Chemical and biochemical characterization and in vivo safety evaluation of pharmaceuticals in drinking water
Abstract
The water constituents that are currently subject to legal control are only a small fraction of the vast number of chemical substances and microorganisms that may occur in both the environment and water resources. The main objective of the present study was to study the health impact resulting from exposure to a mixture of pharmaceuticals that have been detected in tap water at low doses. Analyses of atenolol, caffeine, erythromycin, carbamazepine, and their metabolites in blood, urine, feces, fat tissue, liver, and kidney after exposure to a mixture of these pharmaceuticals in treated drinking water were performed. The effects of this exposure were assessed in rats by measuring biochemical markers of organ injury or dysfunction. Simultaneously, the selected pharmaceuticals were also quantified in both physiological fluids and organ homogenates by liquid chromatography–tandem mass spectrometry (performed in multiple reaction monitoring mode and full scan mode). Following exposure of rats to a concentration of a pharmaceutical which was 10 times higher than the concentration known to be present in tap water, trace levels of some pharmaceuticals and their metabolites were detected in biological samples. This exposure did, however, not lead to significant organ injury or dysfunction. Thus, the authors report an experimental model that can be used to characterize the safety profile of pharmaceuticals in treated drinking water using a multiorgan toxicity approach. Environ Toxicol Chem 2016;35:2674–2682. © 2016 SETAC
INTRODUCTION
Environmental sustainability, and in particular water conservation, is a globally growing and always present issue. Because of the importance of water in human health, national and international regulations and legislation controlling the quality of treated drinking water have been developed to minimize the risk to the consumer.
Despite the growing number of treated drinking water constituents that are currently subject to legal control, these represent only a small fraction of the vast number of chemical substances and microorganisms that may occur in the environment and ultimately in the drinking water. The development of analytical methods that allow the identification and quantification of increasingly lower concentrations of chemical compounds together with data regarding their biological effects has raised our awareness of emerging contaminants. Pharmaceutical compounds belong to this group of contaminants because they reach the aquatic environment through discharge of wastewater-treatment plants, onsite wastewater disposal systems or septic system discharges, and agricultural runoff or leaching 1-5. Because they have been designed to be biologically active often in nanomolar concentrations, their presence—even in these very small amounts—raises the possibility of potentially significant adverse effects after chronic exposure of both ecosystems and humans 6, 7. Thus, the evaluation of their safety profile following chronic exposure is timely and highly relevant in the current environment.
The aquatic environment is probably more susceptible to exposure to pharmaceuticals than drinking water, but the public is more focused on the risks to human health associated with the inadvertent exposure to such compounds via the consumption of contaminated drinking water 8, 9.
Although current risk assessments indicate that very low concentrations of pharmaceuticals in drinking water are very unlikely to pose any risks to human health, there are knowledge gaps in terms of assessing the risks associated with long-term, low-level exposures to pharmaceuticals and possible combined effects of chemical mixtures, including pharmaceuticals. The investigation of possible additive or synergistic effects of mixtures would be necessary for an accurate exposure assessment to determine whether there are any potential risks to human health, taking into account sensitive subpopulations 10.
The Empresa Portuguesa das Águas Livres is the largest drinking water supply company in Portugal and is responsible for the production and supply of treated drinking water to the city of Lisbon, as well as for the bulk supply to 25 municipalities of the greater Lisbon area, comprising approximately 2.6 million people. An intensive treated drinking water quality control program is performed by the Empresa Portuguesa das Águas Livres laboratory throughout the water supply system, from water sources (surface and groundwater) to consumers' tap water in the city of Lisbon. In collaboration with the Faculty of Pharmacy of the University of Lisbon, several occurrence studies of organic compounds in waters from Lisbon network have been performed 11-13. More recently our group studied the occurrence of pharmaceutical compounds in raw and treated drinking water by liquid chromatography–tandem mass spectrometry. Based on that study, 36 pharmaceutical compounds from different therapeutic classes were monitored in the Empresa Portuguesa das Águas Livres's water supply system 11. The sampling points included both raw and treated drinking water. Of the 36 compounds analyzed, carbamazepine, atenolol, propranolol, sulfadiazine, sulfamethazine, sulfapyridine, sulfamethoxazole, erythromycin, caffeine, acetaminophen, ibuprofen, naproxen, gemfibrozil, diclofenac, indomethacine, and nimesulide were quantified in the analyzed samples. The concentrations ranged from 0.03 ng/L to 46 ng/L in raw water samples and from 0.09 ng/L to 46 ng/L in treated drinking water samples 11, 14. However, only 4 pharmaceuticals had a statistically significant presence in the water samples analyzed: carbamazepine, atenolol, erythromycin, and caffeine. The biological effects of these contaminants in treated drinking water are not well known, and the use of animal models for this purpose is still very limited. Therefore, it is important to develop in vivo studies with the mixture of these 4 pharmaceuticals to evaluate the potential toxicity through water consumption.
