An in vitro method for determining the bioaccessibility of pharmaceuticals in wildlife
Abstract
Wildlife can be exposed to human pharmaceuticals via prey that have accumulated the compounds from wastewater, surface water, sediment, and soil. One factor affecting internal absorption of pharmaceuticals is bioaccessibility, the proportion of the compound that enters solution in the gastrointestinal tract. Currently, the bioaccessibility of most pharmaceuticals in prey remains unknown for most wildlife species. The authors evaluated the potential of a 2-compartment in vitro gastrointestinal tract model to compare the bioaccessibility of the antidepressant fluoxetine from invertebrate prey for birds and mammals. Samples of gizzard (or stomach) and intestinal-phase digestive juices were obtained from the in vitro models along with the residual solid material. High-performance liquid chromatographic analysis revealed that the bioaccessibility of fluoxetine in the avian in vitro models was statistically significantly lower than that in the mammalian models as a percentage of what was recovered; however, there were no statistically or biologically significant interspecies difference in terms of the amount recovered per gram of “food” inserted at the start of the simulation. This in vitro model provides a useful method of comparing the bioaccessibility of pharmaceuticals in different prey for species with different gastrointestinal conditions. There is merit for ecological risk assessments in further developing this in vitro approach to improve estimates of internal exposure for organics. Environ Toxicol Chem 2016;35:2349–2357. © 2016 SETAC
INTRODUCTION
Over the last 15 yr there has been increased attention on the dispersal and effects of active pharmaceutical ingredients in the natural environment 1, but the potential risks of pharmaceuticals in the environment to mammalian and avian species are only just starting to be studied 2-6. One approach that has been proposed for estimating the impacts of pharmaceuticals on organisms in the natural environment is to read-across from the wealth of data that are available on the safety and pharmacology of pharmaceuticals for humans and model mammals 7, 8. The read-across approach has already shown utility for evaluating the effects of selected pharmaceuticals on fish 9, 10. Because extensive in vivo testing is usually done on pharmaceuticals using laboratory mammalian species (e.g., rodents), the read-across approach could be particularly helpful in understanding potential impacts on birds and mammals in the environment.
One factor that will need to be considered in applying the read-across approach is bioaccessibility 7, 11. Bioaccessibility is defined as the percentage of the pharmaceutical that goes from the ingested matrix (e.g., tablet, food, or soil) into the digestive juices. Although bioaccessibility does not necessarily equate to bioavailability (the percentage of the dose that reaches systemic circulation), an understanding of how much of the ingested contaminant is available for uptake is an important stepping-stone toward predicting internal concentrations in an organism. Because digestive systems of different species vary in terms of temperature, enzymatic composition, gastrointestinal tract transit time, and physical breakdown 12-14, the degree of bioaccessibility of a pharmaceutical will vary in different species types. To add further complexity, it has also been demonstrated that bioaccessibility will likely vary across different ingested matrices (e.g., soil, plant, fish, and meat) 15-17. Given these many sources of variability in bioaccessibility, in vivo measurements of bioaccessibility relevant to ecologically relevant exposure scenarios are impractical. However, the use of in vitro approaches may provide the solution.
In vitro test systems, such as the physiologically based extraction test (PBET), which simulates gastrointestinal conditions, have already been developed and validated for assessing the bioaccessibility of trace metals for several organisms including humans 12, 14, 17-22, small mammals 23, 24, and birds 13, 25, 26. Physiologically based extraction tests have been used in a variety of applications including testing the safety of children's toys containing metals 27, assessing contaminated land 11, 28, and assessing the risks presented by lead shot to wild birds 13. Assessments for organics have largely been limited to polyaromatic hydrocarbons (PAHs) in humans 29, 30.
It has been suggested that PBETs could play a wider role in the assessment of risks of chemicals to wildlife by helping to adjust for differences in bioaccessibility 31, 32. However, as yet the approach has not been used for environmental risk assessment of pharmaceuticals. In the present study we therefore describe the application of a PBET approach to develop an understanding of the differences between mammals and birds. We illustrate the approach using earthworms containing the selective serotonin reuptake inhibitor fluoxetine. As a secondary amine, the uptake of fluoxetine across lipoidal membranes is strongly affected by pH close to its dissociation constant (pKa) of 10.06 33. At a basic pH, the log octanol–water distribution coefficient of fluoxetine reduces, and fluoxetine becomes increasingly present in its ionized form (AH+ is more easily dissolved in water than the non-ionized A species). The ionized form is less soluble than the un-ionized form and cannot cross lipid cell membranes in the intestine to reach the blood as easily. Thus, the accumulation and toxicity of amines, such as fluoxetine, varies considerably at pH values just below the pKa because of the reduction in hydrophobic non-ionized species with decreasing pH 34.
