Is the Effect Assessment Approach for Fungicides as Laid Down in the European Food Safety Authority Aquatic Guidance Document Sufficiently Protective for Freshwater Ecosystems?
Abstract
In Europe, the European Food Safety Authority aquatic guidance document describes the procedures for the derivation of regulatory acceptable concentrations (RACs) for pesticides in edge-of-field surface waters on the basis of tier-1 (standard test species), tier-2 (geometric mean and species sensitivity distributions [SSDs]), and tier-3 (model ecosystem studies) approaches. In the present study, the protectiveness of such a tiered approach was evaluated for fungicides. Acute and chronic RACs for tier-1 and tier-2B (SSDs) were calculated using toxicity data for standard and additional test species, respectively. Tier-3 RACs based on ecological thresholds (not considering recovery) could be derived for 18 fungicides. We show that tier-1 RACs, in the majority of cases, are more conservative than RACs calculated based on model ecosystem experiments. However, acute tier-2B RACs do not show a sufficient protection level compared with tier-3 RACs from cosm studies that tested a repeated pulsed exposure regime or when relatively persistent compounds were tested. Chronic tier-2B RACs showed a sufficient protection level, although they could only be evaluated for 6 compounds. Finally, we evaluated the suitability of the calculated RACs for 8 compounds with toxicity data for fungi. The comparison shows that the current RACs for individual fungicides, with a few exceptions (e.g., tebuconazole), show a sufficient protection level for structural and functional fungal endpoints. However, more data are needed to extend this comparison to other fungicides with different modes of action. Environ Toxicol Chem 2019;38:2279–2293. © 2019 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals, Inc. on behalf of SETAC.
INTRODUCTION
The regulatory aquatic risk assessment scheme that supports the registration of pesticides in the European Union is based on a tiered approach proposed by the Panel on Plant Protection Products and Their Residues (PPR) coordinated by the European Food Safety Authority (EFSA; 2013). Each tier is characterized by an exposure assessment, which results in a predicted environmental concentration (PEC), and an effect assessment, which results in a regulatory acceptable concentration (RAC) after the application of an appropriate assessment factor to laboratory or semifield toxicity data (Figure 1). By analyzing the PEC and the RAC, it is determined whether the risk related to an intended pesticide use is considered acceptable (PEC < RAC) or nonacceptable (PEC > RAC). The principle of the tiered approach is to start with a simple conservative assessment and to refine the exposure and/or the effect assessment making use of data obtained from more complex and time-consuming experiments (Boesten et al. 2007). The tier-1 aquatic effect assessment for pesticides, as outlined in the EFSA aquatic guidance document (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013), is based on the results of laboratory toxicity tests conducted with a limited number of standard test species. Tier 2 also includes results of laboratory toxicity tests, with additional test species, allowing the geometric mean (tier-2A) approach or the species sensitivity distribution (SSD; tier-2B) approach for the calculation of RACs. The experimental tier-2 studies can be complemented with toxicokinetic–toxicodynamic models to address the risks of time-variable exposures (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2018). In tier 3,the results from model ecosystem experiments (i.e., micro- and mesocosms) are used. In theory, population-level models can be used to complement the results of model ecosystem experiments, but no detailed guidance on mechanistic effect models has yet been provided in EFSA documents. In the tier-3 procedure, the RACs can be derived on the basis of 2 options: 1) the ecological threshold option (ETO-RAC), accepting negligible population-level effects only; and 2) the ecological recovery option (ERO-RAC), accepting some population-level effects under the condition that recovery takes place within a given time frame (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013).
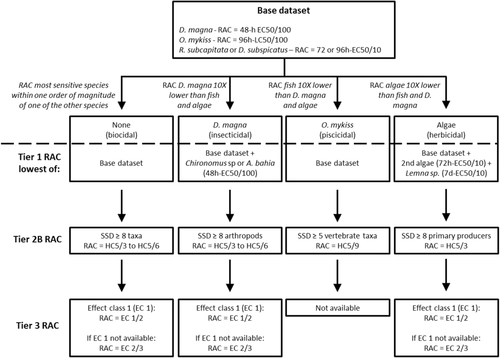
Decision scheme used to calculate acute tier-1, tier-2B, and tier-3 regulatory acceptable concentrations (RACs) according to European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013). Note that for fungicides with a biocidal mode of action, the acute tier-2B RAC was only derived when a minimum of 8 different taxa belonging to at least 6 different families/orders was available. For fungicides with an insecticidal mode of action, the species sensitivity distribution (SSD) was at the first instance based on at least 8 arthropods, but when toxicity data for nonarthropod taxa were available and were found to be sensitive (below the largest toxicity value for arthropods), they were also included in the SSD. When the latter was the case, the SSD had to meet the requirement of at least 8 different taxa belonging to at least 6 different families/orders. EC50 = median effect concentration; LC50 = median lethal concentration; HC5 = hazardous concentration for 5% of the species tested.
In principle, the adequacy of the prospective environmental risk assessment approaches for safeguarding aquatic organisms must be evaluated, for example, by using results from the most appropriate reference tier. According to the protection goals adopted in the EFSA aquatic guidance document, the ecological entity of plants and animals to protect is the population level, but in the acute risk assessment, vertebrates need to be protected at the individual level to avoid visible suffering and mortality due to direct toxicity (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2010). Population dynamics in the field (i.e., biomonitoring data) usually are the result of many environmental factors, and consequently may not allow observed effects to be linked to a single active substance, but only to multiple stressors (Rico et al. 2016). Consequently, safe threshold concentrations for individual active substances derived from aquatic micro- and mesocosm experiments (based on the ETO), have been used to evaluate the adequacy of the lower tier RACs. Previous studies have evaluated the adequacy of the tier-1 and tier-2 approaches, as described in European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), for insecticides (Van Wijngaarden et al. 2015; Brock et al. 2016) and herbicides (Van Wijngaarden and Arts 2018). However, to the best of our knowledge, such an evaluation for fungicides has not yet been performed. Maltby et al. (2009) compared acute hazardous concentration for 5% of the species tested (HC5) values from SSDs with no-observed-effect concentrations (NOECs) and lowest-observed effect concentrations (LOECs) from the most sensitive and relevant population-level endpoint observed in micro-/mesocosm experiments. However, in the EFSA aquatic guidance document, the criteria to derive a RAC both on the basis of the SSD approach (tier 2B) and on the basis of the model ecosystem approach (tier 3) were redefined, so that a new evaluation is warranted. In addition, the question has been raised as to what extent these RACs sufficiently protect aquatic fungi populations and communities, as well as their mediated ecological processes such as organic matter decomposition (Zubrod et al. 2015, 2019; Ittner et al. 2018).
The aim of the present study was to evaluate the adequacy of the experimental effect assessment procedures used in the European Union to protect populations of nontarget aquatic organisms in edge-of-field surface waters from exposures to a single fungicidal compound. To this end, following the guidance provided by the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), acute and chronic tier-1 and tier-2 RACs were calculated and compared with tier-3 ETO-RACs. Tier-2 RACs were only derived on the basis of the SSD approach (tier-2B), because the adequacy of the geometric mean approach described by the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) is difficult to evaluate for biocidal substances for which a wide array of taxonomic groups of aquatic organisms may be sensitive. This topic will be discussed in a follow-up manuscript together with a proposal for the implementation of the weight-of-evidence approach in the aquatic effect assessment for fungicides. The present study also compares the derived RACs with available toxicity data for structural and functional endpoints of aquatic fungi. Finally, some recommendations are given for improving the knowledge that underpins the environmental risk assessment of fungicides.