Mice and rats are 2 of the primary mammalian species used for the evaluation of acute and chronic toxicity, metabolism, and bioavailability in preclinical evaluation of drugs and preregistration assessment of chemicals 15. Therefore, and based on our experience, we chose rats for our in vivo studies.
For the evaluation of the toxic effects of pharmaceuticals in treated drinking water we investigated the effects on a variety of potential targets of toxicity, providing information on the possible health hazards likely to arise from repeated exposure, through determination of the levels of the pharmaceuticals and their metabolites in blood, urine, feces, liver, fat tissue, and kidney of rats. At the same time biomarkers of organ injury and dysfunction were also determined.
MATERIALS AND METHODS
Animals and experimental protocol
Studies were carried out in Wistar rats (Harlan Ibérica), 4 wk old and weighing 60 g to 80 g. Rats received a standard diet and treated drinking water without any limitation or restriction and were cared for in accordance with the Institutional Animal Research Committee Guide for the Care and Use of Laboratory Animals 16, as well as with the European Commission regulations 17. The light to dark cycle was also monitored and established as 12 h each.
To test the effects of pharmaceuticals in treated drinking water, rats (n = 32) were randomly divided into 3 groups: 1) control group (n = 7), rats consumed treated drinking water (pretreated with charcoal to remove any organic compounds); 2) group 1 (n = 18), rats consumed treated drinking water spiked with a concentration of pharmaceuticals 10 times higher than the maximum concentration found in treated drinking water (atenolol 16 ng/L, caffeine 460 ng/L, erythromycin 50 ng/L, and carbamazepine 57 ng/L); and 3) group 2 (n = 7), rats consumed treated drinking water spiked with a concentration of pharmaceuticals equal to the maximum concentration found in treated drinking water (atenolol 1.6 ng/L, caffeine 46 ng/L, erythromycin 5.0 ng/L, and carbamazepine 5.7 ng/L) 11. In all groups, the animals were housed 3 to 4 per cage. The animals were weighed weekly. Water consumption per cage was recorded daily. The present study was performed over 12 wk.
The treated drinking water used in all assays with these 3 animal groups was pretreated with charcoal to remove any organic compounds, which might change the fate and behavior of the target pharmaceuticals. The pretreatment with charcoal and the spiking of treated drinking water were performed daily, with the exception of weekends. Both waters were analyzed by solid-phase extraction ultrahigh-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) 11 to control the efficiency of charcoal (treated drinking water without target pharmaceuticals) and the concentration of pharmaceuticals in spiked and treated drinking waters. Therefore, the daily renewal of these 3 types of water in each animal cage allowed a continuous and consistent exposure.
The duration of exposure tried to mimic the normal period of the individual's exposure to a specific xenobiotic propagated through drinking water, that is, from the first months of life until the adult stage. The target compounds were administered via treated drinking water spiked with the 4 pharmaceuticals. This spiked water was administered orally on a daily basis over 3 mo with concentrations intended to mimic the normal and 10-fold concentrations of pharmaceutical compounds in waters.
The state of health or any signs of toxicity were observed closely. The major toxic effects in tissues and, specifically, effects on kidney, liver, and pancreas were evaluated through measurement of serum biochemical parameters.
Collection of 24-h feces and urine
In the last week of the present study, feces and urine were collected for 24 h, using an individual metabolic cage. There was a complete separation with no urine washover and no chance for urine to enter the feces tube, yielding untainted and reliable samples.
Quantification of organ dysfunction and injury
At the end of the present study, animals were anesthetized with pentobarbital sodium 60 mg/kg intraperitoneally (Eutasil; Sanofi Veterinária), and 2.5 mL of blood was collected by cardiac puncture into a serum SST™ gel and clot activator tube (Becton Dickinson). The blood sample was centrifuged (1000 g for 10 min at room temperature) to separate serum. Serum was analyzed within 24 h in a certified medicine laboratory (J. Chaves, Laboratório de Análises Clínicas, Miraflores, Algés, Portugal).
The following marker enzymes were measured in the serum as biochemical indicators of multiple organ injury or dysfunction: 1) liver injury was assessed by measuring the increase in serum levels of alanine aminotransferase (a specific marker for hepatic parenchymal injury), aspartate aminotransferase (a nonspecific marker for hepatic injury), and alkaline phosphatase (a hepatobiliary marker); 2) renal dysfunction was assessed by measuring the increases in serum levels of creatinine (an indicator of reduced glomerular filtration rate and, hence, renal failure), urea (an indicator of impaired excretory function of the kidney and/or increased catabolism), and lactate dehydrogenase (LDH, its concentrations were usually very high in acute renal failure of widely different pathogenesis); and 3) the increases in the serum levels of lipase, a specific indicator for the development of pancreatic injury, using an automatic analyzer (ADVIA 1200; Siemens).
