Tissue explant coculture model of the hypothalamic–pituitary–gonadal–liver axis of the fathead minnow (Pimephales promelas) as a predictive tool for endocrine disruption
Abstract
Endocrine-disrupting compounds (EDCs) can impact the reproductive system by interfering with the hypothalamic–pituitary–gonadal (HPG) axis. Although in vitro testing methods have been developed to screen chemicals for endocrine disruption, extrapolation of in vitro responses to in vivo action shows inconsistent accuracy. The authors describe a tissue coculture of the fathead minnow (Pimephales promelas) HPG axis and liver (HPG-L) as a tissue explant model that mimics in vivo results. Brain (hypothalamus), pituitary, gonad, and liver tissue explants from adult fish were examined for function both individually and in coculture to determine combinations and conditions that could replicate in vivo behavior. Only cocultures had the ability to respond to an EDC, trenbolone, similarly to in vivo studies, based on estradiol, testosterone, and vitellogenin production trends, where lower exposure doses suppressed hormone production but higher doses increased production, resulting in distinctive U-shaped curves. These data suggest that a coculture system with all components of the HPG-L axis can be used as a link between in vitro and in vivo studies to predict endocrine system disruption in whole organisms. This tissue-based HPG-L system acts as a flexible deconstructed version of the in vivo system for better control and examination of the minute changes in system operation and response on EDC exposure with options to isolate, interrogate, and recombine desired components. Environ Toxicol Chem 2016;35:2530–2541. Published 2016 Wiley Periodicals Inc. on behalf of SETAC. This article is a US Government work and, as such, is in the public domain in the United States of America.
INTRODUCTION
Endocrine-disrupting chemicals (EDCs) pose health risks to humans and wildlife populations, potentially leading to impaired reproduction, endocrine-related disease, and shifts in sex ratios 1-3. Assays that accurately evaluate and quantify the effects and mechanisms of EDC activity are important in understanding the properties of known EDCs as well as screening chemicals with unknown endocrine activity 2. The US Environmental Protection Agency (USEPA) is emphasizing the need to uncover adverse outcome pathways that link molecular initiating events to organismal outcomes through the chain of biological events 4, 5. In vitro screening methods are actively under investigation for evaluating effects of estrogenic compounds through evaluation of estrogen receptor (ER) binding and activation 6-8. In vitro binding assays in cell lines have been developed for studying androgenic and antiandrogenic compounds as well 9, 10. In vitro gene expression of androgen receptors (ARs), ERs, and other components of the hypothalamic–pituitary–gonadal–liver (HPG-L) axis, including vitellogenin (Vtg), has been used to evaluate the effects of EDCs 2, 11-14. These in vitro techniques, although rapid and relevant using high test concentrations, are often not effective when testing environmentally relevant chemical concentrations 2. Further, most in vitro assays measure only a single endpoint of EDC activity on the HPG-L axis and do not provide the complete picture of how a chemical will affect an organism's reproduction.
The HPG axis is responsible for proper development and function of the reproductive organs and controlling the behaviors of reproduction 15. It is a complex interaction of multiple tissues that respond to each other, resulting in dynamic feedback loops 16, 17. A disadvantage of traditional single-cell cultures is that their responses do not reflect the interaction and feedback among these multiple tissues.
In vivo studies are useful for seeing the organismal effects of a chemical 2. However, these studies do not always allow for a mechanistic understanding of a chemical's action. In addition, in vivo studies are laborious and costly, making it impractical to screen every chemical and combination 18. Screening methods that are both sensitive and accurate to organism effects and mechanisms of action are necessary to better screen for EDCs and understand the system biology.
It is desirable to develop and use assays that can be extrapolated across multiple species 19. It is important that the systems studied are conserved between the model species and any species of concern, including humans, such that the information is useful in developing and regulating synthetic chemicals 20. In this regard, the HPG axis is highly conserved across vertebrates, and research on 1 species can often be applied across numerous species 19, 21.
In addition to species extrapolation, assays need to be relevant to ecological impact 4, 22. A link from detailed mechanistic understanding to broader impacts on individuals, populations, and ecosystems should be made such that an event seen at the molecular level can infer outcomes through an adverse outcome pathway. With these caveats in mind, the fathead minnow (Pimephales promelas) is an ideal model organism for teleost toxicology. Studies using this species have been both extrapolated to other organisms and linked to ecosystem-level impact, making it an excellent study species for EDCs 19, 23.
Androgens are important hormones required for the proper development of the reproductive tract and reproductive function in vertebrates 24. The major androgens in teleosts are testosterone (T) and 11-ketotestosterone (11-KT) 25. Endocrine-disrupting chemical binding to the AR can result in androgenic or antiandrogenic responses, resulting in endocrine disruption 24-26. Synthetic chemicals, including androgens and antiandrogens, are abundant worldwide in surface waters, making fish particularly susceptible to their effects 27. One source of androgenic EDCs is feedlot and agricultural runoff that contains excreted trenbolone metabolites 28. Trenbolone acetate (TRB) is an anabolic steroid used for beef cattle growth promotion. The metabolites of TRB bind to the fish AR with high affinity 26.
Evidence of endocrine disruption from TRB and its metabolites has been seen in fathead minnows 26, 29, channel catfish (Ictalurus punctatus) 30, European bullhead 3, Japanese medaka (Oryzias latipes) 31, mosquitofish (Gambusia sp.) 32, eelpout (Zoarces viviparus) 33, and zebrafish 34. Trenbolone-induced changes in androgen and estrogen ratios suggest interference in the HPG axis feedback loop 26. Modeling has shown that exposure to 17β-trenbolone can lead to drastic population declines after only 2 yr of exposure because of hormone interference that results in reduced fecundity 35.
The goal of the present study was to establish a tissue explant coculture system to study the HPG-L axis of fathead minnow and the effects of a known EDC, TRB, on this system. The value of these types of “body-on-a-chip” systems to simulate multitissue chemical interactions is well established, for instance, to determine drug efficacy and toxicity 36. Our aims for a tissue explant coculture system were 2-fold. First, we wished to verify that HPG-L single-tissue cultures continue to physiologically function in response to an appropriate stimulus from another tissue in the system. We tested whether the liver would produce Vtg when exposed to media from an ovary, whether the ovary would produce estradiol (E2) when exposed to media from a hypothalamus and pituitary compartment, and whether the testes would produce 11-KT when exposed to media from a hypothalamus and pituitary compartment.