MATERIALS AND METHODS
Single-species toxicity data mining
An initial fungicide list was created (Table 1), which included 18 compounds for which information on ecological threshold levels is available from micro-/mesocosm studies that allowed derivation of tier-3 ETO-RACs, following the procedures described in the EFSA aquatic guidance document (see the Derivation of tier-3 RACs section). Information on the toxicological mode of action on microorganisms for these fungicides was obtained from the Fungicide Resistance Action Committee (2017). The CAS numbers were added to each fungicide by cross-checking the fungicide names with those contained in the Pesticide Properties Database (University of Hertfordshire 2007). The CAS number list was used to download all aquatic single-species acute and chronic toxicity data available from the US Environmental Protection Agency (2018) ECOTOX Database. Next, the draft assessment reports (European Food Safety Authority 2015) available for the list of selected fungicides were thoroughly searched, as well as the open literature and the toxicity dataset for fungicides collated by Maltby et al. (2009). The single-species toxicity data that were not already contained in the ECOTOX Database were added to the dataset. The dataset was managed using Microsoft Excel.
| Tier 2B acute RAC | Tier 2B chronic RAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mode of action | Compound | Type | Tier 1 acute RAC | Tier 1 chronic RAC | All taxa | Nonvertebrates | All taxa | Nonvertebrates | Tier 3 RAC Exposure category | Open literature references for micro-/mesocosm data (not reported reference means that the data are confidential) |
| Amino acids and protein synthesis | Cyprodinil | IN | xb | xb | x | – | – | – | 3 | |
| Cytoskeleton and motor proteins | ||||||||||
| Carbendazim | BIO | xc | xd | x | x | * | – | 3, 4 | Cuppen et al. (2000); Van den Brink et al. (2000); Slijkerman et al. (2004) | |
| Multisite contact activity | ||||||||||
| Chlorothalonil | BIO | xd | xc | x | x | x | x | 1 | ||
| Mancozeb | BIO | xe | xd | * | x | – | – | 3 | ||
| Metiram | BIO | xd | xc | x | x | x | x | 1 | Lin et al. (2012) | |
| Tolylfluanid | PIS | xd | xd | x | – | – | – | 1 | European Commission (2003) | |
| Respiration | ||||||||||
| Azoxystrobin | BIO | xc | xg | x | x | x | * | 4 | Van Wijngaarden et al. (2014) | |
| Fentin acetate | BIO | xd | xd | x | x | – | – | 2 | Roessink et al. (2006a, 2006b) | |
| Fluazinam | BIO | xc | xd | * | * | – | – | 3 | Van Wijngaarden et al. (2010) | |
| Kresoxim-methyl | BIO | xc | xd | x | x | x | – | 4 | ||
| Picoxystrobin | BIO | xc | xc | x | x | – | – | 3 | ||
| Trifloxystrobin | BIO | xc | xc | x | x | – | – | 3 | ||
| Signal transduction | ||||||||||
| Fludioxonil | HERB | xh | xd | – | – | – | – | 4 | Yin et al. (2018) | |
| Sterol biosynthesis in membranes | ||||||||||
| Fenpropidin | HERB | xh | xh | – | – | – | – | 3 | ||
| Prochloraz | BIO | xd | xc | x | – | – | – | 3 | ||
| Spiroxamine | HERB | xi | xi | – | – | – | – | 3 | European Commission (2009) | |
| Unclear | ||||||||||
| Copper | BIO | xc | xd | * | * | x | x | 4 | European Commission (2007) | |
| Pentachlorophenol | BIO | xd | xd | * | x | x | x | 3 | Willis et al. (2004) | |
- a The x indicates the availability of data to calculate a regulatory acceptable concentration (RAC) in the different tiers; the – indicates that not enough data were available to calculate a RAC; the * indicates that enough data were available but the log-normality test of the species sensitivity distribution (SSD) was rejected at the 0.05 level, so a tier-2B RAC value could not be calculated. The tier-3 RAC exposure categories refer to those described in the Derivation of tier-3 RACs section of the text. Tier 1 RAC is based on toxicity data for:
- b Americamysis bahia (crustacean).
- c Daphnia magna (crustacean).
- d Oncorhynchus mykiss (fish).
- e Raphidocelis subcapitata (green algae).
- f Pimephales promelas (fish).
- g Danio rerio (fish).
- h Desmodesmus subspicatus (green algae).
- i Skeletonema costatum (diatom).
- IN = insecticidal; BIO = biocidal; PIS = piscicidal; HERB = herbicidal.
Prior to analysis, the toxicity data reported as ppb or mol/L were converted to µg/L. Next, only data fulfilling the criteria regarding the measured effect endpoint, the calculated toxicity value, and the test duration described in Table 2 were used for further analysis. These criteria were broadly based on the criteria proposed by the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) for the selection of standard and nonstandard species toxicity data to be used in tier 2. Comparable selection criteria were also used in the evaluation studies conducted with insecticides (e.g., Brock and van Wijngaarden 2012; Van Wijngaarden et al. 2015; Brock et al. 2016) and herbicides (Van Wijngaarden and Arts 2017). When more than one toxicity value was available for a given taxon, the following rules were applied: 1) the geomean was calculated when more than one toxicity value was reported for the same test duration and the same endpoint of a given taxon, and 2) when one taxon was represented by several endpoints, the value corresponding to the most sensitive relevant endpoint included in Table 2 was selected.
| Vertebrates | Invertebrates | Primary producers | ||||
|---|---|---|---|---|---|---|
| Acute | Chronic | Acute | Chronic | Chronic | ||
| Endpoint | LC50 | EC10 or NOEC | EC50 | EC10 or NOEC | EC50/EC10 or NOEC | |
| Measured effect | Mortality | Growth rate, development, behavior, mortality, immobilization | Mortality, immobilization | Growth rate, feeding rate, reproduction, mortality, immobilization | Growth rate (preferred), yield | |
| Test duration (d) | 2–4 | >21 | 2–4 | >7–21a micro/meso-fauna ≥28d macro-invertebrates | 3–5 (algae), 7–8 (macrophytes) | |
- a It was checked whether for short-living organisms such as Rotifera and Nematoda there were EC10 or NOEC values that could be considered chronic (exposure duration more than 4 d), but there were none.
- LC50 = median lethal concentration; EC10 = effect concentration, 10%; EC50 = median effect concentration; NOEC = no-observed-effect concentration.
Our method of RAC derivation
The derivation of tier-1 RACs, tier-2B RACs, and tier-3 ETO-RACs in the present study follows the procedure described for RAC derivation in the EFSA aquatic guidance document (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013). These RACs are based on all relevant scientific information that we could obtain from the open and gray literature, including recent literature. Consequently, the RACs we derived may deviate from the values previously published by the EFSA or European Union Member States in official regulatory documents.