Chemical analysis: Reagents and standard solutions
The standards of the 4 pharmaceuticals—atenolol, caffeine, erythromycin, and carbamazepine—were provided by Fluka and Sigma-Aldrich and were of the highest purity available (≥95%).
Sea sand–washed, thin-grain QP was provided by Panreac. Acetonitrile, ethyl acetate, and n-hexane proanalysis grade were purchased from Merck.
Other chemicals, such as methanol, ultrapure water, formic acid, and 0.2 μm cellulose nitrate membrane, were the same as those previously reported 11.
Sample preparation
To maintain the characteristics of sample collection, a slice of approximately 1.5 g of liver and the right kidney (approximately the same location in all animals) was used in all assays. The sample size of urine and feces from 24 h were animal-dependent. Therefore, the urine volume and feces weight were both recorded, and the results from pharmaceuticals and their metabolites were corrected on the basis of sample size.
The extraction methods were modified on the basis of the published reports for similar matrices (organs and biologic fluids) and resemblance to the target compounds. In this context, some published extraction methods 18-21 were adapted for the extraction of target compounds and their metabolites from the liver, kidney, fat tissue, blood, urine, and feces of rats.
The selection and mixture of solvents aimed to increase the polarity range of solvent extraction to maximize the extraction and recovery of target compounds and their metabolites. In blood samples, acetonitrile was used to precipitate proteins and hence minimize matrix interference. Preliminary investigations regarding solvent proportions were based on recovery of parent compounds in each matrix (>70%).
Table 1 summarizes the extraction conditions for the analysis of target compounds. After collection, all samples were stored at −20 °C until use.
| Matrix | Collection | Sample | Pretreatment | Extraction method |
|---|---|---|---|---|
| Urine | Samples (from 24 h) collected at the end of the study using a metabolic cage | 5 mL | Centrifugation at 4000 g for 10 min to remove residues | 10 mL of ethyl acetate: n-hexane 1:1 |
| Centrifugation at 14 000 g, 4 °C for 5 min | ||||
| Feces | 2.3 ± 0.48 g | Homogenization in equal parts with sea sand | 5 mL of ethyl acetate/centrifugation at 4000 g, 4 °C for 10 min | |
| Kidney | Right organ | 0.80 ± 0.30 g | Add 10 mL of ultrapure water | 5 mL of n-hexane/centrifugation at 4000 g, 4 °C for 10 min |
| Liver | Slice of organ | 1.5 ± 0.39 g | Mix for 6 min at high speed in an ultra-turrax | Mix both organic extracts |
| Fat tissue | — | 2.0 ± 0.68 g | Extract immediately after homogenization | |
| Blood | Whole blood | 1 mL | — | 475 µL acetonitrile + 5 mL n-hexane, vortexed for 5 min, and centrifuged at 14 000 g, 4 °C for 10 min |
| 5 mL ethyl acetate, vortexed for 5 min, and centrifuged at 14 000 g, 4 °C for 10 min | ||||
| Mix both organic extracts |
The organic extracts from all matrices were evaporated to dryness under nitrogen at 35 °C and redissolved in 500 μL of ultrapure water. The redissolved samples were filtered through a membrane filter (Millex 0.45 µm; Millipore), and the filtrate was transferred to high-performance liquid chromatography vials and analyzed by UPLC-MS/MS.
UPLC-MS/MS
Acquity UPLC-MS/MS (Waters) was applied to the study of the pharmaceuticals and their metabolites using multiple reaction monitoring mode (acquisition mode). This equipment was also used in the definition of sample chromatographic profiles using full scan acquisition mode.
The tandem mass spectrometer was operated with an electrospray ionization (ESI) source in positive and negative ionization modes. However, the best instrumental signs were obtained in positive ionization mode. The optimized and previously validated UPLC-ESI-MS/MS methods 11 were adapted. The main changes were related to the chromatographic gradient to achieve a better chromatographic resolution and to allow a well-defined chromatographic profile.
The chromatographic gradients of acidic and basic methods are shown in Table 2. The basic method was only used for the analysis of erythromycin.
| Acid method | Basic method | |||
|---|---|---|---|---|
| Flow: 0.3 mL/min | Flow: 0.5 mL/min | |||
| Time (min) | %A | %B | %A | %B |
| 0 | 95 | 5 | 95 | 5 |
| 5 | 70 | 30 | 70 | 30 |
| 8 | 50 | 50 | 50 | 50 |
| 10 | 30 | 70 | 30 | 70 |
| 12 | 10 | 90 | 10 | 90 |
| 15 | 95 | 5 | 95 | 5 |
A mixture of water–formic acid (acid method) and water–ammonium acetate (basic method) was used for mobile phase A, and methanol was used for mobile phase B, both in different proportions.