After assessing the single-tissue culture responsiveness, the second aim was to challenge a combined HPG-L coculture system with TRB at concentrations that corresponded with those measured in fathead minnow tissue after an in vivo study. We compared the hormonal response of HPG-L coculture to the hormonal response trends of fathead minnow in vivo reported by Ankley et al. 26. The present study will describe and demonstrate the impact and interaction of all tissues of the HPG-L axis in an effort to replicate the feedback loops and hepatic metabolism from in vivo studies over these time periods.
MATERIALS AND METHODS
Organisms
We purchased juvenile fathead minnows from Aquatic BioSystems and reared them in 20-gallon tanks at room temperature with a 16:8-h light:dark cycle 37. Fish were separated at sexual maturity (≥5 mo) into tanks of males and females. All fish used in the present study were sexually mature as indicated by secondary sex characteristics and mature gonads.
Tissue collection and viability
Our goal was to collect fresh tissue from fathead minnows for study cultures and to maintain tissue viability throughout every culture period. All fish were anesthetized using an overdose of tricaine methanesulfonate (MS-222) purchased from Argent Chemical Laboratories. The MS-222 was buffered with NaHCO3. We used a scalpel to slice the caudal artery, allowing the fish to bleed out. We immediately removed and sliced brain, pituitary, liver, and gonads for specified studies detailed in this section using dissecting scissors, forceps, and razor blades. We isolated the forebrain, identified the hypothalamus at the bottom of the forebrain, and sliced coronally, ensuring that each slice included the hypothalamus. We took crosswise slices of the ovaries, testes, and liver. The pituitary was left intact for the present study and its viability considered equal to the brain. A single slice of each tissue was separated as a control for viability assessment. All other slices were kept in L-15 medium (Life Technologies) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin–amphotericin B. Cultures were incubated in a humidified chamber at 18 °C, as previously optimized 37.
Tissue viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, MTT being a tetrazolium salt that is reduced to formazan in active mitochondria. Tissues were incubated in 10 μL MTT per 100 μL medium for 4 h. We removed the tissues, solubilized the formazan in 200 μL methanol, and measured ultraviolet absorbance at a wavelength of 570 nm. We normalized absorbance data using tissue mass. A control slice from each organ was immediately assayed on removal to acquire a baseline measurement at time 0 of culture. We verified tissue viability over time by comparing experimental normalized absorbance values to the control normalized absorbance value on tissue slices from the same organ of the same fish. A viability of 100% meant that the absorbance reading per mass was the same for a cultured slice as that of a tissue slice from the same fish immediately on removal. This value of viability provided a measure of the metabolism of a cultured slice relative to that of a fresh slice, which is proportional to the number of living cells in the tissue sample. Our criterion was that every tissue has a viability above 80% to be included in further analyses and statistics.
Endogenous hormone stimulation
Tissue cultures with gonad stimulation studies
We wished to assess the ability of endocrine tissues to produce hormones over multiple days ex vivo and to study the real-time response of gonadal tissue to media exposed to upstream tissues. Ten ovaries and testes were each sliced into 5 approximately equal pieces: ovary slices were 10 mg to 15 mg each and testis slices were 1 mg to 3 mg each. An MTT assay was immediately performed on 1 slice of each tissue as day 0 control. We cultured the remaining 4 slices in 150 μL of medium in individual wells of a 96-well culture plate. The brain and pituitary from the same fish were cocultured (placed together in the same well). We incubated all tissues from a single fish on the same culture plate to ensure uniform handling and storage for all treatments. Multiple fish cultures were incubated on the same plate up to the plate capacity.
After 24 h of tissue culture (day 1), we removed the medium from all ovary and testis tissue wells and stored it separately at –80 °C, which is the storage temperature throughout the present study. This gave us a baseline of initial steroid production over 24 h. We replaced this medium with the same volume of fresh L-15. After 48 h (day 2), we removed the medium from the brain–pituitary wells and used this to stimulate sex steroid production in the gonads of the same fish. The gonad media were replaced with solutions composed of different ratios of the brain–pituitary culture media to fresh L-15 (brain–pituitary:L-15) as listed in Table 1. We replaced the control group medium entirely with fresh L-15. After 24 h of culture in brain–pituitary:L-15 medium (day 3), we collected and stored media from the ovary and testis wells. All media were again replaced in the gonad wells with fresh L-15. The same procedure was used to stimulate the gonad tissues for another 24-h time period with brain–pituitary:L-15 culture medium on day 9 to day 10 using brain–pituitary medium from day 9 (Table 2). Note that the first media exchange used brain–pituitary medium that had been cultured with brain and pituitary for 2 d (days 0–2), whereas the second media exchange used brain–pituitary medium that had been cultured for 7 d (days 2–9). All stored media from the ovaries and testes were analyzed for E2 and 11-KT, respectively, using fathead minnow enzyme immunoassay kits (Cayman Chemical). We ran the MTT assay at day 10 to test for gonadal tissue viability.
| Brain–pituitary culture media (μL) | L-15 (μL) | % Brain–pituitary | |
|---|---|---|---|
| Control | 0 | 150 | 0 |
| Treatment 1 | 25 | 125 | 17 |
| Treatment 2 | 37.5 | 112.5 | 25 |
| Treatment 3 | 75 | 75 | 50 |
| Day 0 | Day 1 | Day 2 | Day 3 | Day 9 | Day 10 | |
|---|---|---|---|---|---|---|
| Ovary and testis tissues and wells | Tissues removed from fish, cultured in separate wells in L-15 | Media removed and tested, replaced with fresh L-15 | Media replaced with a treatment from Table 1 | Media removed and tested, replaced with fresh L-15 | Media replaced with a treatment from Table 1 | Media removed, MTT assay |
| Brain–pituitary tissues and wells | Tissues removed from fish, cultured together in wells in L-15 | Media removed for ovary/testis challenge, replaced with fresh L-15 | Media removed for ovary/testis challenge, replaced with fresh L-15 | MTT assay |
- MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Tissue cultures with liver stimulation studies
We conducted this experiment to verify that the liver produced Vtg in response to media from all upstream tissues better than media from ovaries alone. Ovaries from 10 fish were each sliced into 4 approximately equal masses, 10 mg to 15 mg each; and 2 brain slices were used, 5 mg to 10 mg each. Livers were sliced into 6 equal pieces, 5 mg to 10 mg each. An MTT assay was performed immediately on 1 slice of each tissue. The remaining tissue slices were placed into plate wells in the combinations shown in Table 3. Three liver slices were each cultured individually in a 96-well plate (wells 1–3; Table 3). One ovary slice was cultured individually (well 4), and the remaining ovary slice was cocultured with the brain and pituitary (well 5). All wells 1 through 5 were filled with 150 μL of L-15.
| Well | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Liver | X | X | X | ||
| Ovary | X | X | |||
| Brain–pituitary | X | ||||
- a Liver slices were cultured individually in wells 1–3. Ovary was cultured individually in well 4. Ovary, brain, and pituitary were cocultured in well 5. X = the tissue was present in that well.