Although the present study has only investigated 1 pharmaceutical, food type, and wildlife species, in the future it could be applied to a wider range of active ingredients, food items, and wildlife species. Fluoxetine is the study compound in a series of in vivo uptake and effects studies we have performed using starlings (Sturnus vulgaris; T.G. Bean et al., unpublished data; Bean et al. 35). In these studies, concentrations of fluoxetine in plasma and tissues of the birds were much lower than anticipated based on uptake into humans. The in vitro investigations conducted in the present study provide us with an opportunity to explore whether the differences observed in vivo are the result of differences in bioaccessibility or whether other factors are at play.
MATERIALS AND METHODS
Test chemicals and soil
Fluoxetine (≥98%), pepsin, pancreatin, malate, bile extract, and sodium bicarbonate extract were obtained from Sigma-Aldrich. Lactic acid, citric acid, acetic acid, and methanol (high-performance liquid chromatography [HPLC] grade 99.9%) were obtained from Fisher Scientific.
A sandy loam (pH 6.47) soil was collected from an unpolluted site (N 53.957045, W –1.137880) for use in the earthworm exposures. Roots and stones were first removed by hand. The soil was air-dried for 24 h before passing through a 2-mm sieve. Details of how moisture content and maximum water holding capacity were determined are given in the Supplemental Data.
Exposure of earthworms to fluoxetine
Earthworm prey were first exposed to fluoxetine. A colony of Eisenia fetida were obtained from the UK Food and Environment Research Agency and maintained at optimal conditions 36 for 3 wk until a sufficient number of individuals weighed 0.5 ± 0.1 g. Then, individuals were removed from the colony, and soil that had adhered to the earthworm was removed by holding the earthworm with a pair of blunt-ended forceps and pipetting deionized water. Earthworms were dabbed dry on paper towels prior to inserting into their individual exposure jars.
To expose individual earthworms to fluoxetine, 50 g of moist soil was weighed out into individual glass jars (approximate volume of jars was 100 mL). Soil was spiked with 1 mL of fluoxetine solution (30 mg/mL fluoxetine dissolved in methanol) to give an expected fluoxetine concentration of 600 μg/g soil. A fluoxetine concentration of 0.37 μg/g is an environmentally relevant concentration in treated sludge 1, whereas 0.019 μg/g is predicted for soil 37, 38. The spiking concentration for soil was based on previous work on uptake of fluoxetine into earthworms 37 and was selected to yield a concentration of fluoxetine in earthworms of at least 60 μg/earthworm. The concentration in earthworms after 21 d was required to be greater than an environmentally realistic level to ensure that levels in digestive juice and residual solid material samples were above the limits of quantification of the HPLC analysis method (see section Chemical analyses, and Supplemental Data). The soil for the control group was spiked with 1 mL of methanol. After spiking, the soil was left for 2 h before stirring with a spatula. Jars containing soil were left for 48 h prior to adding a single earthworm to each jar.
The soil in each jar was made up to 60% of maximum water holding capacity with deionized water on a balance (Sartorius LL4800P); the balance was then tared, and the earthworm was added. The earthworm weight was recorded to 0.01 g. Earthworms were provided with a small amount of food, approximately 0.1 g of dried mashed potato powder, by sprinkling a thin layer onto the surface of the soil. To prevent earthworms from escaping, jars were covered with a square of garden fleece held in place by an elastic band. Earthworms were kept in a controlled environment room (20 °C, 70% humidity, 16:8-h light:dark cycle).
To control for the effect of soil moisture content on uptake into worms, the soil moisture content was maintained at 60% maximum water holding capacity over the 21-d exposure period. This was achieved by adding the required mass of deionized water daily to return the jar to its starting mass 36, 37. A small amount of food (<0.5 g) was also reapplied as necessary, approximately every other day. The start of the experiment was staggered so that the PBETs were carried out on 9 separate days (see Supplemental Data).
PBETs
Relative bioaccessibilities for the mammalian and avian digestive systems were quantified by inserting earthworms, along with soil, which had adhered to the worm, into the human and avian PBETs. The earthworms were not cleaned to mimic avian foraging behavior in the wild.
In total 5 PBETs were conducted: 2 avian tests (siliceous grit gizzard and calcareous grit gizzard) and 3 for mammals (recently fed = pH 4, average = pH 2.5, and fasted = pH 1.3). These PBETs were designed to cover the broad range in digestive tract conditions for birds and mammals. To simulate uptake of fluoxetine by wild birds, we inserted earthworms that had been exposed to fluoxetine in the soil directly into each PBET. The mean mass of adhered soil was 0.155 ± 0.042 g (determined by rinsing the soil from 4 earthworms that did not go into a PBET). To humanely kill the earthworms, the tubes containing them were placed in a –20 °C freezer and brought back up to room temperature prior to digestion in the simulated gastrointestinal tract.