The flow rates were 0.03 mL/min and 0.05 mL/min for the acidic and basic methods, respectively. A sample volume of 20 µL was injected and the UPLC column was an Acquity BEH C18 (2.1 mm × 50 mm, 1.7 mm) from Waters. A Z-spray ESI was used for all analyses (Micromass). The MS/MS parameters (cone voltage and collision energy) and multiple reaction monitoring transitions for each target compound are listed in Table 3 11, 14, 18, 22. The ion source temperature was set at 150 °C with a capillary voltage of 0.5 kV.
| Pharmaceuticals and metabolites | Ionization mode | Cone voltage (V) | Transition multiple reaction monitoring | Collision energy (eV) | Limit of quantification (µg/L) | |
|---|---|---|---|---|---|---|
| Atenolol | + | 35 | MRM1 | 267.2 → 190.1 | 25 | 1.0 |
| MRM2 | 267.2 → 145.2 | 20 | ||||
| Caffeine and metabolites | ||||||
| Caffeine | + | 30 | MRM1 | 195.1 → 138.2 | 20 | 0.69 |
| MRM2 | 195.1 →110.2 | 25 | ||||
| AAMU | + | MRM1 | 199.0 → 124.0 | 20 | – | |
| AFMU | + | MRM1 | 227.0 → 157.0 | 20 | – | |
| 1X | + | MRM1 | 167.0 → 110.0 | 20 | – | |
| 1U | + | MRM1 | 183.0 → 155 | 20 | – | |
| 17U | + | MRM1 | 197.0 → 140.0 | 15 | ||
| Carbamazepine and metabolites | ||||||
| CBZ | + | 35 | MRM1 | 237.2 → 194.1 | 20 | 0.05 |
| MRM2 | 237.2→192.2 | 20 | ||||
| 9-HM-10-Ca-O-Glu | + | MRM1 | 431.0 → 255.0 | 20 | – | |
| CBZ-N-Glu | + | MRM1 | 413.0 → 220.0 | 20 | – | |
| MRM2 | 413.0 → 237.0 | 20 | – | |||
| 10,11-DHD-CBZ | + | MRM1 | 271.0 → 210.0 | 20 | – | |
| MRM2 | 271.0 → 253.0 | 20 | – | |||
| CBZ-E | + | MRM1 | 253.0 → 180.0 | 20 | – | |
| MRM2 | 253.0 → 210.0 | 20 | – | |||
| Erythromycin | + | 30 | MRM1 | 716.0 → 158.1 | 30 | 0.53 |
| MRM2 | 716.0 → 558.5 | 15 | ||||
- AAMU = 5-acetylamino-6-amino-3-methyluracil; AFMU = 5-acetylamino-6-formylamino-3-methyluracil; 1X = 1-methylxanthine; 1U = 1-methyluric acid; 17U = 1,7-dimethyluric acid; CBZ = carbamazepine; 9-HM-10-Ca-O-Glu = 9-hydroxymethyl-10-carbamoyl acridan O-glucuronide; CBZ-N-Glu = N-glucuronide of carbamazepine; 10,11-DHD-CBZ = trans-10,11-dihydrodiol-carbamazepine; CBZ-E = carbamazepine-10,11-epoxide; MRM = multiple reaction monitoring.
The peak areas of the parent compounds and their metabolites in all biological samples were compared, as were the profiles of full scans of these samples within the group and between rat groups.
Quality of LC-MS/MS analysis
As one of the objectives of the present study was the analysis of chromatographic profiles of various biological samples, the recovery studies in various matrices were only performed with the parent compounds (carbamazepine, atenolol, erythromycin, and caffeine). Therefore, the distribution of the parent compounds and their metabolites in the different biologic matrices was performed based on the areas of the chromatographic peaks of target compounds on these matrices.
During the present study, other studies with rats were performed in our laboratory. Studies in progress do not always require all the organs and biological fluids of animals. For this reason, various biological samples were reserved for recovery.
Analyte recovery was determined by spiking 1.5 g of organs (liver, kidney), 2 g of fat tissue, and 2 g of feces with a mixture of the 4 parent compounds at 2 concentration levels: 1) low level, 50 pg/g, 30 pg/g, 3 pg/g, and 30 pg/g of atenolol, caffeine, carbamazepine, and erythromycin, respectively, and 2) high level, 150 pg/g, 100 pg/g, 8 pg/g, and 80 pg/g of atenolol, caffeine, carbamazepine, and erythromycin, respectively. Those lower spiking concentrations represent 3 times higher than the instrumental limit of quantification of each parent compound. The spiked samples were homogenized and extracted.
