Aryl hydrocarbon receptor-mediated activity of gas-phase ambient air derived from passive sampling and an in vitro bioassay
Abstract
The gaseous fraction of hydrophobic organic contaminants (HOCs) in ambient air appears to be responsible for a significant portion of aryl hydrocarbon receptor (AhR)-mediated activity, but the majority of compounds contributing to this activity remain unidentified. The present study investigated the use of polyethylene passive samplers to isolate gaseous HOCs from ambient air for use in in vitro bioassays and to improve our understanding of the toxicological relevance of the gaseous fraction of ambient air in urban and residential environments. Concentrations of polycyclic aromatic hydrocarbons (PAHs) and organic flame retardants were measured in polyethylene passive sampler extracts. Extracts were also analyzed using an in vitro bioassay to measure AhR-mediated activity. Bioassay-derived benzo[a]pyrene (BaP) equivalents (BaP-Eqbio), a measure of potency of HOC mixtures, were greatest in the downtown Cleveland area and lowest at rural/residential sites further from the city center. The BaP-Eqbio was weakly correlated with concentrations of 2-ring alkyl/substituted PAHs and one organophosphate flame retardant, ethylhexyl diphenyl phosphate. Potency predicted based on literature-derived induction equivalency factors (IEFs) explained only 2 to 23% of the AhR-mediated potency observed in bioassay experiments. Our results suggests that health risks of gaseous ambient air pollution predicted using data from targeted chemical analysis may underestimate risks of exposure, most likely due to augmentation of potency by unmonitored chemicals in the mixture, and the lack of relevant IEFs for many targeted analytes. Environ Toxicol Chem 2019;38:748–759. © 2019 SETAC
INTRODUCTION
Hydrophobic organic contaminants (HOCs) sorbed to particulate matter in ambient air pose a health risk to humans via several pathways, and activation of the aryl hydrocarbon receptor (AhR) by polycyclic aromatic hydrocarbons (PAHs) is strongly associated with the carcinogenicity of ambient atmospheric particulate matter (Matsumoto et al. 2007; Andrysík et al. 2011). However, health risks associated with HOCs in the gaseous phase remain poorly understood. Humans are exposed to gaseous air pollution directly via respiration and dermal uptake (Weschler and Nazaroff 2012). This is especially concerning in urban areas with heavier vehicular traffic and greater population density, as well as in indoor environments. Furthermore, gaseous HOCs are freely available to partition into other media, including plants (Kobayashi et al. 2007), and dietary uptake from crops has been identified as a route of human exposure (Kobayashi et al. 2008).
The gaseous fraction of ambient air has a distinct composition compared with the particle-bound fraction (Boström et al. 2002). The summed mass of PAHs in the gaseous phase is typically greater than in the particulate phase. However, gaseous PAHs are generally dominated by lower molecular weight 2- to 3-ring PAHs, whereas the particulate-bound fraction is dominated by more hydrophobic 4- to 5-ring PAHs (Boström et al. 2002; Klein et al. 2005; Ramírez et al. 2011; Barrado et al. 2013; Gungormus et al. 2014).
In addition to PAHs, recent studies have demonstrated that many organic flame retardant (OFR) compounds are also ubiquitous in ambient urban air, and that one particular class, the organophosphate esters (OPEs), are present at unexpectedly high levels in urban ambient air (Salamova et al. 2014; Shoeib et al. 2014). Furthermore, some currently used chlorinated OPEs are expected to be present predominantly in the gaseous phase (Brommer et al. 2014; Salamova et al. 2014; Peverly et al. 2015). O'Connell et al. (2014) used silicone wristbands as personal monitoring devices for exposure to gas-phase HOCs and frequently detected OPEs, along with several 2- to 3-ring PAHs.
Chronic exposure to gas-phase OPEs and other OFRs in ambient air is of concern because several studies have provided evidence that many OPEs, including tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), tris(1-chloro-2-propyl) phosphate (TCIPP), triphenyl phosphate (TPHP), and tris(2-ethylhexyl) phosphate (TEHP), can disrupt normal development, metabolism, immune response, and hormone function (Farhat et al. 2013, 2014; Liu et al. 2013; Porter et al. 2014). Studies have also indicated that TDCIPP is carcinogenic and/or mutagenic (Gold et al. 1978; Farhat et al. 2014), and, along with tris(2-chloroethyl) phosphate (TCEP), has been designated a carcinogen under California Proposition 65 (California Office of Environmental and Health Hazard Assessment 2017). Some OFRs, including tris(methylphenyl) phosphate (meta) (TmMPP) and TDCIPP, have also been associated with changes in expression of genes regulated by AhR in a few past studies, although evidence of this is sparse (Liu et al. 2013; Porter et al. 2014). Previous studies indicate that some polybrominated diphenyl ether (PBDE) congeners are also weak or moderate AhR agonists, and that binding affinity appears to depend on the degree and position of bromination (Chen and Bunce 2003; Gu et al. 2012). Recent work has also indicated that concentrations of PBDEs may be positively correlated with dioxin-like activity in dust samples, possibly due to the co-occurrence of polybrominated dioxins/furans (Wong et al. 2016).