After 24 h of culture, media were removed from liver wells 1 through 3 for further use and replaced with fresh L-15. After an additional 24 h, all media was removed from the liver cultures and replaced with 50% medium from 1 coculture and 50% fresh L-15 as described in the treatments in Table 4. After 24 h, media were collected from all wells, stored at –80 °C, and replaced with fresh L-15 medium. This sequence was repeated again on day 10 (Table 5) using media that had been cultured with a tissue for 8 d (day 2 through day 10). Media from liver cultures were analyzed for Vtg at the end of the present study with an enzyme-linked immunosorbent assay (ELISA; Cayman Chemical). Medium was collected from the ovary cultures and cocultures and tested for E2. Tissues were subjected to the MTT assay at the end of the present study to ascertain viability.
| Coculture media (μL) | L-15 (μL) | |
|---|---|---|
| Control | 0 | 150 |
| Treatment 1 | 75 from well 4 | 75 |
| Treatment 2 | 75 from well 5 | 75 |
| Day 0 | Day 1 | Day 2 | Day 3 | Day 10 | Day 11 | |
|---|---|---|---|---|---|---|
| Wells 1–3 (liver) | Tissues removed from fish, cultured in L-15 | Media removed for analysis, replaced with fresh L-15 | Media replaced with treatment 1 or 2 from Table 4 | Media removed and replaced with fresh media | Media replaced with treatment 1 or 2 from Table 4 | Media removed, MTT assay |
| Well 4 (ovary) and well 5 (ovary, brain, and pituitary) | Tissues removed from fish, cultured in L-15 | Media removed and added to liver or saved for analysis, replaced with fresh L-15 | Media removed and added to liver or saved, replaced with fresh L-15 | Media removed, MTT assay |
- MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
TRB chemical challenge
Tissue culture and TRB challenge
Our goal was to challenge the HPG-L coculture with a known EDC and compare the resulting hormone production trend to that of previously reported in vivo hormone response trends as well as to the hormone production trend of individual gonad cultures. Trenbolone acetate (from USEPA Mid-Continent Ecology Division) was dissolved into a stock solution of phosphate-buffered saline by sonicating for 2 h as recommended by the Ankley laboratory (USEPA, Duluth, MN, personal communication), to make a stock solution of 32 μg/mL. This stock solution was added to L-15 medium in 3 different doses such that the resulting TRB concentrations in media were 0.006 μg/mL, 0.04 μg/mL, and 0.32 μg/mL. These doses were chosen based on tissue concentrations in fathead minnow after exposure to 3 different TRB solution concentrations in an in vivo study by Ankley et al. 26. The solution concentrations in that study were 0.5 μg/L, 5.0 μg/L, and 50 μg/L, which resulted in wet weight tissue concentrations of 6.58 μg/kg, 44.1 μg/kg, and 321 μg/kg 17β-trenbolone, respectively. To mimic the in vivo tissue concentrations of TRB found by Ankley et al. 26, we used a volume of 200 μL medium, which is slightly greater than the whole-fish blood volume but sufficient for the necessary assays.
Individual testis/ovary and tissue combinations were cultured in 200 μL control medium (0 μg/mL TRB) or TRB treatment medium in 96-well culture plates in a humidified chamber at 18 °C. Culture plates were placed on a rocking device within the incubator to promote movement of medium among tissues in the same well. Six individual gonad tissue slices and 6 cocultures containing brain, pituitary, liver, and gonad were established for each treatment and time point. Samples were collected at 24 h and 3 d. Culture media from the samples were evenly divided into 4 aliquots for separate hormone analyses and stored at –80 °C until analysis. Tissues were subjected to the MTT assay at the end of the present study to verify that all samples were alive throughout the culture period.
Hormone analyses
All media samples for both male and female fish tissues were analyzed for E2, T, and Vtg using E2 and T enzyme immunoassays and Vtg ELISA. In addition, males were analyzed for 11-KT, the major male androgen. Quality control was checked and verified with parallelism and recovery. The E2 enzyme immunoassay for ovary stimulation had 10.0% intra-assay variation, 18.8% interassay variation, and a detection limit of 24 pg/mL. The 11-KT enzyme immunoassay had an intra-assay variation of 14.2%, an interassay variation of 18.7%, and a detection limit of 0.18 pg/mL. The T enzyme immunoassay had an intra-assay variation of 10.4%, an interassay variation of 19.0%, and a detection limit of 1.9 pg/mL. The Vtg ELISA had an intra-assay variation of 7.7%, an interassay variation of 13.0%, and a detection limit of 0.39 ng/mL. The detection limits are sufficient to analyze the expected concentrations from production of even fractional tissues.
Statistical analysis
Viability values were used as a relative indicator of living cells in a tissue sample, and hormone concentrations were normalized by dividing by the viability value to provide a measure of hormone production per amount of living tissue. Although hormone production is frequently normalized by the tissue weight 38, these studies are performed in vivo and the entire tissue is relevant. Instead, the present studies must account for the very real possibility that not all the tissue mass is continuing to function properly. Based on the MTT assay, all tissues maintained viable cells (>80% of the day 0 control) throughout the specified study period except for 1 liver culture in the liver stimulation experiment. The medium from this sample was not included in the data analysis.
All statistical analyses were conducted using SYSTAT 13 (Systat Software) with statistical significance set at p < 0.05. All experiments were analyzed using analysis of variance (ANOVA) to compare means after verifying normality. When significant effects were detected within an experiment, post hoc one-way ANOVA followed by Tukey's least significant difference analyses were performed. Data are reported as mean ± standard error of the mean.
