Effect of Microcystis aeruginosa–Associated Microcystin-LR on the Survival of 2 Life Stages of Freshwater Mussel (Lampsilis siliquoidea)
Abstract
Microcystin-LR is a toxin commonly produced by the cyanobacterium Microcystis aeruginosa. It is present in harmful algal blooms and is a concern for both human and environmental health in Canadian freshwater systems. Previous studies have investigated the toxicity of microcystin-LR to other organisms such as fish; however, it is important to assess its toxicity to native freshwater mussels (family Unionidae), which are considered imperiled. The present study examined the toxicity of microcystin-LR to fatmucket mussels (Lampsilis siliquoidea) at 2 different life stages. Juvenile mussels were exposed to microcystin-LR in a 28-d chronic test, and glochidia underwent a 72-h acute toxicity test. There was no significant relationship between glochidia viability and microcystin-LR concentration. The median lethal concentration (LC50) value for juvenile mussels after 28 d of exposure was 2.1 µg/L. To determine the environmental relevance of the observed toxicity, an environmental exposure distribution was created using Canadian and Canadian–US Great Lakes microcystin measurements. The 28-d LC50 value (2.1 µg/L) was greater than those values that occurred in the environment 95% of the time; however, the LC10 (0.45 µg/L) and LC25 (0.97 μg/L) values were not greater than the measured microcystin environmental values. This finding indicates that microcystins may exert toxic effects on juvenile mussels at environmentally relevant concentrations. Further investigation should be considered in terms of prolonged exposure to persistent microcystin-LR, and toxicity to sensitive species at different life stages. Environ Toxicol Chem 2019;38:2137–2144. © 2019 SETAC.
INTRODUCTION
The harmful effects of eutrophication and toxic algal blooms is a global phenomenon (Pearl and Otten 2012). Algal blooms can produce toxic substances such as microcystin. Microcystis sp. produce the hepatotoxin microcystin, which has been a threat to global environmental and human health (Kotak and Zurawell 2007; Svircev et al. 2010). An increased presence of Microcystis sp. has been noted in recent years because it grows optimally at higher temperatures and increased nutrient levels (Davis et al. 2009; Orihel et al. 2013). Anthropogenic activities such as agriculture and climatic change contribute to these more favorable conditions (Davis et al. 2009; Pearl and Otten 2012). In previous years, concentrations of microcystins as high as 400 µg/L have been reported in Hamilton Harbour (ON, Canada) at peak blooms (Kotak and Zurawell 2007). This far exceeds Health Canada's acceptable drinking water concentration of 1.5 µg/L for microcystin-LR (Kotak and Zurawell 2007). However, concentrations are variable and range greatly depending on a variety of environmental factors (Niu et al. 2018). Microcystins vary in structure, resulting in different variants of the toxin (Chen et al. 2007; Ortiz et al. 2017). The majority of studies have discussed the most common variant, microcystin-LR, which can induce human illness and death (Chen et al. 2007; Ortiz et al. 2017). Other variants of microcystin such as microcystin-dmLR, microcystin-YR, and microcystin-LA, also exist, but there are few studies available concerning their effects and toxicity (Ortiz et al. 2017). Shimizu et al. (2014) investigated the toxicity of 16 microcystin variants on rat hepatocytes and observed greater than 3 orders of magnitude difference in toxicity across the 16 microcystin variants. A number of studies have only considered the total concentration of microcystins or the concentration of the cyanobacteria Microcystis sp. instead of a specific variant of microcystins in their assessment of toxicity (Zurawell, 2010; Clearwater et al. 2012).
As a hepatotoxin, microcystin has been reported to cause liver damage and necrosis, and to promote tumor growth in multiple species (Nishiwaki-Matsushima et al. 1991; Svircev et al. 2010). Saraf et al. (2018) reported that microcystin caused abnormal development, depressed heart rates, and mortality in Oryzias latipas embryos after exposure to environmentally relevant concentrations. The accumulation of microcystin in tissues and its toxicity has been observed in previous studies and may be ecologically relevant in terms of food web dynamics (Martins and Vasconcelos 2009). It is therefore important to examine the toxicity of microcystin to organisms at lower trophic positions, such as freshwater mussels.