Recoveries were also determined in 5 mL of urine and 1 mL of blood spiked at 2 concentration levels: 1) low level, 150 ng/L, 100 ng/L, 8 ng/L, and 80 ng/L of atenolol, caffeine, carbamazepine, and erythromycin, respectively, and 2) high level, 500 ng/L, 300 ng/L, 25 ng/L, and 260 ng/L of atenolol, caffeine, carbamazepine, and erythromycin, respectively.
The minimum quantification level of the global method (extraction and UPLC-MS/MS) was calculated after analysis of the different biological samples spiked at the lowest concentration level. The minimum quantification level was determined on the basis of the concentration of spiked sample (CSs) of each target compound, its recovery (Rec) and the concentration factor (CF) of the extraction method according to the following formula: minimum quantification level = (CSs × 100)/(Rec × CF).
Statistical evaluation
All data from in vivo studies are presented as mean ± standard error of the mean of n observations, where n represents the number of animals or blood samples studied. Data without repeated measurements (multiple organ injury and/or failure) were analyzed by one-way analysis of variance, followed by a Dunnett's test for multiple comparisons using a GraphPad Prism statistical package (Ver 6.0). A p value of <0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
UPLC-ESI-MS/MS method: Recoveries and minimum quantification levels
The mean recoveries of the 4 target compounds (atenolol, carbamazepine, caffeine, and erythromycin) added to organs, fat tissue, urine, and feces at 2 spiking levels ranged from 70% to 86% (Table 4). There were no significant differences between the recoveries obtained for the analyzed biological matrices.
| Recovery (%) | Recovery (%) | Relative standard deviation (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target compounds | Spiking concentration (pg/g) | Liver | Kidney | Fat tissue | Feces | Spiking concentration (ng/L) | Blood | Urine | Liver | Kidney | Fat tissue | Feces | Blood | Urine |
| Atenolol | 50 | 79 | 76 | 70 | 80 | 150 | 72 | 78 | 10 | 9.8 | 12 | 11 | 10 | 9.9 |
| 170 | 85 | 82 | 80 | 79 | 500 | 79 | 82 | 9.5 | 9.7 | 11 | 10 | 9.6 | 9.5 | |
| Caffeine | 30 | 74 | 71 | 70 | 73 | 100 | 70 | 71 | 9.8 | 9.0 | 9.2 | 9.5 | 9.8 | 8.2 |
| 120 | 78 | 79 | 72 | 74 | 300 | 75 | 72 | 9.6 | 8.5 | 9.0 | 8.5 | 9.0 | 7.8 | |
| Carbamazepine | 3 | 80 | 85 | 76 | 78 | 8 | 79 | 86 | 5.7 | 6.5 | 6.7 | 6.8 | 7.0 | 5.4 |
| 10 | 77 | 79 | 74 | 79 | 25 | 74 | 78 | 5.1 | 4.8 | 4.9 | 6.7 | 3.5 | 4.2 | |
| Erythromycin | 30 | 75 | 76 | 70 | 77 | 80 | 75 | 80 | 5.3 | 5.8 | 11 | 7.6 | 9.3 | 6.4 |
| 90 | 80 | 78 | 76 | 79 | 260 | 80 | 82 | 5.7 | 6.2 | 9.8 | 7.5 | 9.5 | 5.6 | |
The results of the precision study of the UPLC-MS/MS method using repeatability conditions had relative standard deviations between 5.3% and 12% for the lowest concentration level of the target compounds and between 3.5% and 11% for the highest concentration level. The results are presented in Table 4.
The minimum quantification level of the global method (extraction and UPLC-MS/MS) for each target compound in studied matrices was similar because of their similar recoveries (Table 5). As shown in Table 5, the minimum quantification level values were very different for each target compound, but they remained similar in the several biological matrices.
| Minimum quantification level (pg/g) | Minimum quantification level (ng/L) | |||||
|---|---|---|---|---|---|---|
| Target compounds | Liver | Kidney | Fat tissue | Feces | Blood | Urine |
| Atenolol | 21 | 22 | 24 | 21 | 69 | 64 |
| Caffeine | 16 | 16 | 16 | 16 | 49 | 49 |
| Carbamazepine | 1.0 | 1.0 | 1.1 | 1.1 | 3.2 | 2.9 |
| Erythromycin | 12 | 12 | 13 | 11 | 35 | 33 |
Parent compounds and metabolites in biological samples
The present study assumes that the metabolites of pharmaceuticals are the same in rats and in humans, although they may be present in different proportions. Thus, the research has focused on the analysis of the main metabolites of the target pharmaceuticals. This assumption was confirmed in the analysis of target pharmaceuticals and their metabolites in multiple reaction monitoring acquisition mode.