Derivation of tier-1 RACs
According to the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), acute tier-1 RACs were derived from the core dataset, by taking the lowest of 1) Daphnia magna 48-h median effect concentration (EC50; immobility) divided by an assessment factor of 100; 2) Oncorhynchus mykiss 96-h median lethal concentration (LC50) divided by an assessment factor of 100; and 3) the lowest of Raphidocelis subcapitata (synonyms: Pseudokirchneriella subcapitata and Selenastrum capricornitum) or Desmodesmus subspicatus (synonym: Scenedesmus subspicatus) 72- to 96-h EC50 divided by an assessment factor of 10 (Figure 1). For the selection of the algae EC50 values, growth rate was chosen as the preferred endpoint, and when not available yield was selected. Regarding the algal test duration, 72-h toxicity values were preferably chosen, but 96-h values were also allowed when 72-h values were not available. When the fungicide had an insecticidal mode of action (i.e., toxicity value for D. magna was 1 order of magnitude lower than for the other 2 species), toxicity data (48-h EC50 immobility, mortality) for a second arthropod species (Chironomus spp. preferred, or Americamysis bahia) divided by an assessment factor of 100 was also included. When the compound had an herbicidal mode of action (i.e., toxicity value for the selected algae species was 1 order of magnitude lower than the other 2), a 72-h EC50 (growth rate preferred over yield) for a non–green algae species (e.g., diatom or blue–green algae) and a 7-d EC50 for Lemna sp. divided by an assessment factor of 10 were also chosen. When the compound had a piscicidal mode of action (i.e., toxicity value for O. mykiss was 1 order of magnitude lower than the other 2), no further action was taken (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013; Figure 1). Most of the evaluated fungicides were classified as biocidal, that is, representatives of different taxonomic groups may be potentially sensitive (Maltby et al. 2009), on the basis of the core acute toxicity dataset and the chronic EC50 values for primary producers. Of the 18 selected fungicides, 3 were classified as herbicidal (mode of action: 2 as sterol biosynthesis inhibitors and 1 as a signal transduction inhibitor); 1 as insecticidal (mode of action: amino acids and protein synthesis inhibitor); and 1 as piscicidal (mode of action: multisite contact activity; Table 1).
In line with the guidance of the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), chronic tier-1 RACs were calculated as the lowest of 1) D. magna 21-d effect concentration, 10% (EC10) or 21-d NOEC (lowest relevant endpoint in Table 2, usually reproduction); 2) lowest of R. subcapitata or D. subspicatus EC50 (using the same endpoint and test duration criteria as described above for the acute assessment); or 3) the EC10 or NOEC for a standard fish species from an early life stage test or prolonged exposure duration test (lowest of mortality or growth for >21 d), divided by an assessment factor of 10. When the compound had an insecticidal mode of action, a chronic EC10 or NOEC for the most sensitive invertebrate species evaluated in the acute assessment (D. magna, Chironomus spp., or A. bahia) was chosen. When the compound had an herbicidal mode of action, a 72-h EC50 (growth rate preferred over yield) for a non–green algae species (e.g., the diatom Navicula pelliculosa) and a 7-d EC50 for Lemna sp. were also chosen. When the compound had a piscicidal mode of action, no further action was taken.
Derivation of tier-2B RACs
Acute and chronic SSDs were constructed using the acute and chronic toxicity data for standard and additional test species selected according to the criteria shown in Table 2. The SSDs for biocidal fungicides (i.e., one of the tier-1 test species was not 1 order of magnitude more sensitive than the 2 other tier-1 test species) were constructed by pooling toxicity data for primary producers, invertebrates, and fish together; the SSDs for compounds classified as herbicidal, insecticidal, or piscicidal in the tier-1 assessment were at first constructed by only using data for primary producers, invertebrates, and vertebrates (i.e., fish and amphibians), respectively. Following the recommendations of the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), SSDs were constructed when there were toxicity data available for at least 8 different taxa belonging to at least 6 different families/orders, with the exception of piscicidal compounds, for which 5 vertebrate toxicity values were set as a minimum requirement (Figure 1). In addition to these SSDs, alternative SSDs for biocidal compounds were constructed by considering only toxicity data for primary producers and invertebrates, because they are the taxonomic groups that are usually represented and evaluated in micro-/mesocosms studies, and fish are usually not present in these test systems.
The SSDs, their corresponding median HC5 values (50% confidence), and the lower limit of the HC5 values (95% confidence) were calculated with ETX 2.2 software by using a log-normal distribution, as described by Van Vlaardingen et al. (2004). Normality of the distributions was tested with the Anderson–Darling test for datasets with n ≤ 20, and with the Kolmogorov–Smirnov test for datasets with n > 20. The HC5 values were used in the evaluation if the goodness-of-fit was not rejected at the 0.05 level, as suggested by the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013).
Acute tier-2B RACs were derived using EC50 or LC50 data following the procedure described in Figure 1. For compounds classified as biocidal and insecticidal, acute tier-2B RACs were derived by dividing the median acute HC5 by an assessment factor of 6 (the strictest option in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013). For piscicidal fungicides, the acute RACs were derived by dividing the median acute HC5 by an assessment factor of 9. For herbicidal fungicides, and when the SSD was constructed with EC50 values for primary producers only, the RAC was derived by dividing the median HC5 by an assessment factor of 3. Note that according to the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), a distinction between an acute HC5 and a chronic HC5 cannot be made for primary producers because in the risk assessment EC50 values are used and the toxicity tests to derive them are considered to be chronic. For herbicidal fungicides in our dataset, however, there were not enough EC50 data for primary producers to construct a primary producer–specific SSD (Table 1).
For the chronic tier-2B assessment of biocidal fungicides, SSDs were constructed using selected NOEC or EC10 values (according to Table 2) for primary producers, invertebrates, and vertebrates. For compounds classified as insecticidal or piscicidal, there were not enough chronic EC10/NOEC data for the sensitive taxonomic group to construct arthropod- or vertebrate-specific chronic SSDs (Table 1). Chronic tier-2 RACs were calculated by dividing the median HC5 from an SSD containing chronic toxicity data by an assessment factor of 3 (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013).
Derivation of tier-3 ETO-RACs
Micro- and mesocosm data were obtained from the open gray literature including draft assessment reports (European Food Safety Authority 2015), reports from the Dutch National Institute for Public Health and the Environment (2019), summary reports of European Union member states (e.g., those for The Netherlands; Dutch Board for the Authorisation of Plant Protection Products and Biocides 2019), and scientific studies in the open literature. Available results from industry reports were also used. All micro-/mesocosm studies we used to derive a tier-3 RAC were lentic, so that exposure dynamics may be relatively worst-case, particularly for streams.
Before the derivation of the tier-3 ETO-RACs, each study was classified into one of the following exposure categories (following Maltby et al. 2009): 1) short-term pulse exposure: (median dissipation time [DT50] < 1 d; 2) short-term exposure: single application and dissipation DT50 > 1 d, but <10 d; 3) medium-term exposure: one of the following options, single application and dissipation DT50 > 10 d, but ≤25 d; or repeated applications and dissipation DT50 > 1 d, but <10 d; and 4) long-term exposure: one of the following options, single application and dissipation DT50 > 25 d; or more or less constant chronic exposure.