In addition to the fluoxetine and control earthworms, we ran blank PBETs containing no food. All simulations were performed in triplicate, as is widely used in PBETs 12-15 (see Supplemental Data for details of experimental structure).
From each PBET, samples of stomach (mammals) or gizzard (bird) and intestinal digestive juice were obtained. Digestive juice samples were centrifuged and extracted with solvent, passed through a 0.2-μm polytetrafluoroethylene (PTFE) syringe filter, and analyzed by HPLC with fluorescence detection (see Supplemental Data). The residual solid material remaining at the bottom of the intestinal digestive juice sample was also extracted, as in Martinez-Haro et al. 13. The fluoxetine recovered at the end of the intestinal phase was the total bioaccessible amount, and the total fluoxetine recovered was given by the sum of intestinal and residual solid material fluoxetine.
Mammalian models based on human PBET
The mammalian models were adapted from the human PBETs of Li and Zhang 12 and Ruby et al. 14 and the mammalian adapted human PBETs of Moriarty et al. 24 and Kaufman et al. 23 (see Figure 1A and Supplemental Data for further details). Mammalian PBETs were carried out in 50-mL centrifuge tubes using stomach digestive juice at 3 different pHs, to account for the effect of time since the last meal on stomach pH. Stomach digestive juices were prepared in conical flasks by adding pepsin (1.25 g/L), malate, citrate (both 0.5 g/L), lactic acid (420 μL/L), and acetic acid (500 μL/L) to deionized water. Stomach digestive juice was adjusted to 1 of 3 pH values using concentrated (35%) HCl: pH 1.3 was used to represent fasted conditions, pH 2.5 represented average stomach conditions, and pH 4 represented recently fed conditions and then warming to 37 °C in a water bath 14.
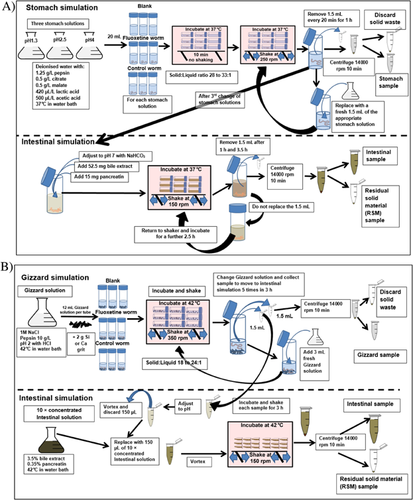
To begin the mammalian PBET simulations, whole earthworms and any adhered soil were added to tubes intact along with 20 mL of stomach digestive juice. The headspace was purged with N2 to create a low-oxygen environment. Tubes were placed in a shaking incubator at 37 °C but were not shaken for 10 min, as in Ruby et al. 14, after which tubes were shaken at 250 rpm for 1 h in total. At 3 time points (every 20 min) during this hour, shaking was stopped and a 1.5-mL aliquot of the stomach digestive juice was taken from each tube and replaced with a fresh 1.5 mL of gastric solution. Stomach digestive juice samples were transferred to a 1.5-mL sealed microcentrifuge tube and immediately centrifuged (10 min at 11 000 g). The supernatant was decanted into a fresh tube and stored at –20 °C.
Once the stomach simulation was complete, the digestive juice in each simulation tube was adjusted to pH 7 with NaHCO3 powder (intestinal pH) 21. For the pH 1.3 simulation, the worm had completely disintegrated after 1 h, at pH 2.5 it had partially disintegrated, but at pH 4 the worm could clearly still be seen intact prior to the intestinal simulation. Once pH had been adjusted, 52.5 mg of bile extract and 15 mg of pancreatin were added to each replicate 12 before returning to the shaker. The 50-mL centrifuge tubes were shaken on their sides at 100 rpm to mimic the slow intestinal passage of food. After 1 h (data not presented) and after 3.5 h of intestinal incubation, 1.5-mL aliquots were taken from each centrifuge tube. Unlike the stomach phase, the intestinal 1.5-mL aliquots were not replaced after sampling. The intestinal samples were centrifuged, the supernatant was aliquoted into a new tube, and the residual solid material was retained. All samples were stored at –20 °C until extraction and analysis.
