The herbicide atrazine induces hyperactivity and compromises tadpole detection of predator chemical cues
Abstract
The ability to detect chemical cues is often critical for freshwater organisms to avoid predation and find food and mates. In particular, reduced activity and avoidance of chemical cues signaling predation risk are generally adaptive behaviors that reduce prey encounter rates with predators. The present study examined the effects of the common herbicide atrazine on the ability of Cuban tree frog (Osteopilus septentrionalis) tadpoles to detect and respond to chemical cues from larval dragonfly (Libellulidae sp.) predators. Tadpoles exposed to an estimated environmental concentration of atrazine (calculated using US Environmental Protection Agency software; measured concentration, 178 μg/L) were significantly hyperactive relative to those exposed to solvent control. In addition, control tadpoles significantly avoided predator chemical cues, but tadpoles exposed to atrazine did not. These results are consistent with previous studies that have demonstrated that ecologically relevant concentrations of atrazine can induce hyperactivity and impair the olfactory abilities of other freshwater vertebrates. The authors call for additional studies examining the role of chemical contaminants in disrupting chemical communication and the quantification of subsequent impacts on the fitness and population dynamics of wildlife. Environ Toxicol Chem 2016;35:2239–2244. © 2016 SETAC
INTRODUCTION
Infodisruption, defined as chemical contaminants disrupting communication within or among organisms, has 2 modes of expression: 1) an external interorganismal disruption that causes a breakdown in detection or production of chemical signals between senders and receivers, and 2) an internal intraorganismal disruption that can affect cell-to-cell communication 1. Several chemical contaminants are infodisruptors, and both internal and external infodisruption can affect organisms' health 1, 2. For many organisms, external infodisruption can interfere with locating predators, mates, and food 1, 2. Similarly, endocrine disruptors, a type of internal organismal infodisruptor (which eventually can also affect communication between the sexes) 1, have been implicated in the collapse of wildlife populations 3. Unlike internal infodisruptors (e.g., endocrine disruptors), external infodisruptors that affect communication among individuals have not been well studied 1.
For freshwater organisms, chemical cues are often crucial 4-6. This is because visual and auditory cues tend to attenuate rapidly through water. In addition, turbidity in freshwater ecosystems can further make visual cues unreliable. Consequently, most freshwater organisms rely heavily on chemical communication to avoid predation and find food and mates 7, 8, which is why external interorganismal infodisruptors have the potential to adversely affect the fitness of freshwater organisms 1, 2.
One of the most common chemical contaminants in freshwater ecosystems is the widely used herbicide 2-chloro-4-(ethylamino)-6-(isopropylamino)-S-triazine (atrazine) 9. Because of its heavy use, persistence, and mobility, atrazine is found commonly in freshwater environments where many fish and amphibians develop 10, 11. Atrazine has a variety of effects on freshwater organisms, including fish and amphibians. For example, atrazine has been reported to affect behavior 12, 13, physiology, and growth 14, 15; elevate mortality 16-18; increase infections and suppresses immunity 19-22; and induce community-wide, indirect effects 23-26. A recent meta-analysis revealed that all of these effects appear to be general, with the exception of elevated mortality 9.
More specifically, atrazine has been reported as both an intraorganismal and an interorganismal infodisruptor. Atrazine has been documented as an endocrine disruptor of freshwater fish and amphibians 9, 27, 28. In addition, several studies provide evidence that exposure to ecologically relevant concentrations of atrazine can reduce the olfactory abilities of fish and crayfish 29-35. For example, atrazine impaired olfaction in salmon, with adverse effects on imprinting, homing, and migratory behaviors 34. Other studies have shown that atrazine exposure decreased the response of male salmon to reproductive priming hormones released in the urine of female salmon 29, 33 and decreased the response of goldfish to chemical cues signaling predation 32. Several fish studies using electro-olfactograms, which measure the changing electrical potentials of the olfactory epithelium, found that short term, low-level exposure to atrazine reduced electro-olfactogram responses and olfactory-mediated behaviors 30, 31. In contrast, olfaction of American toad (Anaxyrus americanus) tadpoles was not significantly affected by atrazine 36. Additional studies are needed on amphibians to assess whether atrazine generally has adverse effects on olfaction in freshwater vertebrates. In the present study, we evaluated whether exposing Cuban tree frog (Osteopilus septentrionalis) tadpoles to the estimated environmental concentration of atrazine affects their abilities to detect chemical cues from predatory dragonfly larvae (Libellulidae sp.) and to exhibit appropriate antipredator behaviors. Given that there are more studies suggesting that atrazine is an infodisruptor than not, we hypothesized that exposure to atrazine would decrease tadpole detection of chemical cues from predators.