The effect classes described in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) were used to evaluate the treatment-related responses observed for the most sensitive and relevant endpoint of the micro-/mesocosm study. The effect classes corresponding to the ETO-RAC derivation are as follows: effect class 1 (i.e., highest test concentration at which a NOEC could be derived for the most sensitive population-level endpoint) and effect class 2 (i.e., lowest test concentration with statistically significant, but only slight/transient, effects on an individual sampling occasion for the most sensitive population-level endpoint). An ETO-RAC was derived separately for each fungicide and exposure regime according to the guidance provided by the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013). Briefly, when only effect class 1 values were available, the ETO-RAC was derived by dividing the highest effect class 1 concentration by an assessment factor of 2. When only effect class 2 values were available, the ETO-RAC was derived by dividing the lowest effect class 2 concentration by an assessment factor of 3 (Figure 1). When both effect class 1 and 2 values were available, the ETO-RAC was derived by dividing the lowest effect class 2 value by an assessment factor of 3. When from more than one micro-/mesocosm experiments an effect class 1 value was available for the same compound and exposure regime, the geometric mean of these values was used for the ETO-RAC derivation. When more than one effect class 2 value was available for the same compound and exposure regime from different studies, the lowest value was chosen for the ETO-RAC derivation. Following this approach, 19 tier-3 ETO-RAC values could be derived for the 18 different fungicides evaluated. (Two ETO-RAC values were available for carbendazim based on different exposure categories; Table 1.)
RAC comparison
Acute and chronic tier-1 RACs, as well as the lowest of the 2, were compared with tier-3 ETO-RAC values, and acute and chronic tier-2B RACs derived with all aquatic taxa and with only nonvertebrate taxa were compared with tier-3 ETO-RACs. When the lower tier RACs were found to be less conservative than the tier-3 ETO-RACs, alternative options (different from those proposed in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013) were tested, such as the increase in assessment factor or use of the lower limit of the confidence interval of the HC5.
The EFSA aquatic guidance document (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013) provides the possibility of using acute laboratory toxicity data for the derivation of acute tier-2B RACs when the fungicide is expected to result in single and repeated pulsed exposure regimes under field conditions. For this reason we only used micro-/mesocosm experiments characterized by single or pulsed exposure regimes (i.e., exposure categories 1–3) in the evaluation of acute tier-1 and acute tier-2B RACs. For the chronic tier-1 and chronic tier-2B RACs, comparisons were made with the tier-3 ETO-RACs derived from micro-/mesocosm experiments that considered all exposure categories (i.e., exposure categories 1–4).
Comparisons are illustrated on the basis of scatter plots, in which the 1:1 ratio of both RAC values is indicated (e.g., Figure 2). Thus cases falling below the 1:1 line indicate that calculated tier-1 or tier-2B RACs are sufficiently protective (green traffic light sign), whereas cases in which the data points are above the line are insufficiently protective (red traffic light sign).
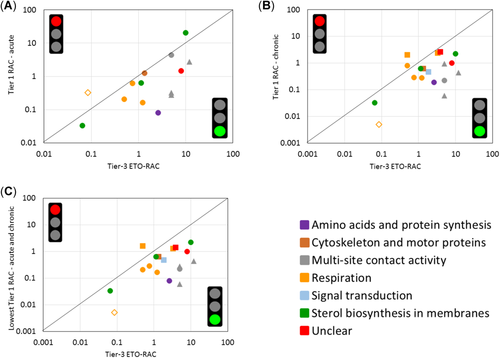
Comparison of tier-3 ecological threshold option regulatory acceptable concentrations (ETO-RACs) with acute tier-1 RACs (A), chronic tier-1 RACs (B), and the lowest of the acute or chronic tier-1 RACs (C). The type of symbol indicates the micro-/mesocosm exposure category, as follows: 1, short-term pulse exposure (triangles); 2, short-term exposure (diamonds); 3, medium-term exposure (circles); 4, long-term exposure (squares). Exposure category 4 is not used for the comparison with acute RACs. The symbol color relates to the mode of action of the compound. The tier-3 ETO-RAC value of fentin acetate, for which a “lower than” value (<0.086 µg/L) is available, is shown as an open diamond.
RESULTS AND DISCUSSION
Comparison of tier-1 and tier-3 ETO-RACs
The majority of the acute tier-1 RACs showed a sufficient level of protection compared with the tier-3 ETO-RACs (12/14 cases), with the exception of a sterol biosynthesis inhibitor and a respiration inhibitor, which had tier-1 RACs 2 to 3 times higher than the tier-3 ETO-RACs (Figure 2A). Note, however, that the value for the respiration inhibitor (the open diamond in Figure 2A) concerns a “smaller than” tier-3 ETO-RAC value for the fungicide fentin acetate (Roessink et al. 2006a, 2006b), a compound that is presently banned for use in agriculture in the European Union. In terms of the comparisons made with chronic tier 1 RACs, the majority of cases (15/17) resulted in a sufficient protection level, when the “smaller than” tier-3 ETO RAC value for fentin acetate was excluded (Figure 2B). The exceptions were 2 respiration inhibitors (one of them azoxystrobin; Van Wijngaarden et al. 2014), which had chronic tier-1 RACs approximately 2 and 4 times higher than the tier-3 ETO RACs. When the lowest of the acute and chronic tier-1 RACs, were compared, 16/17 cases (excluding fentin acetate) resulted in a sufficient protection level (Figure 2C). Again, the exception was azoxystrobin, which was evaluated in a microcosm experiment with a more or less constant exposure regime (Van Wijngaarden et al. 2014; exposure category 4; Figure 2C).
The fact that the acute tier-1 RAC is not protective for fentin acetate may be related to its high sorption capacity and persistence in the sediment compartment. Roessink et al. (2006a, 2006b) evaluated the fate and effects of this substance in microcosms simulating floodplain lakes. These authors reported water DT50 values of approximately 3 d, and identified some oligochaete, rotifer, and mollusk taxa as particularly sensitive to this compound. They attributed such sensitivity to delayed effects from chronic exposure via sediment contact or ingestion of organic matter particles with high accumulated concentrations of the test substance. Because fentin acetate has a high sediment sorption capacity, this compound must also be evaluated under the low-tier effect assessment proposed in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2015) for epibenthic and endobenthic organisms. Following such sediment toxicity assessment, it is expected that the corresponding aquatic exposure threshold would have been much lower, probably below the calculated tier-3 ETO-RAC; however, this was not evaluated in the present study.
In the case of azoxystrobin, Van Wijngaarden et al. (2014) demonstrated that calanoid copepods were highly sensitive to this compound under semifield conditions and a 42-d constant exposure regime. Maximum population-level effects (NOEC of 1 µg/L on abundance) were observed approximately 9 d after the start of exposure. This finding indicates that these organisms are especially sensitive to this compound under prolonged exposure conditions and that the time needed to show immobility/mortality is quite long. Apparently, populations of these sensitive copepods are not sufficiently protected by the tier-1 RACs. The high sensitivity of copepods to azoxystrobin was also noted in outdoor brackish water microcosms (Gustafsson et al., 2010), and in indoor freshwater microcosms exposed to another respiration inhibitor, fluazinam (Van Wijngaarden et al. 2010). Future studies are needed to evaluate whether this high copepod sensitivity is also the case for other (respiration inhibitor) fungicides. If that is the case, it may be an option to select a copepod species as an additional standard test species for fungicides in the near future.