The presence of significant concentrations of other potential metabolites can only be assessed by comparing the chromatographic profiles of biological samples from control animals with animals of group 1 and group 2 obtained in the total ion chromatogram profile by the ESI-UPLC-MS/MS method in full scan acquisition mode.
The main pathway of carbamazepine metabolism in humans and rats involves the formation of the chemically stable carbamazepine-10,11-epoxide and its hydrolysis to trans-10,11-dihydrodiol-carbamazepine. The carbamazepine and its monohydroxylated metabolites suffer conjugation to give O-glucuronide metabolites, such as the N-glucuronide of carbamazepine. The other metabolite is 9-hydroxymethyl-10-carbamoyl acridan O-glucuronide. The O-glucuronide of 10,11-dihydro-10-hydroxy-carbamazepine has been found only as a minor product in uremic subjects 22, 23. The parent compound and these metabolites were analyzed in all samples according to the conditions described by Gaffney et al. 11 and Maggs et al. 22.
Erythromycin base is acid-sensitive and, for this reason, is inactivated by gastric acid, except if administered with a protective enteric coating. Animal studies indicate that erythromycin is well distributed in the body and that the tissue content (e.g., liver, spleen, kidneys, and lungs) is generally higher and remains longer than in serum 24, 25. It is rapidly metabolized in the liver, mainly through a demethylation process, and excreted in the bile as des-N-methyl-erythromycin, the major metabolite, present only in the bile and in the intestinal contents of rats 26. Erythromycin was analyzed in all samples in multiple reaction monitoring acquisition mode 11.
In humans, approximately 50% of an oral dose of atenolol is absorbed from the gastrointestinal tract, the remainder being excreted unchanged in the feces. Atenolol undergoes little or no metabolism by the liver, and the absorbed portion is eliminated primarily by the kidney. Thus, only the parent compound was analyzed in all samples in multiple reaction monitoring acquisition mode 11, 27.
Caffeine is absorbed in the small intestine, metabolized in the liver cell, and distributed to body tissues within a few minutes of ingestion. It is distributed in proportion to tissue water and enters and leaves various organs at different rates. Caffeine breaks down into the following components: paraxanthine (84%), theobromine (12%), and theophylline (4%). Paraxanthine, the most representative metabolite, has the effect of increasing lipolysis (breakdown of lipids), leading to elevated glycerol and free fatty acid levels in the blood plasma. As well as paraxanthine, the major metabolites of caffeine in urine are 1-methylxanthine, 1-methyluric acid, 5-acetylamino-6-formylamino-3-methyluracil, 5-acetylamino-6-amino-3-methyluracil, and 1,7-dimethyluric acid, which are formed by the secondary metabolism of paraxanthine. Caffeine itself is a minor excretion product, accounting for 1% to 2% of the exposure dose 18, 28, 29. The undissociated form of the molecule, which is soluble in the lipoidal gastric membrane, is the form in which caffeine is absorbed and uniformly distributed to tissues. The plasma protein binding for caffeine is approximately 25% to 30%, and the remaining caffeine with a high lipid solubility and low protein binding can penetrate in several tissues rapidly 30.
Full scan
Supplemental Data, Figures S1 through S6, show the total ion chromatogram profile of a representative sample (urine, feces, liver, kidney, fat tissue, and blood) from each animal group (control group, group 1, and group 2) by the UPLC-ESI-MS/MS method in full scan acquisition mode.
The total ion chromatogram peak profile of each biological sample from each animal within a group and between groups (control group, group 1, and group 2) is very similar. The main chromatographic peaks are similar in all total ion chromatograms from the same sample (urine, feces, liver, kidney, fat tissue, and blood) in terms of retention time. In each biological sample, the total ion chromatogram obtained for the group 1 samples did not show more chromatographic peaks or peaks with a higher area when compared with the total ion chromatograms obtained from control group and group 2 samples.
These results suggest that the metabolism in animals was not changed by the fortified water intake at concentrations of pharmaceuticals up to 10 times the concentration found for this type of compound in drinking water (group 1); thus, the type and concentration of metabolites were not significantly altered. Therefore, the profiles of the various biological matrices remained unchanged. If the chromatographic profile between groups of 1 specific biological sample had showed significant differences because of the presence of 1 or more extra peaks, this could be related to a metabolic change in the rats caused by the chronic exposure to a large amount of pharmaceuticals. These changes could be more or less significant depending on the synergetic effects of the tested pharmaceuticals.
Multiple reaction monitoring mode
The parent compounds and the metabolites of the target compounds were analyzed by UPLC-ESI-MS/MS in multiple reaction monitoring mode. The peak areas obtained in each biological sample are shown in Figure 1.