Several studies have examined the accumulation of microcystin in bivalves, yet very few have characterized its toxicity to freshwater mussels. In 2012 to 2015, 37% of the mussels sampled in San Francisco Bay (CA, USA) had some level of microcystin accumulation (Peacock et al. 2018). One study by Clearwater et al. (2012) examined toxicity to juvenile freshwater mussels (New Zealand freshwater mussel, Echyridella menziesii) over a period of 96 h and found a 92% survival rate and a 16% reburial rate at a high concentration of 5298 µg/L. However, many studies report variations in the response of mussel species to microcystins. Mussels can excrete toxins like microcystins and selectively excrete the cyanobacteria Microcystis aeruginosa in pseudofeces as an immune response (Burmester et al. 2012; Kim et al. 2017a, 2017b). Burmester et al. (2012) found that zebra mussels (Dreissena polymorpha) can detoxify and excrete the toxin in concentrations of up to 50 µg/L for up to 7 d of exposure, whereas the Swollen River mussel (Unio tumidus) did not demonstrate this ability to a significant degree. Other studies have reported that the ability to detoxify and excrete microcystins varies with length of exposure, concentration, and species of mussel (Burmester et al. 2012; Kim et al. 2017a, 2017b).
Freshwater mussels belonging to the family Unionidae are some of the most imperiled species in North America, and their sensitivity to microcystins has not been widely examined (Lydeard et al. 2004; Strayer et al. 2004). The present study investigates the effects of M. aeruginosa–associated microcystin-LR on the fatmucket mussel (Lampsilis siliquoidea) at 2 life stages in an acute and chronic study. Juvenile mussels were exposed to varying concentrations of microcystin for a period of 28 d, and their mortality was examined. Glochidia of the same species were exposed to those same concentrations in an acute test for 72 h, to examine mortality of the larval life stage. The information gathered from our study is useful in characterizing the risk of microcystins at environmentally relevant concentrations in freshwater systems. This is especially important as temperatures and nutrient loading increase and result in more favorable conditions for the growth of Microcystis sp. (Davis et al. 2009; Pearl and Otten 2012; Orihel et al. 2013).
MATERIALS AND METHODS
Microcystis aeruginosa and microcystin-LR
Microcystis aeruginosa (from the Canadian Phycological Culture Centre [CPCC] at the University of Waterloo, Waterloo, ON, Canada; strain 300; CPCC 300) was cultured at the Ontario Ministry of Environment, Conservation and Parks (MOECP) in Etobicoke, Ontario, Canada in a BG-11 media (Acreman n.d) following the method of Shahmohamadloo et al. (2019). The concentration of microcystin-LR, microcystin-dmLR, nodularin, anatoxin-A, microcystin-dmRR, microcystin-RR, microcystin-LA, microcystin-LF, microcystin-LY, microcystin-Hi1R, microcystin-LW, microcystin-YR, microcystin, HtYR, and microcystin-WR produced by the M. aeruginosa culture was measured using on-line solid phase extraction and liquid chromatography at the MOECP according to the methods of Ortiz et al. (2017). A sufficient volume of M. aeruginosa to perform each experiment was isolated from the M. aeruginosa culture and stored at 4 °C before being used in the experiment. Nominal treatment concentrations for both juvenile and glochidia tests were calculated based on the measured concentrations in the Microcystis culture. Microcystis aeruginosa were transported to the University of Guelph (Guelph, ON, Canada), where they were kept in a stationary phase in the dark at approximately 6 °C to prevent growth. To account for fluctuations in the concentration of microcystins produced by M. aeruginosa CPCC 300, water samples were taken throughout both juvenile mussel and glochidia experiments and transported back to the MOECP, where their concentrations were confirmed.
Chronic toxicity juvenile test
Juvenile fatmucket mussels were selected due to their fairly abundant dispersal across Ontario's freshwater systems. Juvenile mussels were obtained from the laboratory of C. Barnhart at Missouri State University (Springfield, MO, USA). Glochidia were collected from female mussels from Silver Fork of Perche Creek (Boone County, MO, USA). Largemouth bass (Micropterus salmoides), originating from Chesapeake Fish Hatchery (Mt. Vernon, MO, USA), were used as a host species for the glochidia in the Barnhart laboratory. Juvenile mussels were released from the gills of the fish between 7 and 18 July of 2018. Juveniles were then shipped overnight to the University of Guelph on 5 September 2018. On arrival, mussels were approximately 1.5 to 2 mo old. At the University of Guelph Hagen Aqualab, mussels were transferred to Petri dishes filled with approximately 2 mm of sand, which were placed in an aerated communal tank. In the communal tank, the mussels underwent a 4-d acclimation period. During the acclimation period, mussels were fed daily 200 µg/L of a mixture of 0.7% Nanno 3600TM (Nannochloropsis sp.) and 2.7% Shellfish Diet 1800TM (Isochrysis, Pavlova, Thalassiosira, and Tetreselmis sp.; Reed Marticulture, San Jose, CA, USA).