Comparison of tier-2B and tier-3 ETO-RACs
There were sufficient data to build acute SSDs for 15 fungicides. For 4 of them the log-normality test was rejected, so HC5 values (and their lower limit; 95% confidence) could only be derived for 11 compounds. However, comparisons of acute tier-2B RACs with tier-3 ETO-RACs were only valid for 9 compounds, because for azoxystrobin and kresoxim-methyl, tier-3 ETO-RACs were derived with micro-/mesocosm tests characterized by a more or less constant exposure regime (exposure category 4; Table 1). Acute tier-2B RACs were generally lower than tier-3 ETO-RACs (and hence protective) for compounds in exposure category 1 (Figure 3A; all with a multisite contact activity mode of action). However, this was clearly not the case for 3 compounds characterized by exposure category 3 in micro-/mesocosm tests (Figure 3A). Therefore, alternative acute tier-2B RAC calculations (not included in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013) were derived by applying a larger assessment factor to the median acute HC5 or by using the lower limit of the confidence interval of the HC5. The lowest assessment factors that resulted in a sufficient protection level for almost all fungicides in acute tier-2B RAC derivation were as follows: assessment factor of 20 applied to the median acute HC5 (Figure 3C), and assessment factor of 6 applied to the lower limit of the acute HC5 (Figure 3E). Subsequently, these alternative approaches (not yet included in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013) may provide sufficiently protective acute tier-2B RACs for compounds with a moderate water persistence (10 d ≤ DT50 ≤ 25 d) or for those that are less persistent but characterized by repeated pulse exposures in edge-of-field freshwater ecosystems.
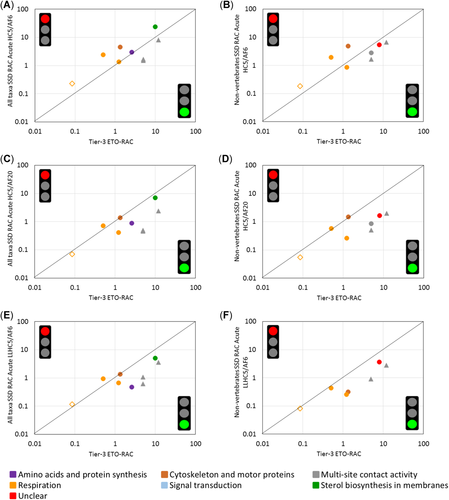
Comparison of tier-3 ecological threshold option regulatory acceptable concentrations (ETO-RACs) with acute tier-2B RACs calculated by dividing the hazardous concentration for 5% of the species tested (HC5) by an assessment factor of 6 and 20, and by taking the lowest limit of the 95% confidence interval of the HC5 divided by an assessment factor of 6. Comparisons are done with species sensitivity distributions (SSDs) built with all taxa (A, C, and E) and with nonvertebrate taxa (B, D, and F). The type of symbol indicates the micro-/mesocosm exposure category, as follows: 1, short-term pulse exposure (triangles); 2, short-term exposure (diamonds); 3 m medium-term exposure (circles). Colors represent different microbial modes of action. Note that the RAC comparisons for fentin acetate (open diamond) are indicative, because the tier-3 ETO-RAC is only provided as a “lower than” value (<0.086 µg/L). AF = assessment factor.
The evaluation performed on the basis of acute SSDs constructed excluding vertebrates (mainly fish) slightly improved the situation, with only 3 of the 8 evaluated cases being not protective (Figure 3B, D, and F). For fentin acetate, and for 2 fungicides (one respiration inhibitor and carbendazim) evaluated in micro-/mesocosms with an exposure category 3, the acute tier-2B RACs were not sufficiently protective (Figure 3B). The application of an assessment factor of 20 to the acute median HC5 or an assessment factor of 6 to the lowest confidence limit of the acute HC5 was also a suitable measure to prevent such a situation (Figure 3D and F). Fish have been demonstrated to be less sensitive than invertebrates and primary producers to the majority of fungicide classes, except for some multisite contact activity compounds with an ethylene bisdithio-carbamate chemical group (Maltby et al. 2009). This finding explains why RACs based on HC5s including fish are less protective. However, the option to only use nonvertebrate data in the SSD approach can be considered as more realistic because it focuses on the taxonomic groups and endpoints that are usually evaluated in micro-/mesocosm experiments.
The results of the present study contrast with those provided by Maltby et al. (2009), who demonstrated that an assessment factor of 3 applied to the median acute HC5, or the lowest confidence limit of the acute HC5, generally suffices for protecting against adverse ecological effects of pesticides (including fungicides) in micro-/mesocosm experiments. Note, however, that the selection criteria of toxicity data to be used in the SSD approach became stricter in the EFSA aquatic guidance document in terms of exposure durations (European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013), minimum number of toxicity values (8 in European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013 vs 6 in Maltby et al. 2009), and minimum number of families/orders represented (6 in European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2013 vs no minimum number in Maltby et al. 2009). In addition, in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), the criteria for the conduct and interpretation of micro- and mesocosm tests were also sharpened, and an assessment factor (2–3) was introduced to derive a tier-3 ETO RAC from threshold levels (effect classes 1–2) observed in these semifield tests. Maltby et al. (2009) used the NOEC and LOEC of the most sensitive and relevant population-level endpoints to derive a NOECeco (= ecosystem-level threshold) from micro-/mesocosm tests. In particular, these LOEC values also concerned treatment-related responses that, following the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013), would now be classified as effect class 3 A, that is, a short-term treatment-related effect followed by recovery (with an observed deviation from controls during <8 wk). Although these effect class 3 A values might be used to derive a tier-3 ERO-RAC (considering recovery of vulnerable populations), such concentrations cannot be used to derive tier-3 ETO-RACs according to European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013).
An observed exceedance of the acute tier-2B RAC relative to the tier-3 ETO-RAC does not provide information on the ecological consequences and duration of the effects that may occur at exposure concentrations resembling the tier-2B RAC. For the substances that were positioned above the 1:1 line in Figure 3A and B (except for fentin acetate), concentrations resembling the acute tier-2B RACs resulted in relatively short-term population-level effects in the micro-/mesocosm experiments. Nevertheless, the present study clearly illustrates that the SSD approach as described in the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) to derive acute tier-2B RACs may not be protective for all populations of freshwater organisms, at least when only the ETO is considered and in case of exposure category 3.
When all relevant taxa were considered, enough chronic toxicity data were available to build SSDs for 8 fungicides; however, the goodness-of-fit was rejected for 2 of them, so comparisons with tier-3 ETO-RACs were only performed for 6 fungicides (Table 1). The application of an assessment factor of 3 to the derived chronic median HC5 values resulted in a sufficient protection level compared with the available tier-3 ETO-RACs. The same assessment with chronic toxicity data for nonvertebrates only allowed a comparison for 4 fungicides, and indicated that the assessment factor of 3 applied to those HC5 values was sufficiently protective as well (Figure 4).