Avian PBETs
To capture the complexity of the avian digestive tract, a dynamic avian gizzard–intestine system containing either 2 g of siliceous grit or 2 g of calcareous grit was simulated following the methods of Martinez-Haro et al. 13. The method used for the avian PBET is summarized in Figure 1B and explained in full in the Supplemental Data. Five samples of gizzard digestive juice were collected every 36 min and aliquoted into 2 microcentrifuge tubes (1.5 mL) during a 3-h incubation at 42 °C on a mechanical shaker (full details in Supplemental Data). At each of the 5 sampling time points, 1 aliquot was centrifuged and the supernatant stored at –20 °C prior to analysis. The other sample was adjusted to pH 6 with a concentrated NaHCO3 solution; one-tenth of the volume was then discarded and replaced with a 10 times concentrated intestinal digestive juice containing bile and pancreatin (see Supplemental Data). Intestinal simulations were returned to the incubated mechanical shaker for 3 h before being centrifuged. The supernatant was aliquoted into a new 1.5-mL tube, and the residual solid material was retained and stored at –20 °C until extraction and analysis.
Chemical analyses
After the PBETs had been run, the samples of stomach (or gizzard) and intestinal digestive juice were thawed and vortex-mixed. A 500-μL aliquot was taken from each sample and combined with 500 μL of methanol using a further vortex mix. Samples of residual solid material were extracted into 1 mL of methanol using sonication for 3 min. Sample and methanol mixtures were centrifuged for 10 min at 11 000 g and filtered using a 0.2-μm PTFE filter.
Extracts were analyzed by HPLC with fluorescence detection (excitation = 230 nm, emission = 305 nm). Separation was achieved using a C-18 column (Kinetex 5 μm C18 150 × 4.6 mm; Phenomenex) and a gradient mobile phase comprising a mixture of water containing 0.1% H3PO4 and methanol ranging in concentration from 90% water/H3PO4 to 10% water/H3PO4. The run time was 23 min, with a retention time of 11.7 min to 11.8 min.
To validate the extraction procedure from digestive juice samples and feces for each of the 5 PBETs, blank digestive juice and fecal samples were generated by running the PBET simulations without the addition of an earthworm (see Supplemental Data).
Data analysis and statistics
Effect of fluoxetine on earthworm growth during 21-d exposure via soil
To meet the assumption of normality, earthworm body weight data were log-transformed, and the effect of fluoxetine or control on growth was assessed using a repeated measures model in R package nlme. The repeated measures were body weight before and after the 21-d exposure period.
Effect of in vitro model type on percentage of fluoxetine in stomach or gizzard, intestine, and feces
An explanation of how the amounts of fluoxetine (micrograms) recovered in the different physiologically based extraction test compartments is presented in Figure 2. The relation between mass of solid material added and amount of fluoxetine recovered was not linear (Pearson's correlation: n = 15, r = 0.265, p = 0.36). This was because there was a significant inverse relationship between mass of solid material added and the percentage of the total fluoxetine that was bioaccessible (Pearson's correlation: n = 15, r = 0.519, p < 0.05). Therefore, the bioaccessibility data were normalized in 2 ways for separate analyses: data set 1, micrograms per gram of solid material (worm + soil) inserted at the start of the physiologically based extraction test, and data set 2, percentage recovery, assuming the sum of bioaccessible and residual solid material fluoxetine (micrograms) was the total fluoxetine added (Figure 2).
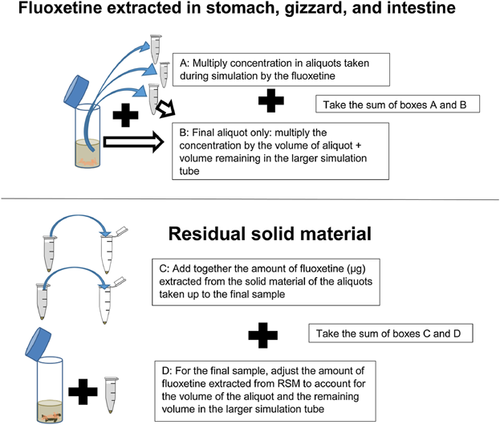
Normality was assessed from Q-Q plots for both data sets (micrograms per gram solid material and in percentage terms), and no transformation was applied. The effect of physiologically based extraction test model on fluoxetine recovered in the different physiologically based extraction test compartments was initially assessed using a multivariate analysis of variance (MANOVA). The response variables used in the MANOVA were the micrograms per gram or percentage of the total micrograms of fluoxetine recovered in the physiologically based extraction test compartments (stomach or gizzard, the intestinal phase minus the stomach or gizzard, at the end of the intestinal phase [total bioaccessibility]). For the micrograms per gram analysis, the residual solid material remaining at the end of the intestinal phase and the total (intestinal fluoxetine + residual solid material) were also included in the MANOVA. They were not included in the MANOVA for the analysis of percentages because the total would always be 100% and percentage residual solid material is just 100% minus the intestinal percentage. Because we obtained an overall effect of the physiologically based extraction test model in the MANOVAs, individual ANOVAs were used to identify which of the response variables contained significant differences between models. Finally, for the PBET compartments where there were significant differences in the individual ANOVAs, post hoc Tukey's honestly significant difference tests (all on 4 degrees of freedom [df]) were used to identify which physiologically based extraction test models (i.e., mammalian pH 1.3, pH 2.5, pH 4, bird siliceous grit, and bird calcareous grit) these were for. Multivariate ANOVAs, ANOVAs, and post hoc analyses were performed in SPSS (Ver 23); and significance was tested at the 5% level.