At the end of the acclimation period, mussels were exposed to nominal microcystin concentrations of 2.5, 5, 10, 25, 50, and 100 µg/L. The concentrations of the microcystin solutions were made based on the concentration of microcystin-LR measured in the M. aeruginosa culture before a sufficient volume of cyanobacteria was isolated from the M. aeruginosa culture for testing and by diluting with culture water from the Hagen Aqualab (Supplemental Data, Table S1). For each concentration, 3 1-L beakers were filled with approximately 2 mm of sand and 700 mL of microcystins solution. Two control treatments with 3 replicates each were used in the experiment, a negative control, and a BG-11 media control to ensure that any observed effects were not due to the M. aeruginosa culture media. Each beaker contained 10 juvenile mussels that were aerated and given 200 µL of algae food mixture, as described above, per beaker, per day. Beakers were randomized within a growth chamber at the Hagen Aqualab with a 16:8-h light:dark photoperiod and a temperature of 20 ± 1 °C.
Water was changed weekly to refresh the microcystin concentrations. Weekly water changes occurred on days 7, 14, and 21. During water changes, mussels were removed from sediment and counted as alive or dead under a dissecting microscope. Mussels were considered alive if foot movement was observed in a 2-min time period. During water changes, water samples were taken, and water chemistry parameters were measured at the beginning and end of each 7-d period (Supplemental Data, Table S2). Beakers were rerandomized after each water change. Water samples were frozen and analyzed for microcystin concentration at the MOECP.
Acute toxicity glochidia test
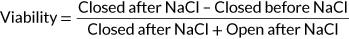 (1)
(1)Pooled glochidia were separated into 250-mL beakers for microcystin-LR concentrations of 2.5, 5, 10, 25, 50, and 100 µg/L, as well as the negative control and media control. Concentrations were made by diluting the culture with moderately hard water. Four replicates were used for each concentration and both controls. Water chemistry parameters were taken at the beginning and end of the 72-h acute test (Supplemental Data, Table S3). Test vessels were kept in a growth chamber at 21 ± 1 °C with a 16:8-h light:dark photoperiod. Viability checks were conducted at the 24, 48, and 72 h by taking a subsample from each beaker and determining the viability as just described. Test vessels were rerandomized every 24 h after observations were completed. The mean viability of glochidia in the negative control and media control remained above 90% at the 24-, 48-, and 72-h sampling points.
Statistical analysis
The geometric means of the measured concentrations of microcystin-LR from day 1 to day 7 were used for the analysis of mussel toxicity during the first 7 d (Supplemental Data, Table S4). For day 14, the geometric means of the concentrations included the measured concentrations on days 1, 7, and 14. For days 21 and 28, the measured concentrations of the previous water change days were included in the calculation of the geometric means (Supplemental Data, Table S4). The measured geometric means and nominal concentrations are compared in the Supplemental Data, Table S5.
A Welch 2-sample t test was used to determine whether there was a significant difference between the controls and media controls. This was done to ensure that the media control was not influencing mussel survival.
Statistical analysis was performed using R Ver 3.5.1. Median lethal concentration (LCx) values of 10, 25, and 50% and their respective standard errors, and 95% confidence intervals were estimated using nonlinear regression. A 4-parameter log–logistic model was fit for a binomial distribution using the drc package in R. Lethal concentrations were based on a mortality response for juveniles and a viability response for glochidia.
To determine the environmental relevance of the lethal concentrations, an environmental exposure distribution (EED) was generated from microcystin-LR monitoring data extracted from Ontario, Alberta, Quebec (Canada), and the Canadian and US Great Lakes (Giani et al. 2005; Chen et al. 2007; Zurawell 2010; Great Lakes Environmental Research Laboratory 2018). These data sources were selected based on public availability and relevance to Canadian freshwater systems. Once collected, data were fit to a log-normal distribution, and the 95th centile was calculated. A second EED was then created using only data extracted from the Great Lakes area (Great Lakes Environmental Research Laboratory 2018).