When concentrations of a hormone were below the limit of detection for an enzyme immunoassay or ELISA, the limit of detection was used for statistical tests for that sample. Means between cocultures and individual culture were not statistically compared with each other for the chemical challenge because the goal of the chemical challenge was to compare overall trends of coculture and gonads alone with previously reported in vivo trends.
RESULTS AND DISCUSSION
Endogenous hormone stimulation
Ovary stimulation
Media from wells containing 1 ovarian tissue slice were analyzed for the amount of E2 produced after 24-h exposure to brain–pituitary medium at 3 d and 10 d in culture. Estradiol concentration was highly variable among different samples, and therefore, the data were normalized to day 1 concentrations of E2 for the same ovary slice. Brain–pituitary culture medium did not significantly increase E2 production compared to control medium after 3 d (Figure 1A, dark bars; F3,64 = 3.075, p = 0.547). However, the ovary alone produced as much as 3 times more E2 than at day 1, and this elevated level likely masks any effects from added gonadotropins from the brain–pituitary medium. In addition, 2 d may not be sufficient time in culture for the brain–pituitary combination to produce an overabundance of gonadotropins to exceed the basal steroidogenesis already occurring in the ovarian tissues, especially at dilutions ≥50%. The E2 concentration did, however, increase significantly in ovaries exposed to 50% brain–pituitary culture medium after 10 d (white bars, F3,64 = 3.563, p = 0.019) compared to the control (p = 0.016) and 17% brain–pituitary culture medium addition (p = 0.015), indicating that E2 production increases with increasing brain–pituitary culture medium percentage, presumably because of an increased amount of gonadotropins. Although it is possible that the brain is producing some E2 itself, we were limited in our ability to measure E2 in the brain–pituitary media because of sample volume. Knowledge of the amount of E2 produced by the brain in this system would benefit future studies.
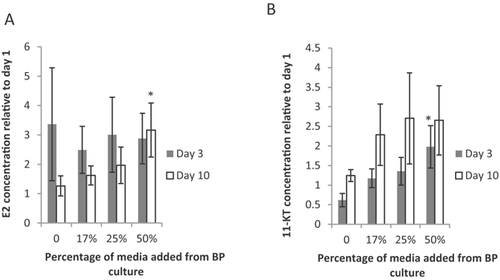
In a previous study, we exposed ovarian tissue to 5 IU pregnant mare serum gonadotropins (PMSG) for 24 h after 2 d in culture 37. Because of the conserved nature of the HPG axis among vertebrates, we expect both PMSG and brain–pituitary medium to contain functional luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which should stimulate gonad development and steroidogenesis. We suggest that follow-on research should strive for direct measurement of LH and FSH in these media to rule out the possibility that other signaling molecules are stimulating the ovary. Nevertheless, the present study's data indicate that the hypothalamus and pituitary function sufficiently after 10 d to produce enough gonadotropins (or other signaling molecules) to stimulate the ovaries, even when diluted by 50%. The production of E2 by 50% brain–pituitary medium is approximately half of the amount of E2 produced when ovaries were cultured with 5 IU PMSG 37. This suggests that undiluted brain–pituitary medium contains approximately 5 IU equivalents of gonadotropic activity of PMSG. Use of 100% brain–pituitary medium would likely produce a similar E2 response as 5 IU PMSG, but sample volume limitations precluded this test. Direct measurement of the gonadotropins FSH and LH in our culture was not performed because of the lack of a suitable fathead minnow ELISA kit for these hormones. However, with more sensitive assays, this system will be ideal for studying neuroendocrine changes. These data indicate that both the brain–pituitary tissue combination and the ovaries are functioning and respond to endogenous signaling after a 10-d period. Viability of these tissues was confirmed using the MTT assay (data not shown).
Testis stimulation
Media from wells containing 1 testicular tissue slice were analyzed for the amount of 11-KT produced after 24 h exposure to brain–pituitary medium after 3 d and 10 d in culture. Production of 11-KT was highly variable among samples and therefore normalized by the day 1 concentration of 11-KT for each testicular sample. There was a significant difference among control and treatment groups at 3 d at 50% brain–pituitary medium (Figure 1B, dark bars; F3,36 = 2.791, p = 0.05). The trend is an increased production of 11-KT by the testis tissue with an increase in percentage brain–pituitary culture medium. There was no significant difference in 11-KT concentration at day 10 among the control or treatment groups (white bars, F3,36 = 0.666, p = 0.578), although again there tended to be more 11-KT produced with an increase in brain–pituitary percentage.
The testis cultures produced more 11-KT (absolute) when stimulated by the 50% brain–pituitary medium compared to those stimulated by PMSG 37, suggesting that the brain–pituitary endogenous hormones elicit a response better than the exogenous PMSG in the testes. As with the ovary and female brain–pituitary culture, the induced sex steroid production indicates that the hypothalamus, pituitary, and testis are all continuing to produce the appropriate hormones for interorgan communication after 10 d. This finding strongly suggests that the system and all its components are both functional and responsive after 10 d in culture.
Liver stimulation
We analyzed Vtg in media from wells containing 1 liver slice exposed to control medium, ovary medium, and brain–pituitary and ovary medium at 3 d and 10 d in culture. We expected media from cultures containing all 3 tissues (hypothalamic, pituitary, and ovarian) to contain the most E2, which, when added to the liver tissue, would stimulate the liver to produce more Vtg than other treatments. These cultures contained all necessary components of the HPG axis and, therefore, were expected to elicit the most relevant response, whereas cultures lacking 1 or more components were expected to produce less E2 and a lower Vtg response. The increased Vtg concentration in liver culture was highly significant among brain–pituitary–ovary media treatments at both 3 d (Figure 2A; dark bars, F3,49 = 7.831, p ≤ 0.001) and 10 d (white bars, F3,49=4.78, p = 0.005). The Vtg concentration was higher in brain–pituitary–ovary coculture medium treatments compared to all other treatments (control, 3 d p = 0.003, 10 d p = 0.002; ovary, 3 d p = 0.001, 10 d p = 0.042), as predicted. There were no significant differences between control and ovary treatment.
