Inhibition effect of Na+ and Ca2+ on the bioaccumulation of perfluoroalkyl substances by Daphnia magna in the presence of protein
Abstract
The authors investigated the individual effects of Ca2+ and Na+ on the bioaccumulation of 6 types of perfluoroalkyl substances (PFASs), including perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), and perfluorododecanoic acid (PFDoA), by Daphnia magna in water with 10 mg L−1 bovine albumin or soy peptone. The bioaccumulation factors of PFASs by D. magna decreased linearly with the increase of Ca2+ and Na+ concentrations. The inhibition effect of Ca2+ was stronger than that of Na+, and the decreasing percentages of the body burden of PFASs in D. magna caused by the increment of 1 mmol L−1 Ca2+ and 1 mmol L−1 Na+ were 41% to approximately 48% and 2% to approximately 5%, respectively, in the presence of soy peptone. The partition coefficients (Kp) of PFASs between protein and water increased with rising Ca2+ and Na+ concentrations. The elevated Kp values led to the reduced concentrations of freely dissolved PFASs. This resulted in a decrease of PFAS bioaccumulation in D. magna, and the body burden of each PFAS was positively correlated with its freely dissolved concentration in water. The present study suggests that cations should be considered in the assessment of bioavailability and risk of PFASs in natural waters containing proteinaceous compounds. Environ Toxicol Chem 2014;9999:1–8. © 2014 SETAC
INTRODUCTION
Over the past 10 yr, perfluoroalkyl substances (PFASs) have increasingly attracted global attention because of their widespread application, environmental persistence, and potential toxicity to animals 1, 2. Some studies have shown that some PFASs such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) may induce potential carcinogenicity and reproductive toxicity on test organisms 3-5. The PFASs are globally distributed in the environment; their concentrations generally range from picograms to nanograms per liter for individual compounds in the aquatic environment, and they can reach micrograms per liter in some waters 6, 7. PFASs have a high bioaccumulation potential in organisms 8. For example, Lasier et al. 9, Rainie et al. 10, and Dai et al. 11 have demonstrated the bioaccumulation of PFASs in some aquatic organisms, including Lumbriculus variegatus, zebrafish (Danio rerio), rainbow trout (Oncorhynchus mykiss), and Daphnia magna.
Moreover, both laboratory studies and field monitoring have shown that PFASs tend to accumulate in protein-rich tissues and blood of exposed organisms 12-15. For instance, Jeon et al. 16 reported that perfluorinated compounds accumulated significantly in liver and serum of Sebastes schlegeli, a kind of black rockfish. Jones et al. 13 have demonstrated that PFOS was strongly bound with serum protein of fishes and birds. Butt et al. 17 also reported that PFASs accumulated in protein-rich tissues including blood, liver, and kidneys in whale and seabird species. Consequently, PFASs are considered as proteinophilic compounds 18, 19. Proteinaceous compounds are not only the main components of organisms including animals and plants but also are ubiquitous in aquatic systems, where they come from the wastewater of slaughterhouse, casein, fish, whey, and vegetable processing industries, with concentrations ranging from 1 mg L−1 to 20 mg L−1 20. Feng et al. 21 reported that the protein concentration ranged from 1.8 mg L−1 to 30.2 mg L−1 in domestic wastewater of a typical region in the Zhejiang province of China. In our previous work 22, we provided evidence that proteins in aquatic environments also combine with PFASs and decrease their bioavailability, and a relatively higher concentration of proteins (>1 mg L−1) will decrease the bioaccumulation of PFASs in D. magna.
In addition, some studies have reported that cations could combine with proteins, and the binding strength differs among different cations. For example, Kretsinger et al. 23 proposed that Ca2+ strongly binds to the protein containing a helix-loop-helix structural EF hand. Poonia and Bajaj 24 reported that Ca2+ has a higher affinity binding even in a 100-fold to 1000-fold excess than Mg2+ to protein. Speroni et al. 25 confirmed that Ca reinforces the protein structure by increasing the stability of existing interactions or promoting the formation of new ones, like hydrogen bonds, electrostatic interactions, or Ca bridges. Vrbka et al. 26 also studied the binding of a series of different proteins with ions and consistently found that sodium (Na+) binds at least twice as strongly to the protein surface as potassium (K+).
Ca2+ and Na+ are major cations in natural aquatic environments, with their concentrations ranging from several to thousands of milligrams per liter 27, 28. As mentioned, cations including Ca2+ and Na+ can affect the structure and conformation of proteins. Thus, we hypothesize that cations will affect the interaction of protein and PFASs and then exert influence on the PFAS bioavailability to, and bioaccumulation by, aquatic organisms. Moreover, the effects of cations will vary with their concentrations and types.
Therefore, the main objective of the present study was to investigate the individual effects of Ca2+ and Na+ on the bioaccumulation of 6 kinds of PFASs, including PFOS, PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), and perfluorododecanoic acid (PFDoA), by D. magna in water with protein. Two types of model protein including soy peptone from plants and bovine albumin from animals were investigated, and the effects of cation concentrations were studied. Furthermore, the binding of PFASs to protein under different concentrations of Ca2+ and Na+ was examined, and the freely dissolved concentrations of PFASs were calculated to explore the effect mechanism of ions on the bioaccumulation of PFASs. The present study is the first to report the effect of ions on the bioaccumulation of PFASs by organisms in water containing protein compounds.