A Kruskal–Wallis rank sum test was conducted to determine whether there were significant differences between concentration treatment groups and controls. A Dunn's test of multiple comparisons using rank sums was then conducted to determine which pairs of treatment groups were significantly different. All tests were conducted to a 5% significance level (α = 0.05).
RESULTS AND DISCUSSION
Comparison of measured and nominal concentrations
Only microcystin-LR and microcystin-dmLR were detected in the M. aeruginosa. The toxicity of M. aeruginosa to juvenile mussels and glochidia has been expressed as concentration of microcystin-LR, because historically this is how toxicity has been expressed and microcystin-LR was present at a greater concentration than microcystin-dmLR (Supplemental Data, Table S2). In the experiment with juvenile mussels, the measured concentrations of microcystin-LR were less than those of nominal concentrations, and typically decreased over the span of 1 wk between water changes (Supplemental Data, Table S2). The mean percentage differences between the nominal and measured concentrations at the start of a week ranged from 32 to 58% (Supplemental Data, Table S2). The nominal concentrations of microcystin-LR were based on measurements of microcystin-LR in the M. aeruginosa culture before cyanobacteria were isolated for experimentation. The percentage difference between nominal and measured concentrations is likely due to variation in the subsample of M. aeruginosa initially taken for the determination of the nominal concentration for experimentation and the volume of M. aeruginosa that was isolated for use in the experiments. The mean percentage difference between the beginning and end of 1 wk ranged from 17 to 52% for all concentrations (Supplemental Data, Table S2), indicating that microcystin-LR and microcystin-dmLR degraded over each 7-d exposure period before renewal or else the microcystins were taken up and/or removed by the mussels. The measured concentrations were consistent at the beginning of each 7-d period after each renewal, which would indicate that microcystin-LR and microcystin-dmLR were relatively stable during the storage of M. aeruginosa at 4 °C.
In the experiment with mussel glochidia, the percentage difference between nominal and measured concentrations of microcystin-LR ranged from 14.4 to 99.0% (Supplemental Data, Table S3). The majority of the measured concentrations were greater than the nominal concentrations (Supplemental Data, Table S3). As mentioned in the above paragraph, the variation between the nominal and measured concentrations at the beginning of the test was likely due to a difference in the concentration of microcystin-LR between the subsample of the M. aeruginosa culture that was used to determine nominal concentrations and the portion of the culture that was isolated for use in the experiment. This highlights the importance of sampling exposure water at the initiation of the test and not simply relying on the measurement of a stock solution, or in this case, a stock cyanobacteria culture.
Chronic toxicity juvenile test
There was no significant difference in survival between negative controls and media controls at 7, 14, 21, or 28 d of exposure (p > 0.05). This finding indicates that the media used to culture the microcystin did not have a significant effect on the mussels alone. There was no mortality in negative controls and media controls after the 28-d test.
During the juvenile mussel chronic experiment, microcystin-LR was observed to have a significant effect on the mortality of juvenile mussels after 21 and 28 d of exposure relative to the control treatment (p < 0.05). On days 7 and 14, no significant effect was observed (p = 0.57). The LC10 values for 21 and 28 d of exposure were determined to be 0.45 and 1.4 µg/L, respectively (Table 1). Interestingly, the LC10 value for 28 d of exposure to microcystin was a value similar to the Health Canada acceptable drinking water concentration of 1.5 µg/L, meaning that toxicity was at similar concentrations to acceptable drinking water guidelines for 10% of juvenile fatmuckets tested (Kotak and Zurawell, 2007). The no-observable-effect concentrations were determined through Dunn's test of multiple comparisons to be 1.6 µg/L for 21 d of exposure and 1.5 µg/L for 28 d of exposure. When higher proportions of mortality were examined, the LC25 and LC50 values were determined to be 7.6 and 42 µg/L for 21 d, and 0.97 and 2.1 µg/L for 28 d (Table 1). At 28 d of exposure, complete mortality was observed at the 2 highest concentrations of microcystins.