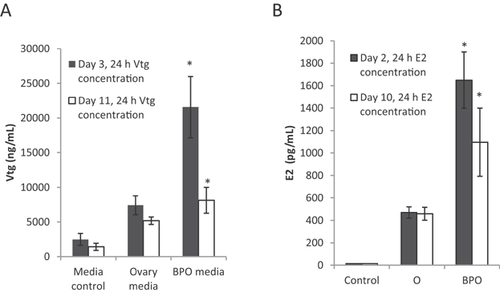
The increase in Vtg production corresponded with an increase in E2 present in the added media (Figure 2B). Unsurprisingly, E2 was not detectable above the lower detection limit in the L-15 medium control at either 3 d or 10 d. The E2 concentration in brain–pituitary–ovary medium was significantly higher than the control at both 3 d (F3,64 = 5.437, p = 0.002) and 10 d (F3,53 = 15.835, p ≤ 0.001) and significantly higher than ovary treatments at day 3 (p = 0.002). There were no significant differences in E2 concentration between control and ovary treatment groups. Estradiol concentrations followed the same trend on day 10, with the brain–pituitary–ovary medium containing a significantly higher concentration of E2 than the other treatments (control p ≤ 0.001, ovary p ≤ 0.001).
Hormone communication and response among the endocrine tissues were maintained in this fathead minnow HPG-L coculture system. The significant stimulation of the liver by brain–pituitary–ovary medium compared ovary alone medium and the increased concentration of E2 in the brain–pituitary–ovary medium compared to ovary alone medium demonstrate the need for additional components of the HPG axis for a properly functioning system, which includes the response of gonads to hypothalamic and pituitary secretions as well as the response of liver to E2. In the present study, we did not look directly at how inclusion of all tissues is necessary for hypothalamic and pituitary feedback response. We believe additional studies including changes in gonadotropin production would complement the present study. Because there are no currently reliable hormone assays to assess gonadotropin production in fish, we propose analyzing the tissue using quantitative polymerase chain reaction to monitor changes in gene regulation. The present study's results demonstrate that important system functions, such as E2 production in the ovaries, require the presence of upstream tissues to be more consistent with whole-fish outcomes. Considering that most in vitro techniques to assess endocrine disruption of steroid production use only 1 cell type, this finding shows that additional information is needed when using single-cell or single-tissue assays and that a single-tissue assay may not be reliable. This coculture system is a very important link between traditional in vitro data and relevant in vivo effects.
TRB chemical challenge
To evaluate the HPG-L coculture as an effective tool to predict endocrine disruption using the fathead minnow as a model, we exposed individual ovarian and testicular tissues to TRB and compared their sex steroid production to that of HPG-L cocultures exposed to TRB. Media formulations were based on using a single “fish mass” and adding TRB such that the concentrations were equivalent to those calculated from an in vivo study 26. We then compared the dose response of these “single fish replicate” cultures to the 21 d in vivo exposure study. Trenbolone did not interfere with any hormone enzyme immunoassay used at any TRB concentration (0.006 μg/mL, 0.04 μg/mL, and 0.32 μg/mL). Trenbolone at a concentration of 32.1 μg/mL does interfere with both the T and 11-KT enzyme immunoassays, suggesting that there is cross-reactivity with this compound at very high concentrations; but this is 100 times greater than any dose used in the present study. Cross-reactivity was calculated for the doses used by determining 2 standard deviations from the lower detection limit of each assay. The cross-reactivity for E2 was <0.007%, for T it was <1.3 × 10−5%, and for 11-KT it was <6 × 10−7%.
Female HPG-L coculture and ovary culture E2 production
Exposure of gonad tissue to TRB was expected to reduce steroidogenesis, mainly by acting as an androgen agonist and triggering the negative feedback loop in the coculture. We did not expect to see this system effect on the individual ovary. We found that exposure to environmentally relevant concentrations of TRB for 24 h caused a response in ovarian and coculture hormone concentrations. Trenbolone caused a significant increase in E2 production in both HPG-L cultures (Figure 3A, black bars; F3,20 = 16.732, p ≤ 0.001) and individual ovary tissue (white bars, F3,20 = 16.615, p ≤ 0.001) after 24 h in culture. We found no evidence in the literature for direct conversion of TRB to E2.
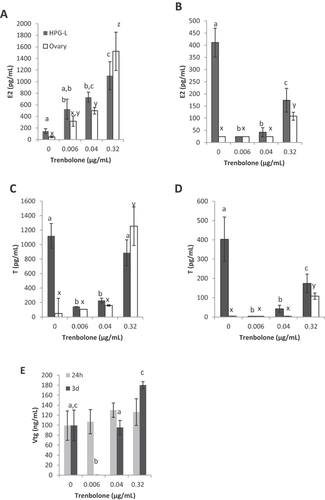
The ovary responded to the doses of TRB after 24 h in a similar way as the HPG-L culture. Both the 0.04-μg/mL dose (p = 0.057) and the 0.32-μg/mL dose (p ≤ 0.001) caused a significant increase in E2 concentration compared to the control. The E2 concentration in the 0.32-μg/mL dosed culture was significantly higher than that at both lower doses, 0.006 μg/mL (p ≤ 0.001) and 0.04 μg/mL (p ≤ 0.001). Based on the 24-h response of both the HPG-L and ovary cultures, we would expect TRB to cause a dose–response increase in E2 production. However, this was not observed in vivo 26.
The 3-d coculture of HPG-L resulted in a significant difference in E2 concentration when exposed to different TRB concentrations (Figure 3B, black bars; F3,20 = 24.560, p ≤ 0.001). Unlike the 24-h culture, all 3 TRB concentrations resulted in a decrease in E2 concentration after 3 d compared to the control (p ≤ 0.001 for all 3 doses). The decrease in E2 over this time period trended toward a U-shaped response in which the lowest dose saw the most dramatic decrease in E2 production, whereas the highest dose had lesser effects. The ovary cultures did not produce detectable amounts of E2 after 3 d in culture except for the 0.32-μg/mL dose. The amount of E2 produced by the 0.32-μg/mL dose was significantly higher than the control and other doses (Figure 3B, white bars; F3,20 = 31.045, p ≤ 0.001). Based on the ovarian data, we would predict that TRB acts as an estrogen inducer. However, on looking at the HPG-L data and in vivo research 26, we see a more complicated interaction of TRB on the reproductive endocrine system. One key difference between the ovary culture and HPG-L coculture is that E2 production continues after 3 d in the coculture control, whereas the ovary no longer produces E2 without external stimulation after 3 d. The remarkable difference in E2 production between ovary alone and the HPG-L coculture highlights the importance of including all relevant tissues when examining hormone production.