MATERIALS AND METHODS
Reagents
Perfluorooctane sulfonate (98%) was obtained from Tokyo Chemical Industries; PFOA (99.9%) and PFDA (99.9%) were obtained from Sigma-Aldrich; and PFNA (97%), PFUnA (95%), and PFDoA (95%) were obtained from Acros Organics. A stock solution containing these PFASs was prepared in a methanol to water solution (80:20, v/v) at a concentration of 200 mg L−1 for each PFAS. Both [1,2,3,4–13C4] perfluorooctane sulfonate (MPFOS) and [1,2,3,4–13C4] perfluorooctanoic acid (MPFOA; purity > 99%) were purchased from Wellington Laboratories and used as recovery indicators. Chromatography-grade methanol was obtained from J.T. Baker. Methyl-tert-butyl ether (99.5%), tetrabutyl ammonium hydrogen sulfate, and ammonium acetate (98%) were obtained from Sigma-Aldrich; they were used to extract PFASs from D. magna. Sodium chloride (NaCl) and calcium chloride (CaCl2) were obtained from Sinopharm Chemical Reagent. Soy peptone and bovine albumin were purchased from AMRESCO and Sigma-Aldrich, respectively. Sodium dihydrogen phosphate monohydrate (NaH2PO4 · H2O) and anhydrous sodium hydrogen phosphate (Na2HPO4) were purchased from Fisher Chemical. Spectra6 dialysis bags of 7000 Da molecular weight were obtained from Sigma-Aldrich.
Cultivation of D. magna
The cultivation of D. magna was performed according to the chemical testing conditions described by Organisation for Economic Co-operation and Development 29. In brief, the cultivation of D. magna was conducted in artificial freshwater containing 0.294 g CaCl2, 0.123 g MgSO4, 0.064 g NaHCO3, and 0.006 g KCl per liter deionized water (with Ca2+ = 2.64 mmol L−1, Na+ = 0.76 mmol L−1, and pH = 7.15). Daphnia magna were cultured under a 16:8-h light:dark photoperiod at 21 ± 0.5 °C and were fed a Scenedesmus subspicatus suspension twice per day. The detailed culture procedure can be found in our previous study 11.
Effect of Ca2+ and Na+ on PFAS bioaccumulation in the presence of protein
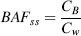 (1)
(1)| Decreasing percentages of the body burden of PFASs | |||||
|---|---|---|---|---|---|
| Treatments | Na+ (mmol L–1) | Ca2+ (mmol L–1) | pH | Bovine albumin | Soy peptone |
| Control without protein | 0.76a | 2.64a | 7.15a | ||
| Effect of Na+ in the presence of 10 mg L–1 soy peptone or bovine albumin | |||||
| Addition of 0 mmol L–1 Na+ | 0.76 | 2.64 | 7.15 | ||
| Addition of 1 mmol L–1 Na+ | 1.76 | 2.64 | 7.19 | 3%–6% | 2%–5% |
| Addition of 5 mmol L–1 Na+ | 5.76 | 2.64 | 7.23 | 21%–33% | 19%–29% |
| Addition of 10 mmol L–1 Na+ | 10.76 | 2.64 | 7.28 | 39%–47% | 32%–43% |
| Effect of Ca2+ in the presence of 10 mg L–1 soy peptone or bovine albumin | |||||
| Addition of 0 mmol L–1 Ca2+ | 0.76 | 2.64 | 7.15 | ||
| Addition of 0.25 mmol L–1 Ca2+ | 0.76 | 2.89 | 7.17 | 9%–21% | 6%–16% |
| Addition of 0.5 mmol L–1 Ca2+ | 0.76 | 3.14 | 7.19 | 32%–43% | 24%–34% |
| Addition of 1.0 mmol L–1 Ca2+c | 0.76 | 3.64 | 7.24 | 46%–62% | 41%–48% |
- a The composition of artificial freshwater for Daphnia magna cultivation.
Dialysis experiments
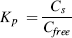 (2)
(2)Extraction and analysis of PFASs
An ion-pairing agent extraction technique was used to extract PFASs from water and D. magna samples, with some modification 30, 31. The extraction was conducted following the procedure descried in our previous study 11. In brief, each polypropylene centrifuge tube containing 10 D. magna or 4.5 mL water sample was spiked with 2 mL Na2CO3 (0.25 mol L−1), 2 mL methyl-tert-butyl ether, 1 mL ion-pairing agent tetrabutyl ammonium hydrogen sulfate (0.5 mol L−1, adjusted to pH 10), and 100 µL internal standards (10 ng MPFOA and MPFOS). The PFASs were analyzed with liquid chromatography–tandem mass spectrometry (Applied Biosystems API 3200 and Dionex Ultimate 3000) in an electrospray negative ionization mode. A 10-µL sample aliquot was injected into an Acclaim 120 C18 Column of 4.6 mm × 150 mm, and the mobile phase was 50 mmol L−1 ammonium acetate and methanol at a 1 mL min−1 flow rate. The detailed procedure can be found in our previous study 32.