| LC value | 7 d | 14 d | 21 d | 28 d | |
|---|---|---|---|---|---|
| LC10 (µg/L) | >100 | 19.6 (14.5) | 1.4 (0.76) | 0.45 (0.13) | |
| 95% CI | –8.89–48.1 | –0.12–2.9 | 0.19–0.71 | ||
| EED exceedance Canada | Ns | 16 | 58 | ||
| EED exceedance Great Lakes | Ns | 19 | 48 | ||
| LC25 (µg/L) | >100 | >100 | 7.6 (2.2) | 0.97 (0.20) | |
| 95% CI | 3.2–12 | 0.58–1.3 | |||
| EED exceedance Canada | Ns | Ns | 1.8 | 25 | |
| EED exceedance Great Lakes | Ns | Ns | 0.83 | 36 | |
| LC50 (µg/L) | >100 | >100 | 42 (16) | 2.1 (0.31) | |
| 95% CI | 9.5–74 | 1.5–2.7 | |||
| EED exceedance Canada | Ns | Ns | 0.16 | 9.8 | |
| EED exceedance Great Lakes | Ns | Ns | 0.01 | 17 |
- a The percentage of the environmental exposure distribution (EED) generated from data in Canada and data focused on Great Lakes that exceeded the LCx was also calculated. The relationship between concentration and mortality was determined to be insignificant at 7 and 14 d of exposure. Standard error is given in parentheses (n = 3).
- Ns = denotes those values with a p-value greater than 0.05.
Freshwater and marine mussels have previously been found to accumulate high concentrations of microcystins in their tissues while demonstrating few negative health impacts (Chen and Xie 2007; Vareli et al. 2012). The ability of mussels to metabolize microcystins varies between species. Burmester et al. (2012) compared the responses of the invasive zebra mussel (D. polymorpha) and the native Swollen River mussel (U. tumidus) when exposed to 10 and 50 µg/L of microcystin-LR in a 7-d exposure study. They found that the invasive species had a stronger detoxification response over the 7-d period (Burmester et al. 2012). However, most detoxification abilities decreased over time and at higher concentrations (Kim et al. 2018).
Previous studies have confirmed that the toxicity of microcystins to freshwater mussels and fish was both concentration and time dependent (Burmester et al. 2012; Kim et al. 2018). Wu et al. (2017) demonstrated the time dependency of the effect of microcystins in a study that examined the cumulative impact of toxic M. aeruginosa and hypoxia on triangle sail mussels (Hyriopsis cumingii) over a period of 21 d. They found that the accumulation of microcystins in the exposed mussels increased over time (Wu et al. 2017). On day 7 of their experiment, triangle sail mussels were observed to have damage in their gills, digestive gland, and stomach (Wu et al. 2017). These effects were amplified as length of exposure increased to 21 d (Wu et al. 2017). Similarly, a study examining the toxicity of microcystins to common carp (Cyprinus carpio) found time-dependent damage in the gills and livers at 10 µg/L after 14 d of exposure (Jiang et al. 2011). Clearwater et al. (2012) observed that 92% of juvenile New Zealand freshwater mussels (Echyridella menziesii) survived after exposure to 5300 µg/L of microcystins for 96 h. Although this concentration was much higher than those used in the present study, it is important to note that Clearwater et al. (2012) quantified total microcystins, not solely microcystin-LR. In the present study, microcystin-LR was the dominant microcystin, but microcystin-dmLR was also present and might have contributed to some level of observed toxicity. Microcystin-dmLR (also referred to as [D-Asp3]-microcystin-LR) has been observed to be approximately 4 times more toxic than microcystin-LR (Shimizu et al. 2014). Although statistical analysis was completed based on measurements of microcystin-LR, microcystin-dmLR was also measured and is reported in Supplemental Data, Tables S2 and S3. The observation of time-dependent toxicity reported in these studies corresponds with what was observed in our study, because mortality in juvenile mussels did not occur until after 21 and 28 d of exposure.
The 28-d LC25 and LC50 values were below concentrations reported in aquatic systems throughout North America. In Mexico's Valle de Bravo Reservoir, microcystin-LR concentrations were reported as ranging from 0.25 to 5.56 µg/L (Alillo-Schanez et al. 2014). In Vancouver Lake (WA, USA), concentrations varied in 2009 and 2010 from 12 to 15 µg/L, well above the calculated 28-d LC50 value of 6.1 µg/L reported in the present study (Table 1; Lee et al. 2015). Within Canada in 2010 and 2011, Quebec's Missisquoi Bay had concentrations ranging from 0.01 to 24 µg/L. Considerably higher concentrations have been observed in western Canada in the past. In 1993, Driedmeat Lake (Alberta, Canada) had peak concentrations of 6400 µg/L of microcystin-LR (Kotak and Zurawell 2007). In 2003, concentrations of 400 µg/L were seen in Hamilton Harbour (ON, Canada; Kotak and Zurawell 2007).