The E2 response of HPG-L culture after 3 d was most similar to females in the in vivo study (Table 6). In the in vivo study 26, the control plasma concentration of E2 was significantly higher than the 0.5 μg/L and 5.0 μg/L TRB doses but not significantly higher than the highest dose (50 μg/L TRB). In the present study, the 3-d HPG-L culture responded with the same U-shaped trend. This U-shaped curve is unusual for in vivo data, yet our coculture was able to replicate this result, whereas the single-ovary culture did not. Importantly, neither the HPG-L nor the ovary culture had the same U-shaped response as the in vivo study after only 24 h, primarily because the control E2 concentration was much lower. The difference in response that we note at 24 h versus 3 d is critical in demonstrating that the exposure time must be selected wisely to provide a complete picture of the effect of TRB on this system. The system clearly requires more than 24 h to respond to TRB and establish a stable feedback equilibrium, likely because of the long steroidogenic pathway to create E2 39. This was also seen by Ekman et al. 39 in a short-term exposure in which changes in ovary production of E2 were not seen until 48 h after exposure. The dose of TRB that corresponded to their high dose (our lowest dose) showed no change after 24 h in the HPG-L coculture but a decrease in E2 after 3 d, similar to a decrease they saw in E2 at that dose after 48 h. Our ovary culture did not follow this same trend after 3 d. Further, using only the ovarian tissue rather than the entire HPG-L axis gives an incomplete picture and an inaccurate representation of the possible TRB mode of action.
| Hormone | Female HPG-L 24 h | Ovary 24 h | Female HPG-L 3 d | Ovary 3 d | Male HPG-L 24 h | Testis 24 h | Male HPG-L 3 d | Testis 3 d |
|---|---|---|---|---|---|---|---|---|
| Estradiol | No | No | Yes | No | Yes | Yes | Yes | Yes |
| Testosterone | Yes | No | Yes | No | No | No | Yes | No |
| 11-KT | N.A. | N.A. | N.A. | N.A. | No | No | No | No |
| Vitellogenin | No | N.A. | No | N.A. | Yes | N.A. | Yes | N.A. |
- a “Yes” means that the general trend of hormone concentration follows the same trend as the in vivo study, and “No” means that the general trend of hormone concentration in response to trenbolone did not follow the trend of the in vivo study for at least 1 of the concentrations tested. Measurement of some hormones was not applicable to certain cultures because of sex or lack of hormone-producing tissue.
- 11-KT = 11-Ketotestosterone; HPG-L = hypothalamus–pituitary–gonad–liver coculture; N.A. = not applicable.
Female HPG-L coculture and ovary culture T production
Unlike the E2 response, T concentrations were substantial in the control HPG-L coculture over both 24 h and 3 d; however, T production ceased in the individual ovary control cultures. Even without exposure to TRB, we were able to see a more biologically relevant system using the coculture than was seen in an individual ovary because T production was maintained in the control HPG-L culture. Again, this reinforces the importance of including all HPG-L tissues in female endocrine disruption evaluations because of the important communication and responsiveness among tissues. As with the E2 production response, we expected the T production to reflect the in vivo androgen response of fathead minnow to TRB. After 24 h of exposure to TRB, the HPG-L concentration of T similarly decreased from control when exposed to 0.006 μg/mL and 0.04 μg/mL TRB (p ≤ 0.001), whereas there was no difference between the control and the high-dose 0.32 μg/mL TRB (p = 0.512), creating a U-shaped curve (Figure 3C).
Unlike the HPG-L culture, the 2 lower doses of TRB in the single-ovary culture did not alter the T concentration from the control value after 24 h (Figure 3C, white bars; 0.006 μg/mL p = 0.440, 0.04 μg/mL p = 0.629). The 0.032-μg/mL dose did differ from control and all other doses with a substantial increase in T concentration (p = 0.010).
Each treated HPG-L culture T concentration was significantly different from the control after 3 d in culture (Figure 3D, black bars; F3,17 = 17.524, p ≤ 0.001), again creating the U-shaped curve. Similar to the E2 concentrations, the T concentrations in individual ovary cultures were not detectable except at the highest dose (0.32 μg/mL TRB). The T value at this high dose was significantly higher than the control and both lower doses (Figure 3D, white bars; F3,19 = 44.329, p ≤ 0.001). We do not have an indication of why TRB increases T when looking solely at the individual ovary culture. The coculture demonstrates a more complicated interaction in which low doses appear to inhibit androgen production, likely through a negative-feedback response caused by the agonistic activity at the ARs in the brain and pituitary, whereas the highest dose is similar to the control HPG-L, where the negative feedback caused by androgen agonism does not dominate. This could be a result of dynamic transcriptions that are affected differently at low and high concentrations of TRB 39. The system may be compensating for reduced steroid hormones at the high dose, whereas the changes seen at the lower doses have yet to trigger the compensatory mechanisms seen at the high dose. Alternatively, the mechanisms of action of TRB might differ at high and low doses 26. The molecular responses of the tissues to TRB were, however, beyond the scope of the present study.
The female HPG-L culture closely resembles the T response in the in vivo study at both 3 d and 24 h, whereas the ovary alone did not respond in the same manner (Table 6). In the HPG-L culture at both time points and in the in vivo study, T decreased significantly at the lowest 0.006 μg/mL (0.5 μg/L) dose and then progressively increased with each increasing dose such that the high dose was not significantly different from the control. The T concentration trend in the single-ovary cultures at 3 d was very similar to the E2 trend. This is likely because E2 and T are derived in the same steroidogenic pathway, with E2 forming as a derivative of T. The single ovary was not able to replicate the nonmonotonic dose response seen in vivo. Therefore, research employing single-gonad cultures may miss or misinterpret important responses and trends of some EDC effects on steroidogenesis.
Female HPG-L coculture Vtg production
Because Vtg is produced in the liver and not by a gonad, we did not compare individual tissue cultures to coculture but rather looked only at the effects of TRB on coculture Vtg production. We expected to see a decrease in Vtg production with TRB exposure in the female HPG-L coculture, as was seen in vivo 26. The Vtg concentration was not different among control and treatments after 24 h (Figure 3E, gray bars; F3,20 = 0.576, p = 0.638); however, significant differences were observed after 3 d in culture (Figure 3E, black bars; F3,19 = 9.028, p = 0.001).