Background on field concentrations of atrazine
Although atrazine has been banned in Europe, it remains one of the most commonly used pesticides in the United States and the rest of the world, where it is used heavily in corn and sorghum production 9. Atrazine is relatively persistent, with an aqueous photolysis half-life of 335 d under natural light and neutral pH and half-lives that can exceed 3 mo in mescosms 9. Atrazine is also relatively mobile, regularly entering water bodies with runoff and rain inputs 9. Atrazine has been detected in rain or air from European and US sites more than any other currently used pesticide 37.
In the present study, we tested the effects of the estimated environmental concentration (EEC) of atrazine on tadpole olfaction. The EEC is the concentration estimated to enter a standardized farm pond that is a standardized distance from the application site (based on particular soil properties) 23. The EEC was calculated using US Environmental Protection Agency (USEPA) GENEEC software (based on applications to corn) and is the concentration used in registering pesticides 23. The measured concentration to which tadpoles were exposed was 178 µg/L of atrazine.
To place this concentration in an ecological context, we provide background on measured concentrations of atrazine in the environment. The maximum reported wet deposition of atrazine is 154 µg/L from Iowa precipitation 38. Data on atrazine concentrations in surface water are more abundant for lotic (streams and rivers) than lentic (lakes, ponds, wetlands, ditches) systems, primarily because of the extensive stream monitoring conducted by the US Geological Survey National Water Quality Assessment project 39. Atrazine has been detected in streams at 200 µg/L and repeatedly has been detected in streams above 100 µg/L 39. However, more amphibians develop in lentic than lotic systems, where water is not replenished and chemicals can concentrate as lentic systems dry. Rohr and McCoy 9 reported that maximum reported atrazine concentrations in lentic systems are often 2.5 to 10 times higher than maximum concentrations in lotic systems. For example, atrazine concentrations in lentic surface waters have been reported at 131 µg/L in Mississippi (USA) 40, 681 µg/L in Ontario (Canada) 41, 850 µg/L in Florida (USA) 42, 1068 µg/L in Kansas (USA) 43, 1096 µg/L in Nebraska (USA) 44, and 2300 µg/L in Iowa (USA) 45. These findings seem to belie probabilistic aquatic ecological risk assessments for atrazine that suggest that concentrations near or above the EEC are extremely rare 46 and demonstrate that measured concentrations of atrazine near and well above the EEC are widespread. In summary, the EEC is the concentration the USEPA estimates to enter farm ponds regularly and is not a worst-case scenario, because it was exceeded in at least 4 states in the United States and in Canada. As such, the concentration of atrazine we test in the present study is ecologically relevant based both on regulatory standards and measured field concentrations.
MATERIALS AND METHODS
Tadpole rearing
Cuban tree frog tadpoles were collected from the Botanical Gardens at the University of South Florida in Tampa, Florida. Once in the lab, tadpoles were maintained under laboratory conditions at 22 °C and a 12:12-h light:dark cycle. Eighty tadpoles (Gosner stages ranging from 25 to 41; mean ± standard error [SE], 34.00 ± 0.53) were kept individually in 250 mL of pond water in a standard 0.47-L glass mason jar. One-half the tadpoles were then exposed to 7.81 µL of either acetone or a 3.26-mg/mL stock solution of atrazine dissolved in acetone, to achieve an estimated actual concentration of 178 µg/L of atrazine (measured with an Abraxis enzyme-linked immunosorbent assay microtiter plate kit; Abraxis). This is the estimated environmental concentration of atrazine for farm ponds. Tadpoles were exposed to acetone control or atrazine for 7 d. They were fed agarose discs containing spirulina and flaked fish food ad libitum, and we checked survival daily.