Activation of AhR is linked to induction and repression of a large number of genes, including modulation of cell growth and proliferation, tumor promotion, immunological effects, cardiotoxicity, and endocrine disruption, with the severity and type of response dependent on the specific ligand and its binding affinity (Denison et al. 2011). Previous studies on health risks of ambient air pollution have used induction equivalency factors (IEFs) to represent the AhR-mediated potency of PAHs relative to benzo[a]pyrene (BaP; Kennedy et al. 2010; Ramírez et al. 2011). This IEF-based approach assumes an additive, rather than synergistic or antagonistic, relationship between multiple ligands. The AhR is activated by binding with variable affinity to several PAHs, with 4- to 5-ring PAHs generally more potent than the 2- to 3-ring PAHs that dominate gas-phase air pollution (Boström et al. 2002). Highly potent PAHs such as BaP are typically present only at very low concentrations in the gas phase due to low volatility. The lower molecular weight PAHs, especially phenanthrene, fluoranthene, and the methylated phenanthrenes/anthracenes, may contribute more significantly to the potency of the gaseous fraction due to their high gas-phase concentrations (Boström et al. 2002).
Despite low concentrations of potent high-molecular-weight PAHs in the gaseous fraction of ambient air pollution, previous studies have shown that this fraction appears to be responsible for a significant portion of the AhR-mediated activity associated with ambient air. In studies of gas-phase air pollution, Ramírez et al. (2011) found that, although concentrations of PAHs known to be most potent with respect to cytochrome P450 1A1 induction were low in the gaseous fraction, this fraction was estimated to contribute 34 to 86% of the total carcinogenicity associated with 16 PAHs based on potency relative to BaP. Previous studies by Klein et al. (2005) and Novak et al. (2009) also observed significant AhR activation from the gaseous, as well as particulate, fraction of ambient air pollutants. Kennedy et al. (2010) found a statistically significant relationship between PAH concentrations and AhR activity in samples of gaseous and fine particulate contaminants, but determined that the specific PAHs targeted in the study accounted for <3% of the observed AhR activity. Similarly, Érseková et al. (2014) found that quantified PAHs accounted for only 3 to 33% of measured AhR activity from ambient air samples. Although some of these studies considered contributions of compound groups other than PAHs, including polychlorinated biphenyls and organochlorine pesticides, none have investigated whether OFRs may explain some fraction of AhR activity.
Previous studies have noted that gaseous HOCs should not be ignored in risk assessments, but all the research was carried out using high-volume air samplers or passive polyurethane foam samplers, which are less selective for gaseous HOCs than polyethylene passive samplers (Melymuk et al. 2011). Studies using less selective sampling strategies could not fully rule out that some fraction of particulate-bound HOCs may have contributed to the measured AhR activity. Polyethylene passive samplers accumulate only gas-phase HOCs and have an affinity for HOCs that is similar to that of fatty tissue, so they have been used in many studies predicting the extent to which HOCs will bioaccumulate (Joyce et al. 2016). The present study is the first to our knowledge to investigate AhR activation caused by the freely gaseous fraction of HOCs taken up by a single-phase sampler (precleaned polyethylene), and our results should expand our understanding of the biological relevance of the truly gaseous fraction of ambient air in urban and residential environments.
Polyethylene passive samplers were deployed throughout the Cleveland (OH, USA) area on the southern shore of Lake Erie from June to September of 2013. Extracts from the samplers were analyzed by gas chromatography coupled with mass spectrometry (GC–MS) for a suite of PAHs and OFRs and were also analyzed via an in vitro bioassay to measure AhR activation. The objectives of the present study were to 1) investigate the use of polyethylene passive samplers as a viable vehicle for isolating gaseous HOCs for use in in vitro bioassays, 2) explore whether AhR-mediated activity of polyethylene passive sampler extracts correlated significantly with any PAHs or OFRs measured in the extracts, and 3) determine what portion of AhR-mediated activity measured via in vitro bioassays could be predicted based on targeted chemical analysis of commonly monitored PAHs.
We expected that AhR-mediated potency and gaseous concentrations of OFRs and PAHs in polyethylene passive sampler extracts would be greatest at densely populated urban sites located near the city center and that some correlation would be seen between gaseous PAH concentrations and potency. However, based on previous studies, we expected that BaP-equivalents calculated from targeted PAH chemical analysis (BaP-Eqchem) would likely underestimate the potency observed in bioassay experiments. We also expected that, unlike in particulate air samples, AhR-mediated potency of polyethylene passive sampler extracts would not correlate significantly with BaP concentrations, because BaP was not expected to be present at significant levels in the gaseous phase. Furthermore, we hypothesized that gas-phase OFRs might account for some fraction of AhR activity unexplained by commonly monitored PAHs, and that this would be indicated by significant correlations between OFR concentrations and AhR activity.
MATERIALS AND METHODS
Passive air sampler deployment
Low-density polyethylene sheeting (United Plastics), 800-μm-thick, was cut into approximately 7.5- × 13-cm pieces and cleaned in solvent (dichloromethane and hexane) to remove background contamination. At each of 9 sampling sites throughout the Cleveland area, 4 polyethylene passive samplers were fastened inside an inverted stainless steel bowl using zip-ties and the bowl was suspended so that the samplers were hanging at a height of approximately 2 m.