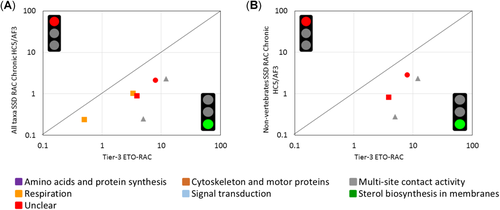
Comparison of tier-3 ecological threshold option regulatory acceptable concentrations (ETO-RACs) with chronic tier-2B RACs calculated by dividing the chronic hazardous concentration for 5% of the species tested (HC5) by an assessment factor (AF) of 3 with all taxa (A) and with nonvertebrate taxa (B). The type of symbol indicates the micro-/mesocosm exposure category, as follows: 1, short-term pulse exposure (triangles); 2, short-term exposure (diamonds); 3, medium-term exposure (circles); 4, long-term exposure (squares). Colors represent different microbial modes of action.
Our observation that the chronic tier-2B RACs (but not the acute tier-2B RACs) for fungicides provide sufficient protection to freshwater organisms in micro-/mesocosm studies (although based on relatively few cases) may be related to several factors. Most fungicide cases for which the acute tier-2B was not protective in our calibration exercise concerned micro-/mesocosm studies with medium-term exposure to the test compound (DT50 > 10 d but ≤ 25 d). In lentic micro-/mesocosm studies without water flow, this may have caused a relatively long-term, and relatively conservative, exposure regime relative to predicted exposure regimens in edge-field ditches and streams. However, it can be concluded that the treatment-related responses in micro-/mesocosm tests for some moderately persistent fungicides are not covered by short-term acute laboratory toxicity tests with a duration of 2 to 4 d, and the SSDs derived from them. The time-to-onset of effects on aquatic organisms may perhaps need to be longer for slow-acting biocidal fungicides than the 2 to 4 d considered in acute laboratory toxicity tests with additional test species (see, e.g., Brock et al. 2008 for a discussion on incipient toxicity). For most fungicides, a wider array of taxonomic groups of aquatic organisms will potentially show greater sensitivity than for insecticides (Maltby et al. 2009), and the test duration to reach incipient toxicity for mortality and immobility has been poorly investigated for fungicidal compounds and taxonomic groups of invertebrates other than crustaceans and insects. In addition, the particular light, temperature, and microbial communities of micro-/mesocosms can influence the formation of fungicide break-down products that are usually not measured and that may have contributed to the observed toxicity (Boudina et al. 2003).
Is the current effect assessment approach protective for aquatic fungi?
In Table 3, freshwater fungi toxicity data (NOECs reported for organic matter decomposition, biomass growth, or sporulation/germination of conidia) available for the fungicides evaluated in the present study are compared with their lower and higher tier RACs. Overall, it appears that tier-1, tier-2B, and tier-3 ETO-RACs for the fungicides evaluated are sufficiently protective for responses of structural and functional fungal endpoints observed in micro-/mesocosm tests, or in laboratory tests using fungi monocultures. The only exception was azoxystrobin, for which the acute tier-2B RAC seemed not to be protective for the growth of the oomycete Pythium spp., based on an agar plate test. In contrast, the chronic tier-2B RAC for azoxystrobin (0.2 µg/L) was lower than the laboratory 2-d NOEC for Pythium (2 µg/L). Similar findings were reported by Zubrod et al. (2015), who compared tier-1 RACs for 5 fungicides with NOEC values for fungal biomass, community composition, species abundance, spore production, and leaf decomposition using microcosms incubated with inoculated leaf material. In their study, they found that the tier-1 RACs for the fungicides studied were generally protective for fungal endpoints, except for tebuconazole, for which a chronic tier-1 RAC was calculated of 1 µg/L (based on toxicity data for D. magna). According to their results, exposure to this tier-1 RAC concentration would result in increasing microbial leaf decomposition compared with control. However, this effect was not observed at higher test concentrations (nor was a decreasing leaf decomposition rate). Zubrod et al. (2015) argued that higher tier RACs, which are used to assess risks of approximately 20% of fungicides in the European Union, may exceed tier-1 RACs by a factor of 10. They also speculate that these higher tier RACs may not be sufficiently protective for fungi.
| Fungicide name | RACs (µg/L) | Test | Type | Endpoint | Taxonomic division | Taxon | NOEC (µg/L) | Reference |
|---|---|---|---|---|---|---|---|---|
| Azoxystrobin | Tier 1 acute RAC: 1.6 | Litter | F | OM | AS, BA, OO | Community | ≥60 | Gustafsson et al. (2010) |
| Tier 1 chronic RAC: 2.0 | Leaf | F | OM | AS, BA, OO | Community | 20 | Zubrod et al. (2015) | |
| Tier 2B acute RAC: 5.8–11.5 | Leaf | S | BIOM | AS, BA, OO | Community | 100 | Zubrod et al. (2015) | |
| Tier 2B chronic RAC: 0.2 | Pure | S | BIOM | AS | Trichoderma hamatum | 460 | Dijksterhuis et al. (2011) | |
| Tier 3 ETO-RAC: 0.5 (Exp. Cat. 4) | Pure | S | BIOM | AS | Fusarium sporotrichioides | 29 | Dijksterhuis et al. (2011) | |
| Pure | S | BIOM | AS | Helicoon richonis | >5000 | Dijksterhuis et al. (2011) | ||
| Pure | S | BIOM | AS | Helicodendron tubulosum | >5000 | Dijksterhuis et al. (2011) | ||
| Pure | S | BIOM | BA | Cryptococcus flavescens | 460 | Dijksterhuis et al. (2011) | ||
| Pure | S | BIOM | OO | Pythium spp | 2 | Dijksterhuis et al. (2011) | ||
| Pure | S | BIOM | ZY | Mucor hiemalis | 230 | Dijksterhuis et al. (2011) | ||
| Carbendazim | Tier 1 acute RAC: 1.3 | Litter | F | OM | AS, BA | Community | 100 | Cuppen et al. (2000) |
| Tier 1 chronic RAC: 0.6 | Leaf | F | OM | AS, BA | Community | 35 | Zubrod et al. (2015) | |
| Tier 2B acute RAC: 4.6–9.2 | Leaf | S | BIOM | AS, BA | Community | ≥1715 | Zubrod et al. (2015) | |
| Tier 2B chronic RAC: – | Leaf | S | GER | AS, BA | Community | 1000 | Chandrashekar and Kaveriappa (1994) | |