The female Vtg concentration response to TRB differed slightly between the in vivo study and the present study for the HPG-L 3 d culture (Table 6). The Vtg levels of female fish in vivo were consistently suppressed at all TRB doses. In our coculture system, however, Vtg decreased significantly at the 0.006-μg/mL dose but remained at control levels at the higher doses such that it resembled the U-shaped trend of female E2 and T production at 3 d. The increase in Vtg at the highest TRB dose likely reflects the increased E2 in culture produced at this dose. However, the same increase in E2 was seen in the in vivo study without a concomitant increase in Vtg. Because Vtg production is farther along the steroidogenic pathway, perhaps 3 d is not sufficient time to see a physiologically accurate response. This test should be run again at a longer time point to determine if the coculture simply requires more time to respond with Vtg production. This reflects the delayed reduction seen by Ekman et al. 39. They did not see a reduction in Vtg until 4 d after exposure. An alternative explanation is that this model is ideal for assessing sex steroid changes but does not reflect the protein changes that are seen in vivo as a result of changes in transcription.
Male HPG-L coculture and testis culture E2 production
Neither the testis tissue nor male HPG-L coculture produced any significant amount of E2 after 24 h in the control wells (Figure 4A), but both responded strongly to the addition of TRB by producing E2. Male HPG-L cocultures produced a significant difference in E2 concentration on TRB treatment after 24 h (Figure 4A, black bars; F3,19 = 17.218, p ≤ 0.001). The 0.32-μg/mL dose caused an increase in E2 compared to the control and the 2 lower doses (p ≤ 0.001 for all). The 0.04-μg/mL dose also caused a significant increase in E2 concentration compared to the control (p = 0.044). Testis cultures did not have a significant increase in E2 concentrations over the control after 24 h with the 2 lowest TRB exposures (Figure 4A, white bars; F3,20 = 31.069, p ≤ 0.001). The 0.32-μg/mL dose, however, caused a significant increase in E2 concentration compared to all other doses (p ≤ 0.001 for all).
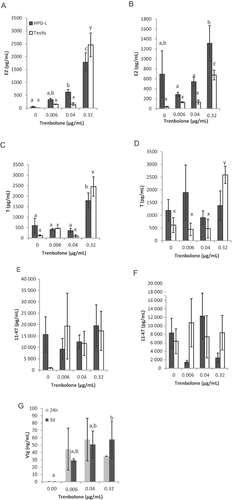
The 3-d coculture of male HPG-L tissues did not result in a significant difference in E2 concentration among treatments (Figure 4B, black bars; F3,20 = 2.551, p = 0.085), but the trend did follow the familiar U-shaped response curve as noted above in Female HPG-L coculture and ovary E2 production. The 0.32-μg/mL dose caused a significant increase in E2 compared to the control and both lower doses (Figure 4B, white bars; F3,19 = 35.511, p ≤ 0.001).
In males, the E2 concentration followed a similar trend as the in vivo study for both HPG-L coculture and single-testis culture at both 24 h and 3 d (Table 6). The testis culture response did vary slightly at 3 d in its appearance as a U-shaped curve. There was a significant increase in E2 concentration at the high dose compared to the control, lower doses, or both for all of the systems and time points. The similarity of both the testis and male HPG-L in E2 response at both time points suggests that the testis replicates in vivo trends better than the ovary and would be the more illustrative tissue to use if limited to only 1 gonad tissue type. The versatility and validity of the male system at both time points and in both culture types provide important validation for studies that use E2 changes in the testis alone as an endocrine disruption indicator.
Male HPG-L coculture and testis culture T production
Testosterone production was significantly different at the highest dose of a TRB-treated culture than the control or the lower TRB doses in both HPG-L (Figure 4C, black bars; F3,19 = 9.257, p = 0.001) and testis (Figure 4C, white bars; F3,20 = 28.590, p ≤ 0.001) cultures after 24 h. No differences in T concentration were observed in HPG-L cultures after 3 d at any TRB dose (Figure 4D, black bars; F3,19 = 0.427, p = 0.736). The testis cultures, however, did have a significant increase in T concentration produced in response to the highest TRB dose after 3 d compared to all other doses (Figure 4D, white bars; F3,20 = 11.603, p = 0.001).
The T concentration of the male HPG-L culture followed the same trend as the in vivo study at 3 d, where the T concentration was not different from the control at any dose. The testis culture alone did not follow this trend at either time point nor did the male HPG-L culture at 24 h because both had a significant increase in T at the highest dose compared to the control (Table 6).
Male HPG-L coculture and testis culture 11-KT production
There were no significant differences in 11-KT concentration in HPG-L coculture (Figure 4E, black bars; F3,19 = 0.516, p = 0.677) or testis cultures (Figure 4E, white bars; F3,20 = 1.043, p = 0.395) after 24 h or 3 d (Figure 4F, HPG-L black bars; F3,20 = 2.525, p = 0.090; testis white bars, F3,20 = 0.219, p = 0.882). The 11-KT concentrations did not follow the in vivo study trend for either the HPG-L culture or testis cultures at 24 h or 3 d (Table 6). In the in vivo study, the concentration of 11-KT decreased at the highest TRB dose, with no differences among any other doses and the control. There were no differences among any of our culture systems and time periods in the present study. It is possible that the conditions of the tissue explant culture were not ideal for the oxidation of T into 11-KT, interfering with the biochemical pathway of 11-KT production. This would account for the high variability of 11-KT in both control and treatment cultures. A system with higher oxygen concentrations may be necessary for proper function of this pathway. Careful and controlled maintenance of oxygen concentration was not performed in the present study but will be a focal point in our continuing research.
Male HPG-L coculture and testis culture Vtg production
The Vtg production response of the male HPG-L coculture did not replicate Ankley et al.'s in vivo study 26 as well as the T and E2 responses in males, although at 3 d it did show a similarity. No statistical differences in Vtg concentration were observed among HPG-L control and treatment groups after 24 h in culture (Figure 4G; F3,19 = 1.641, p = 0.213). The Vtg concentrations produced by the HPG-L tissue coculture, however, tended to increase with increasing TRB dose after 3-d exposure (Figure 4G; F3,18 = 3.539, p = 0.034). The 0.32-μg/mL dose of TRB caused an increase in Vtg concentration compared to the control (p = 0.034). This was the only concentration difference that was significant for males at 3 d. This statistically resembles the in vivo study, where no or very little Vtg was detected among all treatments except the highest dose. In the present study, however, although the highest TRB dose produced a significant Vtg increase over the control, Vtg production was noted at all non-0 doses.