To calculate ambient air concentrations from concentrations measured in deployed polyethylene passive samplers, performance reference compounds (PRCs) are often added to the samplers for in situ calibration of sampling rates. However, PRCs could not be added to the samplers intended for bioassays because these compounds would interfere with bioassay response. Therefore, 50-µm-thick polyethylene passive samplers, preloaded with PRCs by incubation in an 80:20 methanol:water solution, were codeployed at each site, and sampling rates determined for these 50-µm samplers were used to interpret results from 800-µm samplers.
A map of the study region is shown in the Supplemental Data (Figure S1), and characteristics of the deployment sites are summarized in Table 1. Deployments took place from June to September of 2013, with each set of samplers deployed for approximately 60 d. After deployment, the samplers were removed from the protective bowl, wrapped in precombusted aluminum foil, and shipped on ice overnight to the University of Rhode Island Graduate School of Oceanography (Narragansett, RI, USA), where they were kept frozen until extraction.
| Location name | Latitude (°N) | Longitude (°W) | Deployment date range | Volume air sampled (m3)a | Site class | Nearby population densityb |
|---|---|---|---|---|---|---|
| Cleveland Lakefront 1 | 41.507 | –81.703 | 6/30/13–9/7/13 | 7466 | Urban | 359397 |
| Cleveland Lakefront 2 | 41.492 | –81.733 | 7/11/13–9/11/13 | 6588 | Urban | 342363 |
| Cleveland Downtown 1 | 41.492 | –81.679 | 7/1/13–9/5/13 | 7013 | Urban | 453257 |
| Cleveland Downtown 2 | 41.477 | –81.682 | 7/1/13–9/5/13 | 5994 | Semi-urban | 481527 |
| Cleveland Downtown 3 | 41.447 | –81.660 | 7/1/13–9/5/13 | 7023 | Semi-urban | 497567 |
| University Heights | 41.488 | –81.549 | 7/2/13–9/8/13 | 4938 | Semi-urban | 510538 |
| Fairport Harbor Lakefront | 41.758 | –81.277 | 7/3/13–8/29/13 | 4562 | Residential | 68591 |
| Kent | 41.164 | –81.361 | 7/2/13–9/10/13 | 4934 | Residential | 118272 |
| Cuyahoga National Park | 41.162 | –81.543 | 7/2/13–9/7/13 | 7026 | Rural/park | 168225 |
- a Volume of air sampled calculated using the sampling rate for phenanthrene, which was estimated based on performance reference compound loss data from codeployed thin polyethylene passive samplers multiplied by the deployment length.
- b Population density determined by calculating the total number of people within a 10-km radius using the GRUMPv1 database from Columbia University Center for International Earth Science Information Network 2011).
Sample preparation
Each 800-µm polyethylene passive sampler was extracted twice in pentane, each time for 18 to 24 h. The 50-µm samplers were extracted once for 18 to 24 h in pentane. Every batch of samplers was extracted along with a laboratory blank, which was a polyethylene passive sampler that had been cleaned alongside the field samples and then stored frozen in precombusted aluminum foil. All 4 800-µm samplers deployed simultaneously at the same site were composited into one extract and concentrated to 1 mL in a warm water bath under a gentle stream of nitrogen. Extracts from the 800-µm samplers appeared to contain a white precipitate, possibly from co-extracted polyethylene material. To remove the particulate, extracts were serially frozen, causing the precipitate to solidify at the bottom of the vial, and the overlying liquid was removed via a Pasteur pipette and reconstituted to 1 mL with pentane. Two aliquots were removed from the 1-mL solution, one for chemical analysis and the other for biological analysis. A schematic summarizing sample preparation is shown in the Supplemental Data (Figure S2).
Chemical analysis by GC–MS
The fraction of polyethylene passive sampler extract intended for chemical analysis was spiked with the internal standards acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12 and analyzed on an Agilent 6890 GC device coupled to an Agilent 5973 Mass Selective Detector (MSD) in electron impact (70 eV) mode for 22 PAHs, 18 alkylated PAHs, and (in a separate GC–MS run) 12 OPEs using an Agilent J&W DB-5 fused capillary column (30 m × 0.25 mm I.D.). The PAHs were quantified using an 8-point calibration curve with linearity r2 > 0.990 for all compounds. The OPEs were quantified using a 10-point calibration curve with linearity r2 > 0.997 for all compounds except TDBPP, which was not detected in samples and is omitted from further discussion.
Extracts were also spiked with non-native polybrominated diphenyl ethers (BDEs 35, 77, 128, and 183) and analyzed on an Agilent 7890 GC device coupled to an Agilent 5977 MSD in negative chemical ionization mode with methane reagent gas for 12 BDEs and 8 novel halogenated flame retardants (NHFRs), as well as 3 polybrominated biphenyls, which were used as PRCs in sampling rate determination for codeployed thin polyethylene passive samplers. A complete list of target compounds and abbreviations is available in the Supplemental Data (Table S1). The BDEs and NHFRs were quantified using an 8-point calibration curve with linearity r2 > 0.995.
To avoid interference with biological assays, samples were not spiked with internal standard prior to extraction and so were not corrected for internal standard recoveries. Concentrations presented for polyethylene passive sampler extracts were not blank-subtracted before use in data interpretation. This was considered appropriate because our primary interest was in determining the actual concentration present in the bioassay exposure solution.