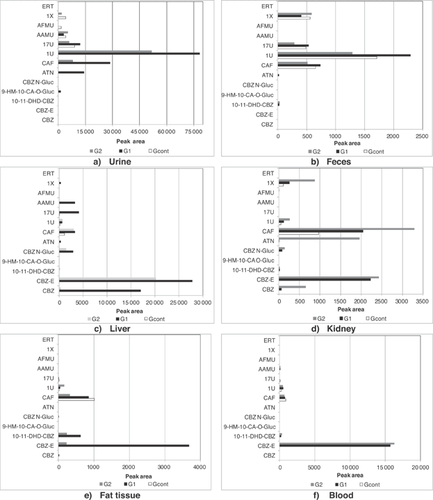
Chronic exposure to the 4 target compounds differed because the occurrence of these compounds in the treated drinking water was also different. Therefore, it is expected that the distribution profile correlates to the exposure pattern or, in other words, that a higher concentration in the treated drinking water will lead to a greater concentration in organs and biological fluids (concentration profile: group 1 > group 2 > control group). However, this distribution is also affected by the physicochemical properties of target compounds, such as lipophilicity and hydrophilicity, but also by the physicochemical properties of organs and biological fluids (for example, fat and water content). Last but certainly not least, the distribution of the target compounds (and their metabolites) is inherent to their pharmacokinetics.
Polar and hydrophilic compounds, such as atenolol and the metabolites of caffeine, exhibit a preferential distribution for the urine and feces matrices (high water content) as a result of their high polarity. Therefore, the distribution profile of both biological fluids was quite similar.
In the urine and feces analysis, no statistically significant differences were observed within each group of rats. The main parent compounds found in these matrices were atenolol and caffeine. However, the areas of these target compounds in feces were lower than those obtained in urine (40 times smaller). While caffeine was observed in all groups studied, atenolol was found only in group 1 (Figure 1). In all animals, the main metabolites found in urine and feces were those of caffeine, but the concentrations of these metabolites were higher in group 1 as a result of the higher concentration of this compound in spiked drinking water. In both matrices 1-methyluric acid was the main caffeine metabolite.
The liver is the principal site of drug metabolism; overall, metabolic processes will convert the drug into a more water-soluble compound by increasing its polarity. This is an essential step before the drug can be excreted in the body fluids such as urine or bile. However, no statistically significant differences were observed within each group of rats. The liver showed 3 of the 4 parent compounds, carbamazepine, atenolol, and caffeine. The main parent compound found in liver was carbamazepine (Figure 1). In all animals under study, the major number of metabolites found in liver belonged to caffeine, but the more representative metabolite was carbamazepine-10,11-epoxide. From caffeine, 1,7-dimethyluric acid and 5-acetylamino-6-amino-3-methyluracil were the most representative metabolites found in liver.
In the kidney, no statistically significant differences were observed within each group of rats. The parent compounds found in kidney were caffeine, atenolol, and carbamazepine (Figure 1); however, these parent compounds and their metabolites were higher in group 2 rats (lowest spiked level). The metabolites of carbamazepine were also found in kidney, namely carbamazepine-10,11-epoxide and its O-glucuronide metabolites. The main caffeine metabolites in kidney were 1-methylxanthine and 1-methyluric acid.
In fatty tissue, no statistically significant differences were observed within each group of rats. Caffeine was the only parent compound found in fat tissue (Figure 1). The main metabolites belonged to carbamazepine (carbamazepine-10,11-epoxide and trans-10,11-dihydrodiol-carbamazepine). Carbamazepine-10,11-epoxide was the main metabolite found in fat tissue.
In blood, no statistically significant differences were observed within each group of rats. As observed in fatty tissue, caffeine was the only parent compound found in blood (Figure 1). The distribution of caffeine in all animals of the 3 groups under study was quite similar. However, the main metabolites in each group were quite different. The main metabolites belonged to carbamazepine (carbamazepine-10,11-epoxide and trans-10,11-dihydrodiol-carbamazepine), with carbamazepine-10,11-epoxide being the main metabolite in blood. The 1-methyluric acid metabolite was found in all groups, but it was higher in group 1. The 1,7-dimethyluric acid and 5-acetylamino-6-amino-3-methyluracil metabolite was found only in group 1 and group 2 but in small proportions.
The carbamazepine-10,11-epoxide proportion in blood was quite similar in group 1 and group 2; however, it was higher in group 2. The area of trans-10,11-dihydrodiol-carbamazepine was 9 times lower than the area of carbamazepine-10,11-epoxide for both groups (group 1 and group 2). The concentrations of carbamazepine in blood were generally much lower than those in the liver, kidney, fat tissue, and biologic fluids. This suggests that carbamazepine is incorporated into organ tissues and retained there in its unchanged form.
Analysis of the compounds and their metabolites in the different organs and biological fluids confirmed that the animals ingested and metabolized these compounds, although by different metabolic processes, because no metabolites were present in the water samples that were freshly prepared on a daily basis and the bottles were covered with aluminum foil.