Predator cue collection
Aquatic dragonfly larvae regularly prey on frog tadpoles 24 and are often the dominant predator in temporary pond ecosystems. We collected 12 Libellulidae dragonfly larvae from Spectrum Pond at the University of South Florida and maintained them in an 11.36-L aquarium filled with 5.68 L of dechlorinated tap water for 24 h. This tank also contained 12 Cuban tree frog tadpoles to provide food for the dragonfly larvae. The water in this tank served as our predator cue source.
Behavioral observations
For behavioral observations, 20 plastic shoeboxes were laid out in a 5-by-4 grid, and each was filled with 2 L of pond water. Observations were double blind, as the observer did not have access to the treatment groups of the individual tadpoles at the time of the observations. One tadpole was placed in each shoebox; 10 of the tadpoles were exposed to acetone control, and the other 10 were exposed to atrazine. Scan samples were conducted for 8 min before cues were added, recording the location of tadpoles in each shoebox (left or right side of the arena) and whether individual tadpoles were moving.
After the initial 8-min period, a repeat pipetter was used to add 3 mL of either a control (water) or experimental predator cue to 1 side of the container, while an equal amount of water was added to the opposite side of the container. Cue and control solutions were added carefully and simultaneously so that the liquid ran down the side of the container to minimize disturbance. One-half the predator cue addition in each treatment occurred on the right side of the container, and one-half was added to the left side. Thus, one-half the atrazine-exposed and one-half the acetone-exposed tadpoles received predator cue on 1 side of the container and the other tadpoles received water on both sides of the container. Having water on both sides allowed us to quantify activity levels in the absence of predator cue.
Immediately after cue additions, which took approximately 3 min, we performed scan samples for 20 min, again recording the location and activity of tadpoles. Given that 1 experimenter could only observe 20 tadpoles at a time, and we wanted the same experimenter making all observations, we conducted 3 additional temporal blocks in sequence, so that all 80 tadpoles were exposed to the cues in a timeframe of approximately 4 h. Immediately after observations in the shoeboxes were complete, all tadpoles were returned to their mason jars containing their assigned chemical treatment. The next day, the same procedure was repeated for all 80 tadpoles, except that any tadpole that was exposed to the predator cue on day 1 received water on both sides of the arena on day 2 and vice versa. This experimental design allowed us to use each tadpole as its own internal control for both activity and locational preference. After the 2 d of observations were complete, tadpoles were euthanized with MS-222 solution. We then recorded the snout–vent length, mass (g), and Gosner developmental stage 21 of each tadpole and preserved tadpoles individually in 70% ethanol.
To determine the mean time it took the predator chemical cues to diffuse across the test chamber, in separate trials, we added 5 drops of food coloring to a randomly selected end of 5 test chambers each containing a tadpole. Time was recorded when the dye had diffused to the halfway point of the tank and again when the dye reached the opposite end of the tank.
Data analysis
We first transformed the data, giving observations on the same side of the predator cue a value of 1 and on the opposite side as the cue a value of –1. Hence, negative values refer to avoidance and positive values refer to attraction. If a tadpole was moving during an observation, it received a 1, and if it was not moving it received a 0. Hence, the average of the activity data reflects the proportion of observations where the tadpole was moving.
Next, we conducted separate 2-way analyses of variance (ANOVAs) on average location and activity before any olfactory cues were added, testing for main and interactive effects of the atrazine and predator treatments. This allowed us to determine whether there were any differences between treatments before the predator cues were added. We knew that the predator cue would eventually diffuse across the entire test arena within the 30-min trial. Thus, we were expecting to detect an atrazine-by-predator-by-time interaction, because we hypothesized that the solvent-exposed but not atrazine-exposed animals would avoid the predator chemical cue early in the trial when there was a clear gradient, but avoidance would eventually subside once the cue fully dispersed. To conduct these analyses, we averaged the location and activity data for each of the four 5-min intervals of the 20-min trials. This enhanced our likelihood of meeting the assumptions of ANOVA (because of the central limit theorem). We then conducted a repeated measures ANOVA with individual as a random effect (so we could compare location and activity of each individual in the presence and absence of predator cues) and tested for the main and interactive effects of atrazine treatment, predator treatment, and time on location and activity responses. Thirteen tadpoles died during this experiment and thus were excluded from any statistical analyses. All analyses were conducted using Statistica 6.0 (Statsoft).