| Tier 3 ETO-RAC: 1.7 (Exp. Cat. 3)– | Pure | S | BIOM | AS | Trichoderma hamatum | 260 | Dijksterhuis et al. (2011) | |
| 1.3 (Exp. Cat. 4) | Pure | S | BIOM | AS | Fusarium sporotrichioides | 1000 | Dijksterhuis et al. (2011) | |
| Pure | S | BIOM | BA | Cryptococcus flavescens | 8200 | Dijksterhuis et al. (2011) | ||
| Pure | S | BIOM | OO | Pythium spp | ≥5000 | Dijksterhuis et al. (2011) | ||
| Pure | S | BIOM | ZY | Mucor hiemalis | ≥8200 | Dijksterhuis et al. (2011) | ||
| Chlorothalonil | Tier 1 acute RAC: 0.3 | Pure | S | BIOM | AS | Trichoderma hamatum | ≥260 | Dijksterhuis et al. (2011) |
| Tier 1 chronic RAC: 0.06 | Pure | S | BIOM | AS | Fusarium sporotrichioides | ≥260 | Dijksterhuis et al. (2011) | |
| Tier 2B acute RAC: 1.7–3.3 | Pure | S | BIOM | BA | Cryptococcus flavescens | ≥260 | Dijksterhuis et al. (2011) | |
| Tier 2B chronic RAC: 0.3 | Pure | S | BIOM | OO | Pythium spp | ≥200 | Dijksterhuis et al. (2011) | |
| Tier 3 ETO-RAC: Confidential | Pure | S | BIOM | ZY | Mucor hiemalis | ≥260 | Dijksterhuis et al. (2011) | |
| Copper | Tier 1 acute RAC: 1.4 | Leaf | S | BIOM | AS, OO | Community structure | <1271 | Duarte et al. (2008) |
| Tier 1 chronic RAC: 2.6 | Leaf | F | OM | AS, OO | Community | 1271 | Duarte et al. (2008) | |
| Tier 2B acute RAC: – | Leaf | S | GER | AS, OO | Community | 1271 | Duarte et al. (2008) | |
| Tier 2B chronic RAC: 0.9 | Pure | S | BIOM | OO | Halophytophthora vesicular | 1000 | Leaño and Pang (2010) | |
| Tier 3 ETO-RAC: 3.9 (Exp. Cat. 4) | Pure | S | GER | OO | Halophytophthora vesicular | <1000 | Leaño and Pang (2010) | |
| Pure | S | BIOM | OO | Halophytophthora elongata | 10000 | Leaño and Pang (2010) | ||
| Pure | S | GER | OO | Halophytophthora elongata | <1000 | Leaño and Pang (2010) | ||
| Pure | S | BIOM | OO | Halophytophthora spinosa var. lobata | 1000 | Leaño and Pang (2010) | ||
| Pure | S | GER | OO | Halophytophthora spinosa var. lobata | 1000 | Leaño and Pang (2010) | ||
| Pure | S | BIOM | OO | Halophytophthora sp. | 10000 | Leaño and Pang (2010) | ||
| Pure | S | GER | OO | Halophytophthora sp. | >100000 | Leaño and Pang (2010) | ||
| Pure | S | BIOM | AS | Heliscus submersus | 156000 | Azevedo and Cássio (2010) | ||
| Pure | S | BIOM | AS | Tricladium chaetocladium | 121333 | Azevedo and Cássio (2010) | ||
| Pure | S | BIOM | AS | Varicosporium elodeae | 19063 | Azevedo and Cássio (2010) | ||
| Pure | S | BIOM | AS | Ypsilina graminea | 34667 | Azevedo and Cássio (2010) | ||
| Cyprodinil | Tier 1 acute RAC: 0.08 | Leaf | F | OM | AS | Community | 40 | Zubrod et al. (2015) |
| Tier 1 chronic RAC: 0.2 | Leaf | S | BIOM | AS | Community | 8 | Zubrod et al. (2015) | |
| Tier 2B acute RAC: 3.0–5.9 | ||||||||
| Tier 2B chronic RAC: – | ||||||||
| Tier 3 ETO-RAC: Confidential | ||||||||
| Fluazinam | Tier 1 acute RAC: 0.6 | Litter | F | BIOM | AS, BA, OO | Community | 50 | Van Wijngaarden et al. (2010) |
| Tier 1 chronic RAC: 0.3 | Pure | S | BIOM | AS | Trichoderma hamatum | 60 | Dijksterhuis et al. (2011) | |
| Tier 2B acute RAC: – | Pure | S | BIOM | AS | Fusarium sporotrichioides | 60 | Dijksterhuis et al. (2011) | |
| Tier 2B chronic RAC: – | Pure | S | BIOM | BA | Cryptococcus flavescens | 60 | Dijksterhuis et al. (2011) | |
| Tier 3 ETO-RAC: 0.8 (Exp. Cat. 3) | Pure | S | BIOM | OO | Pythium spp | 100 | Dijksterhuis et al. (2011) | |
| Pure | S | BIOM | ZY | Mucor hiemalis | 60 | Dijksterhuis et al. (2011) | ||
| Mancozeb | Tier 1 acute RAC: 4.4 | Leaf | S | GER | AS, OO | Community | 1000 | Chandrashekar and |
| Tier 1 chronic RAC: 0.2 | Kaveriappa (1994) | |||||||
| Tier 2B acute RAC: – | ||||||||
| Tier 2B chronic RAC: – | ||||||||
| Tier 3 ETO-RAC: Confidential | ||||||||
| Metiram | Tier 1 acute RAC: 2.8 | Litter | S | BIOM | AS | Anguillospora longissima, Tetracladium | ≥324 | Lin et al. (2012) |
| Tier 1 chronic RAC: 0.4 | setigerum | |||||||
| Tier 2B acute RAC: 8.1–16.2 | ||||||||
| Tier 2B chronic RAC: 2.3 | ||||||||
| Tier 3 ETO-RAC: 12 (Exp. Cat 1) | ||||||||
| Tolylfluanid | Tier 1 acute RAC: 0.3 | Litter | F | OM | AS, OO | Community | ≥214 | European Commission (2003) |
| Tier 1 chronic RAC: 1.0 | ||||||||
| Tier 2B acute RAC: 1.5 | ||||||||
| Tier 2B chronic RAC: – | ||||||||
| Tier 3 ETO-RAC: 5 |
- a Tier-2B RACs were calculated with the hazardous concentration for 5% of the species tested (HC5) derived from a species sensitivity distribution (SSD) with all aquatic taxa divided by assessment factors of 6 and 3 in the acute assessment, and an assessment factor of 3 in the chronic assessment, except for tolyfluanid (piscicidal) for which an assessment factor of 9 was used in the acute assessment. Tier-3 ecological threshold option (ETO)-RACs were obtained for the exposure categories indicated in Table 2. Values from confidential reports are not provided.
- Exp. Cat. = exposure category; Litter = litter bags in microcosms; Leaf = laboratory microcosms with a focus on leaf decomposition and associated microbes; Pure = pure culture, usually in agar plates; S = structural; F = functional; OM = organic matter decomposition; BIOM = biomass (growth); GER = sporulation and germination of conidia; AS = ascomycetes; BA = basidiomycetes; OO = oomycetes; ZY = zygomicota; - = not enough data were available to calculate a RAC.
In our study, comparisons of higher tier RACs and fungi functional or structural NOECs were possible for 9 fungicides (representing 5 microbial modes of action). It appeared that for these compounds, even at exposures resembling tier-3 ETO-RAC concentrations, toxic effects on aquatic fungal structural or functional endpoints will be small (Table 3). This, however, may not be the case for other fungicides for which tier-3 ETO-RACs based on the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) methodology could not be derived, such as for tebuconazole and other triazole fungicides.