RESULTS AND DISCUSSION
Earthworm uptake and growth
The mean fluoxetine concentration in earthworms (537.1 ± 47.3 μg/g) was 0.94 times the mean concentration measured in soil (572.7 ± 9.4 μg/g). The biota–sediment accumulation factor estimated by Carter et al. 37 using radiolabeled fluoxetine and earthworms that had been given 24 h to depurate their gut was 0.3. Our value of 0.94 is of the same order of magnitude as that of Carter et al. 37 and would be closer had we depurated the earthworms prior to extraction. Earthworms were not given a chance to depurate because the aim of the PBETs was to mimic how fluoxetine would be encountered by a wild bird, which in reality would consume both the worm and associated soil.
The fluoxetine and control earthworms had similar masses before the 21-d exposure period (fluoxetine, n = 20, mean ± standard error [SE] = 0.53 ± 0.008 g; control, n = 20, mean ± SE = 0.51 ± 0.008 g). After 21-d exposure, the earthworms in the fluoxetine-treated soil had lost weight, with mean body weights ± SE of 0.50 ± 0.024 g, whereas the control earthworms gained weight (0.70 ± 0.030 g). There was a significant effect of treatment on body mass change from pre-exposure to postexposure time (F38,1 = 33.49, p < 0.0001). Effects on growth have been observed in invertebrates exposed to fluoxetine in sediments before with lowest-observed-effect concentrations (LOECs) of 1.3 mg/kg and 5.6 mg/kg being reported for Chironomus tentans and Hyalella azteca, respectively 33. Our fluoxetine concentration was measured at 572 mg/kg, 2 orders of magnitude higher than these LOECs. However, we observed weight loss and not just growth inhibition, which is the endpoint typically investigated. The findings indicate that fluoxetine has the potential to affect invertebrate growth, the mechanism for which is uncertain 33. However, all concentrations at which fluoxetine has been found to affect growth are several orders of magnitude higher than predicted in the environment (0.019 mg/kg) based on Carter et al. 37, 38, so these effects may not occur in reality.
Differences between PBETs
Concentrations of fluoxetine in samples from the control earthworms and blank treatments were below the limit of detection (<0.2 μg/mL), so no blank correction was applied. The masses of solid material (worm + soil) and the amount of fluoxetine (micrograms) recovered in each PBET compartment are reported for the avian and mammalian PBETs in Table 1. These data were used to compute the 2 versions of the bioaccessibility data analyzed: data set 1, micrograms per gram of solid material added, and data set 2, percentage of fluoxetine recovered. The MANOVAs for both data sets 1 and 2 indicated that there were significant differences between PBETs (micrograms per gram: Pillai's score = 2.41, F = 3.79, p < 0.001; for percentage, Pillai's score = 2.35, F = 8.99, p < 0.001).
| Solid material added to testa (g) | Gizzard or stomachb (μg) | Intestinec (μg) | Intestine–gizzard or stomachd (μg) | RSMe (μg) | Totalf (μg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBET | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| Bird Ca | 0.64 | 0.04 | 105.82 | 15.85 | 255.70 | 38.82 | 132.94 | 20.96 | 69.72 | 11.35 | 325.43 | 49.61 |
| Bird Si | 0.50 | 0.04 | 165.67 | 3.45 | 201.28 | 4.84 | 22.60 | 7.81 | 63.84 | 3.68 | 265.12 | 6.42 |
| Mammal pH 1.3 | 0.70 | 0.03 | 245.07 | 31.38 | 246.10 | 28.86 | 1.03 | 6.75 | 28.75 | 3.63 | 274.85 | 32.45 |
| Mammal pH 2.5 | 0.72 | 0.09 | 159.34 | 18.95 | 267.40 | 24.05 | 108.07 | 5.15 | 36.27 | 6.48 | 303.67 | 29.20 |
| Mammal pH 4 | 0.61 | 0.02 | 71.42 | 3.42 | 226.89 | 11.51 | 155.47 | 13.43 | 30.25 | 2.87 | 257.14 | 10.74 |
- a The mass of solid material added included earthworm and an average of 0.155 g of soil which had adhered to the earthworm; the mean ± standard error concentration of fluoxetine in earthworm tissue was 537.1 ± 47.3 μg/g and that in soil, 572.7 ± 9.4 μg/g.
