs have different potentials for bioaccumulation.
It is generally accepted that alterations of MNMs through stabilizing agents are undesirable but that in some cases natural or dissolved organic matter, at the lowest possible concentration, may be used on a case-by-case basis (Organisation for Economic Co-operation and Development 2019). The results of the present study demonstrated that the use of BSA in the preparation of the stock suspensions significantly reduced the identification of toxicity, but it is unclear if this is attributable to functional differences between the materials or to an alteration of stability profiles of the colloidal suspensions. In general, the dispersion SOP without BSA resulted in test suspensions with poor stability and rapid sedimentation of the CNFs, making it difficult to draw conclusions on CNF hazards and indicating a need for a stabilizing agent to investigate CNF ecotoxicity. This makes the use of BSA in the dispersion SOP a more suitable technique in establishing a stable stock suspension because it allows for the testing of hydrophobic materials. In the context of ecotoxicology, however, the use of BSA is not recommended because of the difficulties in establishing environmental representation; and additional research is required to define a standardized dispersant for ecotoxicity testing. Presently, work is being conducted to identify a more suitable dispersant for environmental testing, with some notable examples being carboxymethylcellulose sodium salt and gum arabic (Bourdiol et al. 2012). In the context of hazard and risk assessment, the present study investigated safety in the context of the initial MNM. To fully determine the hazards associated with the CNFs, a link needs to be made with the final product and associated with risks across the life cycle of the product.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4537.
Acknowledgment
The research contained within this publication was funded by the European Union's Horizon 2020 research and innovation program NANoREG2 under grant agreement 646221.
Disclaimer
The sole responsibility for this publication lies with the author. The European Union is not responsible for any use that may be made of the information contained therein.
Open Research
Data Accessibility
Data, associated metadata, and calculation tools are available from the corresponding author ([email protected]).