RESULTS
Before any predator cues were added, the tadpoles showed no significant difference in their average location in the atrazine or predator cue treatments (p > 0.05; Table 1 and Figure 1A). After the predator cue was introduced, tadpoles exposed to solvent, but not those exposed to atrazine, significantly avoided the predator chemical cue for the first 10 min of observations but exhibited no avoidance during the last 10 min (Figure 2). This resulted in a significant atrazine-by-predator cue-by-time interaction (F3,201 = 3.20, p = 0.024; Table 1). It took an average of 8.8 ± 0.97 min (± SE; n = 5) for the food coloring to diffuse fully across the test chamber. Hence, tadpole avoidance of the predator cue seemed to last for approximately as long as the cue gradient persisted.
| Effect | df | F | p |
|---|---|---|---|
| Pre-predator cue | |||
| Intercept | 1 | 1.64 | 0.204 |
| Atrazine | 1 | 0.74 | 0.392 |
| Error | 67 | ||
| Predator | 1 | 0.02 | 0.883 |
| Predator × atrazine | 1 | 0.44 | 0.507 |
| Error | 67 | ||
| Post-predator cue | |||
| Intercept | 1 | 0.21 | 0.648 |
| Atrazine | 1 | 1.82 | 0.182 |
| Error | 67 | ||
| Time | 3 | 0.37 | 0.778 |
| Time × atrazine | 3 | 0.76 | 0.519 |
| Error | 201 | ||
| Predator | 1 | 0.67 | 0.416 |
| Predator × atrazine | 1 | 0.12 | 0.730 |
| Error | 67 | ||
| Time × predator | 3 | 2.10 | 0.102 |
| Time × predator × atrazine | 3 | 3.20 | 0.024 |
| Error | 201 | ||
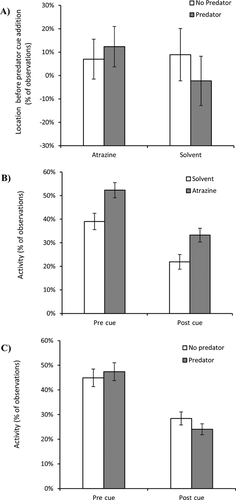

Before any predator cues were added, tadpoles exposed to atrazine were significantly more active than tadpoles exposed to solvent control (F1,67 = 7.84, p = 0.007; Figure 1B); but activity levels did not differ significantly between tadpoles assigned to receive predator cue or not (Table 2). After the predator cue was introduced, tadpoles exposed to atrazine remained significantly more active than tadpoles exposed to solvent control (F1,67 = 7.13, p = 0.009; Figure 1B). The tadpoles, however, did not significantly reduce their activity in response to the predator cue (Figure 1C) but did tend to exhibit less activity later than earlier in the trial (F3,201 = 4.45, p = 0.005; Figure 1C). There were no significant statistical interactions on tadpole activity (Table 2).
| Effect | df | F | p |
|---|---|---|---|
| Pre-predator cue | |||
| Intercept | 1 | 370.63 | <0.001 |
| Atrazine | 1 | 7.84 | 0.007 |
| Error | 67 | ||
| Predator | 1 | 0.30 | 0.583 |
| Predator × atrazine | 1 | 1.04 | 0.313 |
| Error | 67 | ||
| Post-predator cue | |||
| Intercept | 1 | 167.63 | <0.001 |
| Atrazine | 1 | 7.13 | 0.009 |
| Error | 67 | ||
| Time | 3 | 4.45 | 0.005 |
| Time × atrazine | 3 | 0.29 | 0.834 |
| Error | 201 | ||
| Predator | 1 | 2.05 | 0.157 |
| Predator × atrazine | 1 | 2.67 | 0.107 |
| Error | 67 | ||
| Time × predator | 3 | 0.51 | 0.674 |
| Time × predator × atrazine | 3 | 0.45 | 0.717 |
| Error | 201 |
DISCUSSION
We found evidence that atrazine exposure impairs the ability of Cuban tree frog tadpoles to detect chemical cues from predators. Tadpoles exposed to solvent control significantly avoided predator chemical cues, whereas tadpoles exposed to atrazine did not (Figure 2). In addition, we revealed that exposure to ecologically relevant concentrations of atrazine caused hyperactivity in Cuban tree frog tadpoles compared with tadpoles exposed to solvent control (Figure 1B). These results have many similarities with other studies examining the effects of atrazine on the behavior and olfactory abilities of amphibians and fish.