- b Gizzard for birds, stomach for mammals.
- c Total bioaccessible amount of fluoxetine.
- d The difference between the amount of fluoxetine in the intestine and that which had already been extracted in the gizzard or stomach.
- e The residual solid material remaining at the end of the intestinal phase.
- f The total amount of fluoxetine recovered was given by the sum of fluoxetine in the intestine and the residual solid material.
- Ca = calcareous; PBET = physiologically based extraction test; RSM = residual solid material; SE = standard error; Si = siliceous.
Data set 1: Micrograms per gram of solid material added
Figure 3A shows the amount of fluoxetine recovered in each PBET compartment when the data were normalized in terms of the amount of solid material added. There were no significant differences between any PBETS in the individual ANOVAs for the amount of fluoxetine micrograms per gram of solid material added at the end of the intestinal phase (df = 4, F = 0.29, p = 0.88) or in terms of the overall amount of fluoxetine recovered (sum of intestinal fluoxetine and residual solid material; df = 4, F = 1.41, p = 0.30). Importantly, this means there were no interspecies or intraspecies differences in bioaccessibility relative to the amount of solid material added (and thus approximately the amount of fluoxetine added; because of differences in uptake into earthworms, we cannot definitively give the amount of fluoxetine inserted). Therefore, the significant differences were only in terms of whether fluoxetine entered solution in the first compartment (stomach or gizzard: df = 4, F = 11.66, p = 0.001) or second compartment (intestine–stomach or gizzard: df = 4, F = 24.43, p < 0.001) of the PBET and the amount remaining in the residual solid material (df = 4, F = 15.35, p < 0.001). These differences are likely to be of less biological significance than total bioaccessibility. Table 2 summarizes where the differences occurred (interspecies or intraspecies) and the nature of the differences from post hoc Tukey's honestly significant difference tests; the mean micrograms per gram values can be found in Supplemental Data, Table S2. In general terms, the PBETs with a more acidic stomach or gizzard (mammal pH 1.3 and bird siliceous), regardless of species, recovered more fluoxetine per gram of solid material added in the stomach or gizzard than the less acidic models (mammal pH 2.5, pH 4, and bird calcareous). The reverse was true for the difference between the total bioaccessible fluoxetine recovered at the end of the intestine and that which had already entered solution in the stomach or gizzard.

| Test compartment | Difference | Solid material (μg/g)a | % of total fluoxetine recoveredb | |
|---|---|---|---|---|
| Gizzard or stomachc | Interspecies | B and M | 1.3>Ca*, Si>4** | 1.3>Ca***, 2.5>Ca*** 1.3>Si***, Si>2.5*, Si>4*** |
| Intraspecies | B and B | Si>Ca* | Si>Ca*** | |
| M and M | 1.3>4** | 1.3>2.5***, 1.3>4***, 2.5>4*** | ||
| Intestine (total bioaccessibility)c | Interspecies | B and M | None | 1.3> Ca***, 2.5>Ca**, 4>Ca** 1.3> Si***, 2.5> Si***, 4> Si*** |
| Intraspecies | B and B | None | None | |
| M and M | None | None | ||
| Intestine minus (gizzard or stomach)c | Interspecies | B and M | Ca>1.3***, 2.5>Si*, 4>Si*** | Ca>1.3***, Ca>4**, 2.5>Si*** 4>Si*** |
| Intraspecies | B and B | Ca>Si** | Ca>Si*** | |
| M and M | 1.3>2.5, 1.3>4 | 2.5>1.3*** 4>1.3***, 4>2.5*** | ||
| RSMc | Interspecies | B and M | Ca>1.3**, Ca>2.5*, Ca>4*; Si >1.3**, Si>2.5**, Si>4** | Ca> 1.3***, Ca>2.5**, Ca>4**; Si> 1.3***, Si> 2.5***, Si>4*** |
| Intraspecies | B and B | None | None | |
| M and M | None | None | ||
| Totalc | Interspecies | B and M | None | NA |
| Intraspecies | B and B | None | NA | |
| M and M | None | NA | ||
- a Data normalized by micrograms of fluoxetine recovered relative to the mass (grams) of solid material inserted to the test as worm and soil.
- b Data normalized as a percentage of the total amount of fluoxetine recovered (intestine + residual solid material).