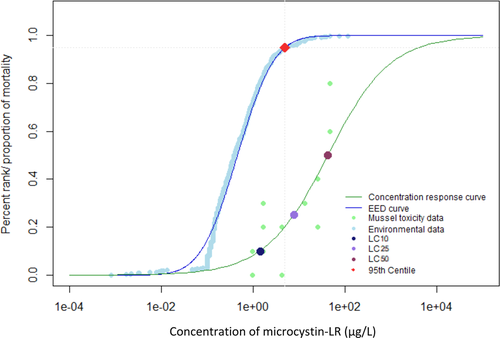
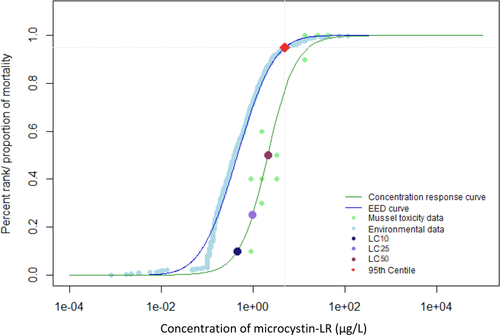
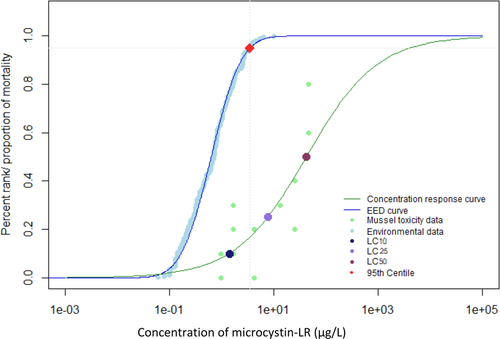
When environmental monitoring data from Alberta, Quebec, and the Great Lakes were examined, it was evident that reported concentrations greater than 100 µg/L were not the most prominent exposure concentrations (Giani et al. 2005; Kotak and Zurawell 2007; Chen et al. 2007; Zurawell 2010; Great Lakes Environmental Research Laboratory 2018). The 95th centile of the EED indicates that 95% of reported environmental concentrations were less than 4.9 µg/L for the Canadian data (Figures 1 and 2) and 3.5 µg/L for the Great Lakes data (Figures 3 and 4). The 28-d LC50 value of juvenile mussels in the present study was greater than the 95th centile of the EED (Figure 2). However, the 21- and 28-d LC10 and 28-d LC25 values were below the 95th centile of the Canadian EED (Figures 1 and 2). This trend is further emphasized by the percentage of the EED that exceeded the LCx values (Table 1). Furthermore, the half-life of microcystin was 90 to 120 d/m depth of the water column, although it can degrade more quickly in shallow water that is exposed to sunlight (Walker and Steinberg, 2000). More frequent monitoring could better characterize the persistence of microcystins and how they may fluctuate throughout the seasons. Because microcystins could be more persistent than the length of the present 28-d chronic study, future experiments should consider extending exposure periods to better characterize the effects of this toxin (Walker and Steinberg 2000; Schmidt et al. 2014). Future studies should consider the fluctuations throughout the seasons and the type of water body because the present EED does not represent exposure under all seasonal conditions, or varying water depths.
Environmental exposure distribution (EED) for microcystin-LR in Canada. Environmental data taken from Giani et al. (2005), Chen et al. (2007), Zurawell (2010), and Great Lakes Environmental Research Laboratory (2018). Concentration–response curve for juvenile mussels exposed to microcystin-LR for 21 d. Concentrations of microcystin-LR that induced a lethal concentration of 10% (LC10), 25% (LC25), and 50% (LC50) mortality in juvenile mussels are highlighted by colored points. Red point represents the 95th centile for the environmental data. The 95th centile was determined to be at a concentration of 4.9 µg/L.