In an outdoor microcosm study, Dimitrov et al. (2014) did not demonstrate effects on fungal biomass or sediment community structure in systems treated with tebuconazole at the acute HC5 level (238 μg/L, as derived by Maltby et al. 2009 using nonfungi toxicity data). However, this concentration reduced conidia production and altered fungal community composition associated with leaf material, which resulted in a decreased feeding rate of Gammarus pulex on exposed leaf material (Dimitrov et al. 2014). This observation for the fungicide tebuconazole is in line with the results of the laboratory study by Zubrod et al. (2015) discussed just above. Other laboratory studies have also indicated that triazole fungicides may alter food processing, reduce energy reserves, and affect survival of leaf-shredding macroinvertebrates at relatively low concentrations (Bundschuh et al. 2011; Rasmussen et al. 2012; Zubrod et al. 2014, 2015; Feckler et al. 2016). For instance, Bundschuh et al. (2011) noted a preference of Gammarus fossarum for control leaf disks compared with those treated with a concentration as low as 50 μg/L of tebuconazole. Similar findings on decomposer food-chain–related endpoints have been reported for some other classes of fungicides, but at relatively high exposure concentrations. Zubrod et al. (2014) assessed the impact of azoxystrobin, carbendazim, cyprodinil, quinoxyfen, and copper on the feeding rate of G. fossarum, and found EC20 values approximately 1 order of magnitude higher than the calculated RACs that we have derived for these compounds. This information suggests that the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) approach may be sufficiently protective for decomposer food-chain–related endpoints for most fungicides investigated, except perhaps for triazoles (such as tebuconazole).
CONCLUDING REMARKS AND RECOMMENDATIONS
Several studies that compared the tier-1 and tier-2 RACs with tier-3 ETO-RACs for insecticides (Van Wijngaarden et al. 2015; Brock et al. 2016) and the tier-1 RACs for herbicides (Van Wijngaarden and Arts 2018) indicate that the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) approach, with few exceptions, offers the required protection level for exposure to individual active ingredients under semifield conditions, at least when the exposure time tested in the micro-/mesocosms is sufficiently realistic. Cases in which the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) approach has shown a low protection level are mainly related to 1) compounds that have a high sediment sorption capacity and persistence and thus the acute assessment is not sufficiently protective for sediment dwelling or feeding organisms (e.g., pyrethroid insecticides; Brock et al. 2016); 2) compounds that have shown latency of effects (e.g., benzoylurea insecticides and other insect growth regulators; Van Wijngaarden et al. 2015); and 3) compounds that have been found to be particularly toxic to nonstandard test species (e.g., neonicotinoid insecticides to ephemeropterans; Van Wijngaarden et al. 2015).
In our study we have identified fungicides with high hydrophobicity that require a sediment assessment to complement the acute aquatic assessment (i.e., fentin acetate), and compounds that are particularly toxic to copepods (i.e., respiration inhibitor fungicides), which may require further considerations in the acute tier-1 assessment (e.g., selecting a copepod as an additional standard test species for fungicides). In addition, we have demonstrated that acute tier-2B RACs are not protective for some compounds that have moderate persistence under semifield conditions (DT50 > 10 d and <25 d), demanding a larger assessment factor to extrapolate the acute HC5, stricter guidance with respect to the toxicity data to include in the SSD (e.g., considering incipient toxicity), or an evaluation with chronic SSDs. The chronic tier-2B RAC for all compounds evaluated shows a sufficient protection level, but the observation is based on a very limited number of cases.
Overall, the present study shows that the number of adequate fungicide micro-/mesocosm studies available for the lower tier RAC calibration is relatively low compared with insecticide studies, partly because the risk may in some cases be triggered by fish in the lower tiers so that a micro-/mesocosm study with a focus on treatment-related responses of primary producers and invertebrates becomes less relevant. Further research is needed to evaluate the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) approach with a larger number of micro-/mesocosm studies conducted with a wider array of fungicidal compounds differing in toxic mode of action, including studies performed under lotic conditions. Attention must be given to sterol biosynthesis compounds, which dominate the European Union market together with the multisite contact compounds (EUROSTAT 2017). In particular, further information is required on the relationship between the exposure regime of individual fungicides in edge-of-field surface waters and the protectiveness of the tier-2B RAC. In addition, it must be taken into account that our evaluation of the tiered approach conducted on the basis of micro-/mesocosms disregards potential direct and indirect effects on fish populations. Therefore, further studies are needed to evaluate the risk of fungicides to fish, particularly for those fungicide classes that are clearly more toxic to fish (multisite contact activity compounds with an ethylene bisdithio-carbamate chemical group; Maltby et al. 2009). This might be done by using validated toxicokinetic–toxicodynamic models (e.g., European Food Safety Authority, Panel on Plant Protection Products and Their Residues 2018) and population models. Finally, amphibians should also be further considered because studies have demonstrated effects of strobilurins (respiration inhibitors) on Bufo cognatus tadpoles and juveniles at environmentally relevant concentrations (Belden et al. 2010).
To the best of our knowledge, very few experimental studies have been performed to assess population- and community-level effects of mixtures of fungicides and other pesticides in general, particularly those that are applied in tank mixtures or that are applied jointly for the same crop and season. Wang et al. (2018) demonstrated synergistic effects of cyprodinil and kresoxim-methyl on zebrafish embryos. Nørgaard and Cedergreen (2010) concluded that some sterol biosynthesis compounds (imidazoles and some triazoles) can enhance the effects of pyrethroid insecticides to D. magna when sprayed together in tank mixtures. In a field study in which 15 fungicides and 4 insecticides were monitored in streams of a German vineyard area, it appeared that the structure of microbial and shredder communities as well as fungal biomass changed along the fungicide toxicity gradient (Fernandez et al. 2015). Therefore, the evaluation of cumulative pesticide stress under field conditions including fungicides should be further investigated (e.g., Arts et al. 2006; Focks et al. 2014; Fernandez et al. 2015).
So far, fungi or other microorganisms are not included as standard test organisms in the prospective aquatic effect assessment for fungicides. With some exceptions (i.e., tebuconazole), the present study shows that lower and higher tier RACs derived according to the European Food Safety Authority, Panel on Plant Protection Products and Their Residues (2013) provide a sufficient protection level for most fungal structural and functional endpoints. However, the number of toxicity studies performed with fungicides and aquatic fungi is still very limited, and several modes of action have not yet been properly evaluated (Ittner et al. 2018). Research also indicates that subtle fungal community changes, including alterations in sporulation and germination efficiencies, may alter the palatability of leaf material for macroinvertebrate shredders at concentrations close to regulatory thresholds and at exposure levels monitored in the environment (Zubrod et al. 2015), so further research on the impact of realistic exposure regimes of (mixtures of) fungicidal compounds on the decomposer food chain is strongly recommended.
Acknowledgment
A Rico is supported by a postdoctoral grant provided by the Spanish Ministry of Science, Innovation, and University (IJCI-2017-33465). The contribution of TCM Brock was funded by the BO-20-002-001 project of the Dutch Ministry of Agriculture, Nature, and Food Quality. MA Daam acknowledges the support of the Portuguese government (Foundation for Science and Technology) through a postdoctoral grant (SFRH/BPD/109199/2015) and the Foundation for Environmental and Sustainability Research (UID/AMB/04085/2019).
Disclaimer
TCM Brock was one of the authors of the guidance document prepared by the European Food Safety Authority Panel on Plant Protection Products and their Residues (2013); the regulatory acceptable concentration derivation procedure in that document is evaluated in the present study.
Open Research
Data Accessibility
Data, associated metadata, and calculation tools are available from the corresponding author ([email protected]).