Calculation of ambient air concentrations
The composition of HOCs accumulated in polyethylene differs from the ambient composition of gas-phase HOCs in air because the concentration in polyethylene is dependent not only on gas-phase concentrations, but also on the affinity of each compound for the polyethylene passive sampler matrix and the rate at which the compound is absorbed into the sampler. To compare the composition of solutions used in bioassay experiments with the actual composition of gaseous HOCs expected in ambient air, gaseous HOC concentrations were calculated based on the results of chemical analysis of polyethylene passive sampler extracts and sampling rates determined from codeployed PRC-loaded polyethylene passive samplers. Concentrations were blank-subtracted using the co-extracted laboratory sampler blank. After blank subtraction, concentrations <25% of the sampler blank were considered <detection limit, and all <detection limit values were replaced with 0.
To translate concentrations within the polyethylene passive sampler to concentrations in ambient air, the volume of air sampled by each sampler during deployment was estimated using data on the percentage of loss of labeled PRCs from codeployed 50-µm-thick samplers. From the PRC loss data, the best-fit value for the thickness of the diffusive boundary layer (DBL) at the air–polyethylene passive sampler interface was determined. Because all the samplers were deployed under the same conditions and the thickness of the sheet does not affect air-side resistance, the DBL thickness determined for thin sheets was then used in a 2-film model describing sampler-side and air-side mass transfer rates to calculate the percentage of equilibration reached by each target compound in the 800-µm-thick samplers. This approach for estimation of percentage of equilibration from PRC loss data has been described in detail in previous work (McDonough et al. 2016).
Biological analysis by reporter cell bioassay
Aliquots for biological analysis were mixed with 200 µL of dimethyl sulfoxide (DMSO) and blown down under a gentle stream of nitrogen to constant volume. This stock solution was then used to create a 10-point dilution curve (0.01–120 g polyethylene passive sampler/mL) for each sample, including the sampler blank (Supplemental Data, Figure S1).
The AhR reporter cell line used was H1G1.1c3, a murine hepatoma cell line consisting of Hepa-1c1c7 cells stably transfected with AhR-responsive green fluorescent protein (GFP) reporter gene (Nagy et al. 2002). Cells were plated in 96-well plates (3 × 105 cells/well; Costar 96-well black plate with a clear bottom) and allowed to attach overnight at 37 °C in selective medium (Nagy et al. 2002). The medium was then changed to nonselective medium, and the cells in 100 μL of medium were treated with 1 µL of each sample dilution for a final vehicle concentration of 1% DMSO. All wells were prepared in triplicate and incubated at 33 °C. For each test extract, the cells in 3 wells were treated with 1 µL of DMSO as a negative control, and the cells in another set of 3 wells were left untreated to control for any natural cell fluorescence. On each plate, 3 wells were treated with BaP at a final well concentration of 120 nM dissolved in DMSO as a positive control. On one plate, a 10-point dilution curve was also run for BaP (1.2 × 10−5–12 000 nM), and the results were normalized to the positive control 120 nM BaP (Supplemental Data, Figure S3).
Activity mediated by AhR was measured by reading the GFP fluorescence emitted by the cells at 515 nm using a Spectra Max M3 plate reader at 24 and 48 h post dosing (hpd). The mean fluorescence value of the DMSO-treated negative control triplicate wells was subtracted from each sample's fluorescence reading, and the response was expressed as a ratio over the mean fluorescence value for the triplicate 120-nM BaP-positive controls run on the same plate to control for plate-to-plate differences in cell response.
Calculation of extract potency
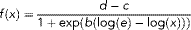 (1)
(1)In addition to the EC50, the ECBaP50 was calculated as an alternative measure of potency. The ECBaP50 is the concentration resulting in 50% of the effect observed for the plate-specific positive control (120 nM BaP). The ECBaP50 was identified as a more useful metric than EC50 because the concentration–response curves of the extracts were not parallel and maximum efficacy varied among curves.
Dosing solutions were prepared so that each sample was representative of the same amount of extracted polyethylene passive sampler to facilitate comparison with the sampler blank and control for any interference caused by background contamination in the sampler matrix. However, due to site-to-site variability in sampling rates, the volume of air represented by each sample differed among sites (Table 1). For this reason, after determination of ECBaP50 from the concentration–response curve fit, ECBaP50 values were normalized based on the volume of air sampled at each site. Aliquots of polyethylene passive sampler extracts used in dosing solutions were representative of 1900 to 3100 m3 of air, and were all normalized to 2000 m3.
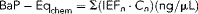 (2)
(2)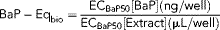 (3)
(3) (4)
(4)RESULTS AND DISCUSSION
Chemical composition of passive sampler extracts
Concentrations of all compounds in polyethylene passive sampler extracts are presented in the Supplemental Data (Table S2) for PAHs, OPEs (Supplemental Data, Table S3), and halogenated flame retardants (HFRs; Supplemental Data, Table S4). Concentrations of PAHs and OPEs in the sampler extracts are displayed in Figure 1A and B along with estimated ambient air concentrations (Figure 1C and D). All concentrations for field samples were normalized to an air volume of 2000 m3 to facilitate comparison between sites.