As expected, the proportion of different metabolites was different in the various biological matrices. These results also allowed us to evaluate which compounds underwent further elimination and which ones accumulated in target organs. Caffeine and its metabolites were found in both urine and feces, but carbamazepine and its metabolites were more commonly found in liver, kidney, and fatty tissue. However, only caffeine and 2 metabolites of carbamazepine (carbamazepine-10,11-epoxide and trans-10,11-dihydrodiol-carbamazepine) accumulated significantly in fatty tissue.
Erythromycin was not found in any of the biological samples tested in the present study. This may be because of its inactivation in the rat stomach (gastric acid) 24, 25, because of its instability in spiked water, or secondary to the low concentrations of metabolites (lower than the limit of detection).
As a polar, poorly metabolized compound, atenolol is almost exclusively excreted in unchanged form into urine. Therefore, there is no risk of atenolol accumulation in organs.
The present data, in our opinion, are a significant step forward in the characterization of the main metabolites of some key pharmaceutical compounds and their accumulation in main organs/tissues as a result of chronic exposure to these target compounds at low concentrations in treated drinking water.
Biochemical markers of organ injury and/or dysfunction
When compared with the control group, groups treated with a 1-fold (group 2) and a 10-fold (group 1) concentration of pharmaceuticals in drinking water showed no significant rises in the plasma levels of urea and creatinine (renal dysfunction), aspartate aminotransferase (very nonspecific, similar to LDH), alanine aminotransferase and alkaline phosphatase (liver injury), lipase (pancreatic injury), or LDH (nonspecific organ injury; Figure 2).
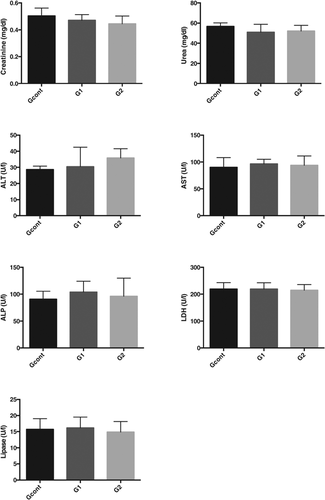
Despite the presence of the pharmaceuticals and their metabolites in biological samples, in vivo results showed that, at least under our experimental conditions, exposure of rats to a 10-fold concentration of pharmaceuticals observed in treated drinking water did not induce any significant changes in the serum levels of key biochemical markers of renal dysfunction or liver and pancreatic injury. These results can give important information relating to the risk associated with the use of treated drinking water containing these specific characteristics in terms of pharmaceutical contaminants.
Both results (chemical and biochemical analyses) suggested that appreciable adverse health impacts to humans are very unlikely from exposure to trace concentrations of these target pharmaceuticals (atenolol, caffeine, erythromycin, and carbamazepine) that might potentially be found in treated drinking water.
These data are consistent with our previous study on occurrence of pharmaceuticals in a treated drinking water supply system and related human health risk assessment 14, which is in accordance with the conclusions of the World Health Organization 10 and other human health risk-assessment studies that have been performed 31, 32.
CONCLUSIONS
A fast and sensitive chromatographic tandem mass spectrometric method (UPLC-ESI-MS/MS) for measuring 4 pharmaceutical compounds (carbamazepine, atenolol, caffeine, and erythromycin) and their major metabolites was applied in biological samples (urine, feces, liver, kidney, fat tissue, and blood) from rats after exposure to different concentrations of these target compounds over 3 mo. Although trace levels of some target pharmaceutical compounds and their metabolites were detected in biological samples, risk to rats' health because of this route of exposure was not observed based on biochemical parameter data representative of toxicity.
Because of the likelihood of increase of use or consumption of several pharmaceuticals (including those tested in the present study) in the future, other studies with other mixtures should be performed to enhance our understanding of the potential chronic (adverse) effects arising from the presence of trace levels of these compounds in treated drinking water.
The present results are therefore especially important as they represent the ranges of concentrations that can normally be present in a water supply system and the worst-case scenario for waters with concentrations of pharmaceutical compounds 10 times higher than the concentrations found in the occurrence studies for such waters.
We propose, for the first time, an in vivo model that can be useful in the characterization of the risk arising from exposure to mixtures of pharmaceutical compounds by treated drinking water.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3451.
Acknowledgment
We thank Fundação para a Ciência e a Tecnologia (Portugal) for funding the present study through a BD fellowship (SFRH/BDE/30804/2009) and Infarmed for providing the consumption data.
Data Availability
The data are available on request from the authors ([email protected]). Data resulted from a collaboration between a university (Universidade de Lisboa, Faculdade de Farmácia) and an enterprise (Empresa Portuguesa das Águas Livres). Some of the data belong to the Empresa Portuguesa das Águas Livres.