- c Physiologically based extraction tests: Ca = bird calcareous grit; Si = bird siliceous grit; 1.3 = mammal pH 1.3; 2.5 = mammal pH 2.5; 4 = mammal pH 4. Compartments: gizzard or stomach, intestine (total bioaccessible fluoxetine), intestine minus gizzard or stomach, residual solid material, and total (intestine + residual solid material).
- * p < 0.05
- ** p < 0.01
- *** p < 0.001
- B = bird; Ca = calcareous; M = mammal; NA = not applicable; None = no significant differences; RSM = residual solid material; Si = siliceous.
A greater amount of fluoxetine per gram of solid material was recovered in the residual solid material from both avian models than in any of the mammalian models. It is believed this was the result of sorption to the grit in the avian models (absent from mammalian models; see Supplemental Data, Figure S1), which seemed to produce residual solid material (bird n = 30, mean ± SE = 0.074 ± 0.002; mammal n = 18, mean ± SE = 0.043 ± 0.002; independent samples t test: df = 46, t = –10.64, p < 0.001).
Data set 2: Percentage of fluoxetine recovered
Figure 3B shows the percentage of fluoxetine recovered in the different compartments of the PBETs. Individual ANOVAs found that there were significant differences between all PBET compartments. Importantly, this means that, in percentage terms, there were significant differences in bioaccessibility (df = 4, F = 36.21, p < 0.001). Table 2 shows that all significant differences in terms of total bioaccessibility were interspecies rather than intraspecies differences (the mean percentages can be found in Supplemental Data, Table S2). Percentage bioaccessibility in mammalian models ranged from 88.2% to 89.6%, but for the avian models they were 78.6% (calcareous grit) and 75.9% (siliceous grit; Figure 3B; Supplemental Data, Table S2). For the avian models, the percentage of fluoxetine in residual solid material was 21.4% and 24.1%, which was significantly higher than in the mammalian models, which ranged from 10.4% to 11.8% (Figure 3B; Supplemental Data, Table S2). In terms of significant differences in the area of the gastrointestinal tract where fluoxetine was recovered (Table 2), the only models with similarities were bird calcareous with mammalian pH 2.5 (intestine–stomach or gizzard) and pH 4 (stomach or gizzard) and bird siliceous grit with mammalian pH 1.3 (intestine–stomach or gizzard). Therefore, in percentage terms, there were interspecies differences for bioaccessibility and residual solid material and both intraspecies and interspecies differences in terms of whether fluoxetine entered solution in the stomach or gizzard or intestine.
How could PBETs be used to support read-across for ecological risk assessment in wildlife
The bioaccessibility of fluoxetine in the avian system was actually very similar to the bioaccessibility in the human system. The statistically significant lower bioaccessibility in the avian models (9.6–13.7%) is unlikely to be of significance for ecological risk assessment. When the data were normalized based on the amount of solid material added, there were no significant interspecies differences; in fact, the avian models actually extracted 25.6 μg/g to 58.7 μg/g of solid material more than the mammalian PBETs. Our data highlight the challenges of conducting in vitro assessments of bioaccessibility when trying to introduce food items that have accumulated contaminants via an environmentally relevant exposure route.
Regardless of whether the data were examined in percentage terms or relative to the amount of solid material present, we found significant differences in the area of the gastrointestinal tract where fluoxetine was extracted from the earthworm. We observed that in the stomach or gizzard the lowest pH models (human pH 1.3 and bird siliceous grit) extracted both more fluoxetine per gram of solid material and a greater percentage of that recovered. These acidic models were observed to cause the worm tissues to break up within 30 min to 60 min. Worms in the less acidic models remained intact until the intestinal phase. This finding follows what is expected based on the optimal pH for pepsin (pH 2), the main enzyme in the stomach and gizzard 39.
It is possible that the region of the gastrointestinal tract where fluoxetine becomes bioaccessible would be significant for risk assessment. Generally, the majority of pharmaceuticals are absorbed from the intestine as a result of the presence of microvilli, with uptake from the stomach possible but less efficient 40. Fluoxetine is reasonably well absorbed from the gastrointestinal tract in some mammals, with 72% bioavailable in dogs, but not in others, with only 38% bioavailable in rodents 41. Dogs excrete 10.9% of a fluoxetine dose as fluoxetine; no data could be found for small mammals 42. Our mean percentage in vitro for mammalian residual solid material was 11.4 ± 0.45%, which is interestingly close to that excreted by dogs even if residual solid material is not exactly equivalent to feces in vitro 40, 41.