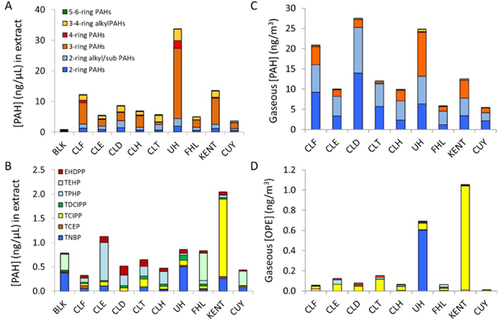
Total alkyl and parent PAHs (Σ40PAH) in polyethylene passive sampler extracts ranged from 3.6 ng/µL for the extract from Cuyahoga National Park to 34 ng/µL for a residential suburban area in University Heights. Concentrations of PAHs were dominated by phenanthrene (0.6–16.3 ng/µL; 10–57%), fluoranthene (0.1–6 ng/µL; 1–18%), 2-methylphenanthrene (0.1–1 ng/µL; 1–6%), and fluorene (0.3–1 ng/µL; 3–9%).
Concentrations of OPEs were much greater than those of HFRs. Total OPEs (Σ12OPE) ranged from 0.4 ng/µL for the extract from Cuyahoga National Park to 2.0 ng/µL for a residential area in Kent. The Σ12OPE was dominated by TPHP at all downtown Cleveland sites (0.09–0.78 ng/µL; 28–69%), whereas Cuyahoga National Park and Fairport Harbor were dominated by TEHP (0.30–0.57 ng/µL; 68%), and University Heights and Kent were dominated by tri-n-butyl phosphate (TNBP; 0.50 ng/µL; 59%) and TCIPP (1.60 ng/µL; 78%), respectively. Concentrations of total BDEs (Σ12BDE) ranged from 10 pg/µL in Cuyahoga National Park to 46 pg/µL at Downtown Cleveland Site 2, and were dominated by BDE 47 and 154. Concentrations of total NHFRs (Σ18NHFRs) were greatest in the polyethylene passive sampler blank due to the presence of 1,2-bis(2,4,6-tribromophenoxy)ethane and Dechlorane Plus, which were not found in any of the field sample extracts.
The 2-, 3-, and 4-ring PAHs, as well as their alkylated and substituted counterparts, were generally correlated in the different extracts (0.3 < r2 < 0.9), whereas the 5- to 6-ring PAHs did not exhibit significant correlation among themselves or with any other group of PAHs (Supplemental Data, Table S5). Correlation among individual PAHs was expected, because they are typically emitted from the same sources. Correlation among PAHs was further confirmed by principal component analysis, which showed that 76% of variation in samples was explained by 2 principal components, the first with loadings primarily from 3- to 4-ring PAHs, and the second with loadings primarily from 2- and 4- to 5-ring PAHs (Supplemental Data, Figure S4). In contrast, individual OPEs were generally not significantly correlated, although some degree of correlation (r2 ≥ 0.3) was observed between TDCIPP and TNBP (Supplemental Data, Table S6). In addition, TNBP, TDCIPP, and ethylhexyl diphenyl phosphate (EHDPP) exhibited some correlation with PAHs (Supplemental Data, Table S7).
Ambient air concentrations
Ambient gaseous concentrations of Σ40PAH ranged from 7.1 ng/m3 in Cuyahoga National Park to 36.2 ng/m3 at urban site Cleveland Downtown 1 and were dominated by the methylnaphthalenes (1.7–8.8 ng/m3; 18–33%), phenanthrene (0.3–9.8 ng/m3; 2–33%), and fluorene (0.5–2.6 ng/m3; 5–14%). Concentrations were similar in range to those measured by Peverly et al. (2015) in Chicago using polyurethane foam passive samplers in 2012 to 2014 (Σ16PAH = 9–52 ng/m3), and by Melymuk et al. (2012) in Toronto in 2007 to 2008 (Σ27PAH = 0.3–51 ng/m3), also using polyurethane foam passive samplers. Concentrations in the present study were similar but lower than previous measurements of total gaseous PAHs using polyethylene passive samplers in the downtown Cleveland area by McDonough et al. (2014) in 2012 (Σ15PAH = 23–80 ng/m3). In larger scale regional studies, atmospheric concentrations of PAHs have often been found to correlate with population density (Hafner et al. 2005; McDonough et al. 2014), but in the present study no significant (p < 0.05) correlation between gaseous PAH concentrations and population density within 5 to 30 km was observed.
Gaseous concentrations of Σ12OPE ranged from 0.01 ng/m3 in Cuyahoga National Park to 1.1 ng/m3 in Kent. This was similar in range to measurements by Peverly et al. (2015) in Chicago using polyurethane foam passive samplers in 2012 to 2014 (Σ13OPE = 0.5–1.5 ng/m3), and slightly lower than measurements of particulate Σ12OPE in the Cleveland area by Salamova et al. (2014) in 2012 (mean Σ12OPE = 2.1 ± 0.4 ng/m3. The most abundant OPE at all sites was TCIPP (0.01–1.0 ng/m3; 9–98%) except at University Heights, where TNBP dominated (0.6 ng/m3; 87%). In a previous study, TCIPP was also found to be the most abundant in Cleveland particulate Σ12OPE (0.85 ± 0.3 ng/m3; Salamova et al. 2014).