Ankley et al. 26 conducted a 21-d in vivo exposure of adult male and female fathead minnows to TRB. The 3 highest doses in that study resulted in significant effects on plasma hormone levels and secondary sex characteristics. The present study attempted to replicate the same TRB tissue concentrations within an HPG-L coculture for a direct comparison of system biofidelity after only 3 d in tissue culture. The data indicate that the results of a chronic in vivo study can be replicated in a much shorter exposure time with this tissue explant assay. Because we demonstrated that the pituitary is required for successful system performance, its small size becomes the limiting factor for assembling multiple systems from a single fish. If the pituitary function could be successfully replicated using cell culture or tissue growth, this system can test multiple treatments using organ slices from a single fish, reducing the number of organisms per assay and the biological variability between samples for different treatments.
The results of the present study demonstrate that the coculture of fathead minnow HPG-L tissues has the same U-shaped response curve as that noted by Ankley et al. 26, who suggested that this response is the result of different mechanisms of action that occur under low-dose and high-dose conditions. One possible explanation is that changes in transcription occur in the ovary to increase aromatase production, allowing ovarian recovery 39. The threshold for change in transcription may not be reached at the lower doses. Future studies would benefit from examining gene regulation in this system to investigate this event in the coculture system. Both of these mechanisms may be acting to reduce the effects of TRB at the highest dose. Both low-dose and high-dose effects of E2 and T are replicated in the coculture by inclusion of the required HPG-L tissues.
Lower production of E2 may be the result of decreases in CYP19A transcription, which reduces aromatase, therefore reducing the conversion of T to E2 39. When this occurs, there is a lower loss of T because conversion of T to E2 is slowed by the interfering TRB. Higher TRB concentrations thus lead to slightly raised T levels. In addition, the interference is potentially compensated by the HPG feedback loop, increasing the amount of cholesterol recruited for sex steroid production. In Ekman et al.'s study 39, after several days of exposure to TRB, CYP19A was up-regulated to compensate for the decreased E2. Based on a gene expression profile for fathead minnow exposure to 17β-trenbolone, a metabolite of TRB, a general down-regulation of the expression of estrogen-responsive genes (hepatic vtg1, brain cyp19a1b, and gonad zp2.2) occurs 40. This corresponds to changes in the biosynthesis of endogenous estrogens. The hydroxysteroid (17β)dehydrogenase 12a is involved in the production of T. This steroid is also responsive to 17β-trenbolone and can serve as a biomarker for TRB exposure. These genetic changes indicate some of the changes in the fish endocrine system that causes the altered hormone levels. Continued use of the culture system developed in the present study can lend itself toward genetic techniques of comparing in vitro effects to in vivo data 40. This system can be used in future studies to identify changes in gene regulation and expression in response to EDCs, which may allow us to understand specific targets and modes of action for EDCs.
Not all TRB studies have resulted in the same hormone changes that were seen in the present study and the Ankley et al. study 26. Zebrafish (Danio rerio) exposure to TRB resulted in a significant decrease in Vtg 34. In the present study, Vtg was depressed in female HPG-L cocultures at 0.006 μg/mL at 3 d but not at any other dose. The Ankley et al. study also showed this depression but with only a slightly lower decrease in Vtg at the highest dose compared to the control. Although model species, such as fathead minnow and zebrafish, may not be representative of all fish species, the present study's data show that comparisons must also account for dose and time-course profiles for a more comprehensive ecosystem-level effect. Ekman et al. 39 found similar hormonal responses to what was found in the present study, including the response at low, but not high, doses. They did find recovery of steroid levels after several days of exposure. This is in contrast to the Ankley et al. 26 study, where both T and E2 were depressed after 21 d. Because neither the present study nor Ankley et al.'s study looked at recovery, the similarity of response of the system after removal of TRB is still unknown. Future studies can accomplish this by removing TRB media and monitoring coculture steroid and Vtg responses over time to determine if they return to nondosed levels. This system could also be used to compare acute versus chronic exposure by conducting time point and recovery studies.
The development of this in vitro HPG-L coculture technique for evaluating EDCs would benefit from more detailed molecular techniques embedded within the system. Studying the HPG-L axis ex vivo permits manipulation of the system by choosing the composite tissues; by in situ, real-time determination of hormones at multiple time points; and by examination of acute, chronic, and recovery effects after EDC introduction. An enhanced coculture system can be designed and interrogated in such a way as to elucidate the mode of action for a chemical. This tissue explant coculture system is demonstrated to be more physiologically true to an in vivo system compared with single-gonad tissue cultures because of its ability to replicate the complex in vivo response over both time and concentration profiles through interactions of multiple tissues not present in the single-tissue culture. Further study with coculture systems may provide the necessary connection from ex vivo response to adverse outcomes at the organismal, population, or ecological level 4, 5, 35.
CONCLUSIONS
In vitro culture systems are important for screening potential EDCs. Use of single cells or tissue types does not, however, give a comprehensive assessment of how EDCs affect the HPG-L axis. We established a coculture system for fathead minnow HPG-L tissues. The cocultured tissues were responsive to each other and to a well-known EDC with similar hormone concentration trends as reported with in vivo data. We demonstrated that the presence of all HPG-L tissues provided a physiologically complex and accurate response that could not be replicated by a single gonad tissue. Further, the equilibration time required to establish the feedback and response system among the tissues is important as well. Importantly, this system is sensitive to TRB at environmentally relevant levels, it can be readily adapted to other organisms, and the entire HPG-L axis is tested, rather than a single component. This tissue explant system can be an important link between traditional in vitro testing and organismal effects seen in vivo, making it a valuable predictive tool for endocrine disruption.
Acknowledgment
We thank J. Flaws, M. Mahoney, and K. Keller for help with the present study. The present study was supported by funding from the US Army Corps of Engineers, Basic Research Program. Research was performed using Protocol 12175 at the University of Illinois, Urbana-Champaign. The authors had no conflict of interest.
Data availability
Data, associated metadata, and calculation tools are available from the corresponding author ([email protected]).