Environmental exposure distribution (EED) for microcystin-LR in Canada. Environmental data taken from Great Lakes Environmental Research Laboratory (2018). Concentration–response curve for juvenile mussels exposed to microcystin-LR for 28 d. Concentrations of microcystin-LR that induced a lethal concentration of 10% (LC10), 25% (LC25), and 50% (LC50) mortality in juvenile mussels are highlighted by colored points. Red point represents the 95th centile for the environmental data. The 95th centile was determined to be at a concentration of 4.9 µg/L.
Environmental exposure distribution (EED) for microcystin-LR in the Canadian and US Great Lakes. Environmental data taken from Great Lakes Environmental Research Laboratory (2018). Concentration–response curve for juvenile mussels exposed to microcystin-LR for 21 d. Concentrations of microcystin-LR that induced a lethal concentration of 10% (LC10), 25% (LC25), and 50% (LC50) mortality in juvenile mussels are highlighted by colored points. Red point represents the 95th centile for the environmental data. The 95th centile was determined to be at a concentration of 3.5 µg/L.
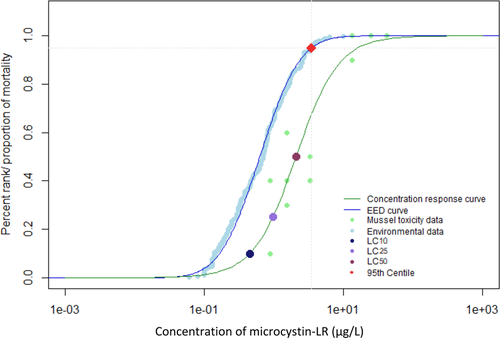
Environmental exposure distribution (EED) for microcystin-LR in the Canadian and US Great Lakes. Environmental data taken from Giani et al. (2005), Chen et al. (2007) Zurawell (2010), and Great Lakes Environmental Research Laboratory (2018). Concentration–response curve for juvenile mussels exposed to microcystin-LR for 28 d. Concentrations of microcystin-LR that induced a lethal concentration of 10% (LC10), 25% (LC25), and 50% (LC50) mortality in juvenile mussels are highlighted by colored points. Red point represents the 95th centile for the environmental data. The 95th centile was determined to be at a concentration of 3.5 µg/L.
Acute toxicity glochidia test
There was no significant difference in the viability of glochidia between controls and media controls at 24, 48, or 72 h of microcystin exposure (p > 0.05). Therefore, the BG-11 media alone had no significant influence on the viability of the glochidia (Supplemental Data, Table S3).
In the acute glochidia test, the effects of the concentrations of microcystin-LR on glochidia viability was not significant after periods of 24, 48, and 72 h (p > 0.05). A decrease in viability was observed for concentrations above 25 µg/L after 48 h of exposure, but there was no statistically significant relationship between viability and concentration. The effect concentrations for the glochidia test were calculated, but these values were not significant (Table 2). The large amount of variability between replicates at the higher nominal concentrations likely contributed to a lack of significant trends.
| Exposure period (d) | LC10 (µg/L) | 95% CI | LC25 (µg/L) | 95% CI | LC50 (µg/L) | 95% CI |
|---|---|---|---|---|---|---|
| 24 | 6.9 (12) | –17–31 | 64 (73) | –78–210 | 599.8 (1300) | –1976–3176 |
| 48 | 4.2 (6.8) | –9.1–17 | 27 (–24) | –20–74 | 170 (220) | –250–600 |
| 72 | 3.7 (5.8) | –7.7–15 | 22 (19) | –7.7–15 | 130 (130) | –130–390 |
- a The relationship between concentration and viability was determined to be insignificant for all exposure periods. Standard error is given in parentheses (n = 4).
- CI = confidence interval.
CONCLUSIONS
The exceedances of the constructed EED's 95th centile by the effect concentrations determined for juvenile fatmucket mussels indicate that freshwater mussels may be at risk from exposure to M. aeruginosa–associated microcystin-LR. Acute exposure may pose a de minimus risk to mussel populations, but chronic exposure of juveniles may pose a considerable risk to mussel populations. A major uncertainty related to this conclusion is that it is based on the concentrations contained with the EED being chronic exposures. The monitoring data currently available do not provide insight into the persistence of peaks in the concentration of microcystins during harmful algal blooms. This highlights the need for data on the frequency, magnitude, and duration of peaks in the concentration of microcystins in regions that overlap with freshwater mussel habitat.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI:10.1002/etc.4527.
Open Research
Data Accessibility
Data, associated metadata, and calculation tools are available from the corresponding author ([email protected]).