Figure 1 compares the chemical composition of the polyethylene passive sampler extracts used in bioassay experiments with that of ambient gaseous PAHs and OPEs. Extracts used in bioassays were enriched in moderately hydrophobic compounds, such as fluoranthene and TDCIPP, which make up a lower percentage of total HOCs in the gaseous fraction of ambient air but have a greater affinity for the polyethylene passive sampler matrix. The different HOC compositions in the gas phase and in the sampler extract illustrate that it is not possible to estimate the total AhR-mediated potency of the mixture that is present in gas-phase air. However, AhR-mediated potency results based on the HOC mixture found in polyethylene passive sampler extracts still represent an important step in understanding the biological relevancy of gas-phase compounds. Furthermore, the composition in polyethylene passive sampler extracts is expected to be more similar to the composition of HOCs diffusing into plant material or skin from air, or accumulating in the body via other mechanisms.
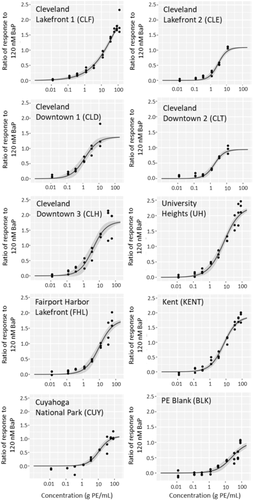
Concentration–response curves
Extracts from all polyethylene passive samplers, including the sampler blank, induced concentration-dependent activation of AhR-dependent GFP. All concentration–response data are displayed along with curve fits and 95% confidence intervals in Figure 2, with response represented as a ratio compared with response elicited by the plate-specific positive control. For all extracts, an initial increase in GFP induction was seen with increasing concentration. However, there was a precipitous decline in the fluorescence for all extracts (except Cleveland Lakefront 1) at the greatest concentrations, possibly due to cytotoxicity or inhibition of fluorescence response at high concentrations of polyethylene passive sampler extract. These points were omitted during concentration–response curve fitting, because we were interested in determining only the induction potencies of the extracts. Most extracts did not exhibit a clear plateau in response, making determination of maximum efficacy, as well as EC50, somewhat uncertain. Furthermore, maximum efficacy of the samples varied from 94 to 230% of positive control response (Table 2). For this reason, ECBaP50, measured relative to the plate-specific positive control, was used to compare the potencies of the samples.
| Sample | ECBaP50 ± SD (g PE/mL) | Maximum efficacy ± SD (% of positive control) |
|---|---|---|
| Cleveland Lakefront 1 | 2.2 ± 1.2 | 188 ± 39 |
| Cleveland Lakefront 2 | 1.9 ± 0.2 | 109 ± 4 |
| Cleveland Downtown 1 | 0.5 ± 0.1 | 138 ± 39 |
| Cleveland Downtown 2 | 1.6 ± 0.2 | 94 ± 13 |
| Cleveland Downtown 3 | 1.1 ± 0.3 | 179 ± 55 |
| University Heights | 1.6 ± 0.3 | 230 ± 18 |
| Fairport Harbor Lakefront | 4.1 ± 0.9 | 178 ± 22 |
| Kent | 2.6 ± 0.4 | 188 ± 18 |
| Cuyahoga National Park | 6.6 ± 1.2 | 110 ± 15 |
- CBaP50 = concentration of the sample resulting in 50% of the effect observed for the plate-specific positive control (120 nM benzo[a]pyrene [BaP]); SD = standard deviation; PE = polyethylene passive sampler extract.
The ECBaP50 of each extract, normalized based on the volume of air sampled at each site, is displayed in Table 2 along with each extract's maximum observed efficacy. Values of ECBaP50 ranged from 0.5 ± 0.1 g polyethylene passive sampler/mL at Downtown Cleveland 1 to 6.6 ± 1.2 g polyethylene passive sampler/mL at Cuyahoga National Park.
The 3 rural/residential sites had the lowest potency (greatest ECBaP50 values), ranging from 2.6 to 6.6 g polyethylene passive sampler/mL, followed by the 2 Cleveland Lakefront sites. The most potent extracts were from the 3 Cleveland Downtown sites and one semi-urban residential site (University Heights, a densely populated suburb). This contrasts with work by Klein et al. (2005), who found no change in potency of gaseous extracts between urban and rural samples with distinct chemical compositions, but is consistent with work by Érseková et al. (2014), who found that extracts from impacted sites were more potent in AhR bioassays than extracts from rural sites. The potency of the polyethylene passive sampler blank (ECBaP50 = 23 ± 5 g polyethylene passive sampler/mL) was significantly lower than that of all field samples. Blank comparisons were done before normalizing for the volume of air sampled so that each sample would be representative of the same mass of extracted polyethylene.
The potency and maximum efficacy of the extracts did not appear to be correlated. This is most likely due to a complex interplay between the unique composition of ligands in each sample, their affinity for the AhR, the resulting ligand–receptor complex's ability to bind other necessary transcription factors, and cytotoxicity of specific components. Response could also be affected by ligands interacting with other pathways that could amplify or dampen AhR response. Klein et al. (2005) also observed a lack of correlation between potency of extracts and maximum efficacy with respect to AhR binding of gas-phase extracts from active air sampling.