Very little is known about the bioavailability and excretion of pharmaceuticals in the avian system. There are a large number of in vitro gastrointestinal tract simulations available to researchers, the majority of which have been developed for inorganics based on a human/child model to simulate exposure via ingestion of lead from soil 18. Relatively few models have been developed for organics (e.g., the Fed ORganic Estimation Human Simulation Test [FOREhST] model used for PAHs 30) or wildlife, and none have been developed specifically for the assessment of organics in wildlife. We encourage future researchers to integrate what is known about the gastrointestinal tract physiologies of wildlife species from the PBETs used in the present study 13, 23, 24 with what is known about in vitro bioaccessibility of organics from the FOREhST model 30.
In terms of the suitability of the avian PBET used in the present study (designed for uptake of lead by waterfowl) 13, we found a good match between the percentage recovered in the residual solid material in vitro and excretion by European starlings from a recent in vivo study (T.G. Bean, K.E. Arnold, J. Lane, E. Bergstrom, J. Thomas-Oates, B.A. Rattner, A.B.A. Boxall, unpublished data). Starlings fed fluoxetine spiked invertebrates excreted 19.3 ± 15.9% of the dose as fluoxetine (n = 3; methods in Supplemental Data). In the avian calcareous grit PBET, 21.4 ± 0.9% of the fluoxetine inserted was recovered in the residual solid material (the diet of the starlings contained a calcium supplement). The good match between avian in vitro and in vivo data were surprising but encouraging given the considerable number of parameters involved in designing and validating a species and contaminant species in vitro model. For example, there is a difference of approximately 5 h in gastrointestinal transit time between waterfowl and small passerines such as starlings 13, 43. Although the mammalian and avian in vitro residual solid material and in vivo excretion data in vitro seem to match well, plasma and tissue residues of fluoxetine in starlings in vivo were found to be up to 2 orders of magnitude lower than would be predicted from human kinetic data (T.G. Bean, K.E. Arnold, J. Lane, E. Bergstrom, J. Thomas-Oates, B.A. Rattner, A.B.A Boxall, unpublished data). To properly validate the PBETs, samples of digestive juice from the bird would have to be collected after the drug has been administered. Not only would digestive juice be challenging to sample but there is limited information on how much digestive juice is secreted by different species (and the timing of secretions). Collecting blood plasma is another option for in vivo evaluation of organics, but this relies on 1) the assumption that the bioaccessible contaminant is 100% bioavailable, and 2) species-specific pharmacokinetic data, such as time to peak plasma concentration, being similar 44.
Future research needs
Expanding the PBET approach from inorganic to organic chemicals is highly relevant to the needs of ecotoxicology to help quantify differences, both between species and between food types, in internal exposure 32, 45. This would enable the accuracy of ecological risk assessments to be improved, the scope of interspecies read-across to be extended, and the number of animals used in routine ecotoxicology studies to be reduced. However, there remain several challenges in terms of both developing and validating PBETs for new species and contaminants. We have identified 5 main challenges to expanding the PBET approach. Firstly, there is a lack of complete data sets on gastrointestinal tract physiology and digestive processes for a range of wildlife species. Secondly, our understanding of how bioaccessibility relates to bioavailability, for example, approaches for assessing the behavior of bioaccessible fluoxetine in cell membranes 46, is limited. Thirdly, it is difficult to obtain licences for in vivo validations and, even then, they are difficult to conduct and standardize. Fourthly, the impact of microbes in the gastrointestinal tract is uncertain but may well be important for organics that are less persistent than fluoxetine 47. Finally, access to existing sensitive data from preclinical and clinical drug trials that could be used to evaluate new PBETs is hard to obtain. Nevertheless, in vitro gastrointestinal tract models such as PBETs are both economically feasible and ethically viable compared with the equivalent in vivo assessments. They provide the potential to improve estimates of internal concentrations of pharmaceuticals and other organic compounds when using read-across approaches to predict risk posed by environmental contaminants to non-target species.
Expanding the PBET approach in terms of numbers of species and compounds included could help to protect both wildlife, by setting safer threshold levels, and laboratory animals (from further tests). We believe the incentives for expanding the PBET approach outweigh the challenges faced. These challenges can be overcome through interdisciplinary research across areas including, but not limited to, ecotoxicology, physiology, and bioaccessibility.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3406.
Acknowledgment
We thank all ASSIST staff at the Food and Environment Research Agency, R. Weaver, G. Bryning, B. Hernout, J. Thomas-Oates, and E. Bergstrom, for their help with the present study. We also thank W.N. Beyer (US Geological Survey, Patuxent Wildlife Research Center) for comments on earlier versions of the manuscript. T.G. Bean was funded by a PhD studentship from the Natural Environment Research Council.
Data availability
Data will be provided to interested parties on request from the corresponding author ([email protected]).