Several other studies have shown that ecologically relevant concentrations of atrazine affect the motor activity of fish and amphibians 9. After just 1 d of exposure to environmentally relevant concentrations of atrazine, red drum fish larva exhibited hyperactivity in the form of high swimming speeds, erratic swimming paths, and increased energy use and metabolic rates 47. Another study investigating the sublethal effects of atrazine in goldfish found that short-term exposures to low atrazine concentrations caused activity increases, including elevated burst swimming and surfacing activities 32. Salamander larva exposed to various concentrations of atrazine exhibited higher activity than control larvae 11, 14, and these effects persisted for several months after atrazine exposure ceased 12, 13.
Our finding that atrazine exposure impairs the ability of Cuban tree frog tadpoles to detect chemical cues that predators release is consistent with several studies showing that ecologically relevant concentrations of atrazine can compromise the olfactory abilities of fish and crayfish 29-35. However, the present study's results are inconsistent with the only other study that examined atrazine-related infodisruption in tadpoles 36. Importantly, every study that detected atrazine-associated infodisruption tested for infodisruption while the fish, crayfish, or amphibian was being exposed to atrazine 29-35. The single study that did not detect infodisruption associated with atrazine-exposed tadpoles transferred the tadpoles to atrazine-free water, provided a 30-min acclimation period, and then introduced chemical cues to elicit behavioral responses 36. If the effect of atrazine on the olfactory organs of vertebrates is short-lived, then 30 min exposure to clean water might have been sufficient time to rinse the atrazine from the olfactory organ, allow for at least partial recovery of olfactory function, or prevent the detection of any atrazine-induced olfactory impairment. However, we cannot rule out species-level differences in sensitivity to atrazine or other differences among the experiments as explanations for the differences in their results. We encourage future studies to evaluate species-level variation in olfactory sensitivity to atrazine and how both durations and concentrations of atrazine exposure affect amphibian olfaction.
Amphibians are in decline globally 48, 49, and although it is theoretically possible that atrazine-induced infodisruption could contribute to these declines, we caution against extrapolating these results to the population viability of amphibians in the wild for several reasons. First, if the effects of atrazine are found to be short-lived, it might suggest that any infodisruption might not have substantial impacts on wildlife populations. Hence, quantifying the duration of atrazine effects on olfaction would offer insight into both discrepancies in the results among studies and the likelihood that any infodisruption would have population-level impacts. Second, if the olfaction of predators is equally or more impaired by atrazine than the olfaction of amphibian prey, then any infodisruption could benefit the prey more than the predators. It is important, therefore, to understand the net effects of pesticides on species' interactions before reliably extrapolating to effects in nature 19. Finally, there are many factors that are not captured in a laboratory study that could affect the effects of atrazine in the wild, such as variation in temperature and organic material on which pesticides can bind 18. Regardless of how any atrazine-induced infodisruption influences populations of vertebrates in the wild, the present study's results contribute to the weight of evidence that atrazine is, indeed, an interorganismal infodisruptor of vertebrates. We encourage further studies to more thoroughly quantify the consequences of such infodisruption and whether they are capable of contributing to declines of biodiversity.
Acknowledgment
We thank B. Roznik and C. Gabor for help and support. This research was supported by grants from the National Science Foundation (EF-1241889), National Institutes of Health (R01GM109499, R01TW010286), US Department of Agriculture (NRI 2006-01370, 2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801) to J. Rohr.
Data availability
Data are available by contacting the corresponding author ([email protected]).