Initial bioassay experiments demonstrated that the fluorescence responses of the treated cells increased over time from 16 to 48 hpd, so all responses reported here were measured at 48 hpd. This is in contrast to other studies of AhR activation for environmental samples, most of which have used a luciferase reporter rather than the GFP reporter we used. For example, Machala et al. (2001) measured the greatest potency at 6 hpd, most likely due to PAH metabolism, and Kennedy et al. (2010) observed steadily decreasing potency in extracts from 24 to 72 hpd. This discrepancy is most likely due to differences in induction kinetics and increased stability of the GFP reporter compared with the luciferase reporter (Han et al. 2004). It is also possible that some of the response observed in this study was due to compounds that were less readily metabolized than PAHs and OPEs.
Bioassay-derived BaP equivalents for polyethylene passive sampler extracts
Figure 3 shows a map of results for BaP-Eqbio alongside maps of total concentrations of PAHs and OPEs in the polyethylene passive sampler extracts (Σ40PAH and Σ12OPE). The BaP-Eqbio values ranged from 21 to 283 ng/µL BaP equivalents and were generally greatest in the downtown Cleveland area and lowest at the rural/residential sites further from the city center.
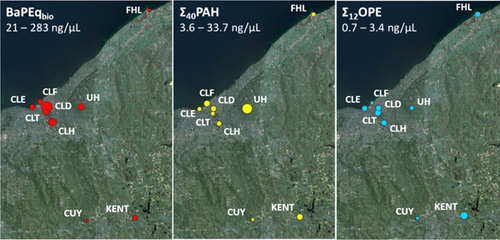
The BaP-Eqbio values were compared with concentrations of PAHs and organic flame retardants (OPEs, PBDEs, and NHFRs) in the polyethylene passive sampler extracts to determine whether there was any significant correlation between potency and chemical composition. Although some correlations were found, few were likely to be driving potency. No correlations with PBDE and NHFR concentrations were observed. The BaP-Eqbio values weakly correlated only with 2-ring alkyl/substituted PAHs (r2 = 0.42; p < 0.1; standard error [SE] = 64; n = 9) and also displayed some correlation with EHDPP (r2 = 0.66; p < 0.01; SE = 49; n = 9). Maximum efficacy of polyethylene passive sampler extracts showed some correlation with concentrations of 3-ring (r2 = 0.61; p < 0.05; SE = 31; n = 9) and 4-ring (r2 = 0.48; p < 0.05; SE = 36; n = 9) parent PAHs. Correlations between BaP-Eqbio values and alkyl/substituted PAHs were only investigated by grouping compounds (2-ring alkyl/substituted PAHs; 3–4-ring alkyl/substituted PAHs) because quantitative standards were not available for all alkylated PAHs. However, it is important to note that AhR-mediated potency differs greatly between PAH isomers. Because a high degree of correlation was observed between different low-molecular-weight PAHs at different locations in the present study (Supplemental Data, Table S5), it was expected that the composition of alkyl/substituted PAHs would most likely be similar between sites, so correlations with BaP-Eqbio values are likely driven by the same compounds at all sites.
Little information is available regarding the biological effects of alkylated PAHs. Recent studies using a yeast reporter assay system and an H4IIE-luc reporter-gene assay suggest that methyl- and dimethyl-substituted phenanthrenes are in some cases more potent with respect to AhR activation than their unsubstituted counterparts (Sun et al. 2014; Lam et al. 2018). The statistically significant correlation between BaP-Eqbio and EHDPP suggests that this compound, or unmonitored compounds with which it covaries spatially, could be contributing to AhR activity. Because no compelling evidence is available for EHDPP as an AhR activator, the presence of other AhR activators that covary with EHDPP is somewhat more likely. Previous studies have shown that levels of OPEs and other OFRs can correlate in air due to their historical use in the same formulations (Salamova et al. 2014). In addition, some OPEs that were not targeted in this study, including mono-substituted isopropyl triaryl phosphate (mITP), have been shown to have relatively strong AhR activity (Gerlach et al. 2014; Haggard et al. 2017).
Predicted BaP equivalents from chemical analysis
The BaP-Eqchem of each polyethylene passive sampler extract was calculated based on concentrations of targeted PAHs from GC–MS analysis. No dataset for the specific cell line we used was available, so IEFs were taken from Machala et al. (2001), who measured PAH-induced AhR-mediated response in a rat hepatoma H4IIE cell line stably transfected with luciferase reporter gene. The IEFs were not available for all PAHs, so calculated BaP-Eqchem values are representative of only 14 compounds (Supplemental Data, Table S8). Although the dataset from Machala et al. (2001) is the most applicable that could be found, these IEFs come from a cell line with a completely different time-dependent expression profile and are not directly applicable to the cell line we used. This contributes greatly to the uncertainty in the derived BaP-Eqchem values, and highlights the need for more studies providing cell–line-specific IEFs for a wide range of ubiquitous environmental contaminants.
The BaP-Eqchem values calculated using potencies from Machala et al. ranged from 1.6 to 7.9 ng/µL BaP, as shown in Table 3. The percentage of BaP-Eqbio accounted for by this BaP-Eqchem is also displayed. The percentages of contributions of individual PAHs to the total predicted BaP-Eqchem are displayed in Figure 4. Among the targeted PAHs, contributions to BaP-Eqchem were dominated by high-molecular-weight PAHs that were present at low concentrations in the polyethylene passive sampler extracts, including dibenz(a,h)anthracene, indeno(1,2,3-c,d)pyrene, benzo(b/k)fluoranthene, and chrysene.
| Sample | BaP-Eqbio | BaP-Eqchem | %BaP-Eqchem BaP-Eqbio |
|---|---|---|---|
| Cleveland Lakefront 1 | 64 | 2.9 | 4 |
| Cleveland Lakefront 2 | 75 | 3.0 | 6 |
| Cleveland Downtown 1 | 283 | 6.0 | 2 |
| Cleveland Downtown 2 | 89 | 6.1 | 7 |
| Cleveland Downtown 3 | 129 | 5.8 | 2 |
| University Heights | 89 | 4.7 | 3 |
| Fairport Harbor Lakefront | 35 | 2.5 | 23 |
| Kent | 54 | 7.9 | 11 |
| Cuyahoga National Park | 21 | 1.6 | 7 |
- BaP-Eqbio = benzo[a]pyrene equivalents (ng/µL) based on bioassay dose–response curve; BaP-Eqchem = benzo[a]pyrene equivalents (ng/µL) estimated based on chemical analysis.
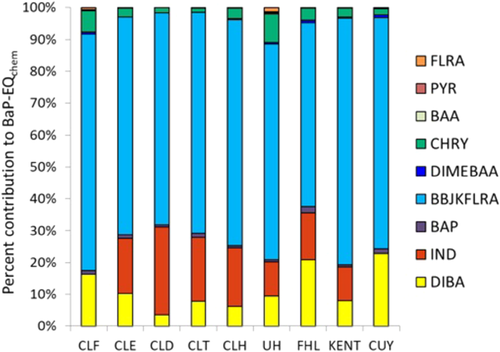
Potencies calculated from known chemical composition using IEFs explained only 2 to 23% of the AhR-mediated potency observed in bioassay experiments (Table 3), and BaP-Eqchem and BaP-Eqbio were not significantly correlated. This finding suggests that other compound groups present in the gaseous fraction of ambient air may also be contributing to BaP-Eqbio of the extracts. These may include additional parent PAHs and alkyl-PAHs not measured in the present study, as well as oxygenated PAHs and N- and S-heterocyclic PAHs (Larsson et al. 2014; Sun et al. 2014; Lam et al. 2018). Compounds other than PAHs may also be responsible for some of the observed AhR-mediated potency. The use of BaP-Eqchem values derived from a different bioassay may also contribute to this discrepancy.
The correlation observed between concentrations of EHDPP and AhR activity suggests that this compound, or other OFRs with a similar source, may be contributing to BaP-Eqbio as well, although further research is needed to understand the AhR-mediated potency of OFRs. Furthermore, a major weakness of predicting potency based on compound IEFs is that it considers only additive interactions, without taking into account synergistic and antagonistic effects, which are highly probable in complex environmental mixtures. This factor, along with the scarcity of IEF values for the targeted compounds, most likely contributed to the discrepancy between observed and predicted AhR-mediated potency.
CONCLUSIONS
The present study demonstrated the use of polyethylene passive samplers coupled with in vitro bioassays as an approach to measure cumulative biological effects of ambient gaseous air pollution. Although some AhR-mediated activity was seen in the sampler blank, the activity of field samples was found to be significantly elevated above blank levels, suggesting that interference from the sampler matrix or typical laboratory contamination did not prohibit the use of polyethylene passive sampler extracts in bioassays for AhR activation. In future studies using this approach, a thinner polyethylene passive sampler sheet (∼50 μm) may be preferable to avoid the extra cleanup steps caused by sampler precipitate in the final extract, because thinner samplers contain less polyethylene passive sampler mass and require less time for extraction. In addition, future work employing effect-directed analysis, as has been used in passive sampling studies of wastewater (Sonavane et al. 2018), could aid in identifying contaminants driving observed biological effects.
The AhR-mediated potency varied significantly between different sites and was greatest in downtown Cleveland. Potency of the extracts displayed some correlation with PAHs common in the gaseous phase, as well as EHDPP, although causative links were difficult to establish. The present study highlights the importance of learning more about the AhR-mediated potency of emerging contaminants that are present at elevated concentrations in urban ambient air, including OPEs and other OFRs. Our results further support those of previous studies suggesting that the BaP-Eqchem approach underestimates risks of exposure to environmentally relevant chemical mixtures, because AhR activation caused by organic contaminants in a mixture may be augmented by other unmonitored chemicals in the mixture and their unforeseen interactions.
Supplemental Data
The Supplemental Data, including a map of study locations, list of all target analytes, summary of concentrations in dosing solutions for all analytes, positive control dose–response curve, and correlation analyses between compounds, are available on the Wiley Online Library at DOI: 10.1002/etc.4361.
Acknowledgment
We acknowledge funding from the US Environmental Protection Agency Great Lakes Restoration Initiative (GLAS 00E00597-0, project officer T. Nettesheim). Funding was also provided by a National Institute of Environmental Health Sciences grant (P42ES007381; Superfund Basic Research Program at Boston University). We thank A. Slitt (University of Rhode Island) for consulting on experimental design and M. Denison (University of California Davis) for providing the H1G1 cells used in bioassays.
Data Accessibility
Data and calculation tools can be made available on request by contacting the corresponding author ([email protected]).