Quality assurance and quality control
The procedure of quality assurance and quality control is described in the Supplemental Data. The limits of quantification (signal to noise [S/N] = 10) for the tested PFASs were between 0.01 µg L−1 and 0.05 µg L−1 with liquid chromatography–tandem mass spectrometry; and all procedural blank areas were lower than half limits of quantification. The repeatability of the standard curves with the correlation coefficients higher than 0.99 was confirmed before each determination. The recoveries of MPFOS, MPFOA, and the tested PFASs from the water samples and protein solutions were in the range of 86% to 108%. Recoveries of the mass spectrometric isotopes added to the D. magna samples ranged from 90% to 95%, and recoveries of the tested PFASs from the D. magna were in the range of 81% to 95% (Table S1). The recoveries of the indicators were used to correct all data of the tested PFASs. In the blank bioaccumulation tests, PFASs in the D. magna samples were below the detection limits. Furthermore, each PFAS accumulated in D. magna accounted for less than 2% of the whole amount added in the system; this indicated that PFAS bioaccumulation in D. magna did not exert significant influences on the PFAS concentrations and their equilibrium between protein and water in the system. For the dialysis bag experiments, PFASs could pass through the dialysis bag freely, with the PFAS concentration difference between the outside and inside of the dialysis bag being lower than 8% without protein in the system, and the mass balance results showed that the PFAS variations were lower than 10% in the system in the presence of protein.
Statistical analysis of data
All statistical analyses of the data were conducted using SPSS 18.0 for windows. The difference between each of the 2 compared groups was tested with one-factor analysis of variance (ANOVA). Duncan's multiple-range test was used to examine the differences among compared groups. Difference was considered statistically significant when the significance level was smaller than 0.05.
RESULTS AND DISCUSSION
Effect of ion concentrations on the PFAS bioaccumulation in D. magna
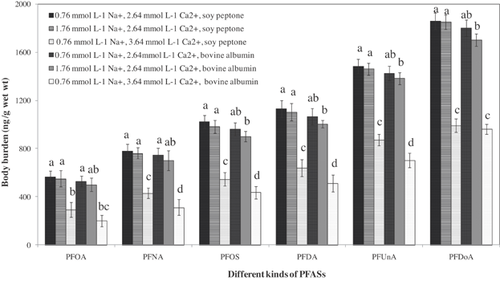
As shown in Figure 1, the body burden of PFASs in D. magna was ordered PFOA < PFNA < PFOS < PFDA < PFUnA < PFDoA for all of the treatments. The bioaccumulation of PFASs was positively correlated with the perfluoroalkyl chain length, and the body burden of PFOS was significantly higher than that of PFNA, with an equal perfluoroalkyl chain length. These findings were consistent with many reported results for Lumbriculus variegates, rainbow trout (Oncorhynchus mykiss), D. magna, and green mussels (Perna viridis) 9, 14, 20, 33. This indicated that the presence of protein and the concentrations of Na+ and Ca2+ did not exert influence on the order of 6 PFAS bioaccumulation in D. magna. In addition, as shown in Supplemental Data, Figure S1, the effect of Ca2+ and Na+ concentrations on the bioaccumulation of PFASs in D. magna was not significant in the absence of protein compounds. This suggests that although Ca2+ and Na+ are essential for D. magna, the variation of Ca2+ from 2.64 mmol L−1 to 3.64 mmol L−1 and the variation of Na+ from 0.76 mmol L−1 to 5.76 mmol L−1 will not exert significant influence on the bioaccumulation of PFASs in D. magna.
Compared with the body burden of PFASs in the absence of proteins, bovine albumin or soy peptone at a concentration of 10 mg L−1 decreased the bioaccumulation of PFASs in D. magna under different Na+ and Ca2+ concentrations. For example, the presence of 10 mg L−1 soy peptone reduced the body burden of PFASs by 7% to approximately 50% under different concentrations of Ca2+. In addition, the decrease of PFAS bioaccumulation caused by the bovine albumin was greater than that caused by soy peptone regardless of the Na+ and Ca2+concentrations (Supplemental Data, Figure S2), and this might be explained by the difference in characteristics of soy peptone and bovine albumin (Supplemental Data, Table S2).
In the presence of soy peptone or bovine albumin, the body burden and BAFss values of PFASs in D. magna decreased with increasing Na+ concentration (p < 0.05; Figure 1 and Supplemental Data, Table S3). For the systems with soy peptone, the body burden of PFASs was decreased by 2% to approximately 5% and 32% to approximately 43% when Na+ increased from 0.76 mmol L−1 to 1.76 mmol L−1, and 10.76 mmol L−1, respectively (Table 1). For the systems with bovine albumin, the body burden of PFASs was decreased by 3% to approximately 6% and 39% to approximately 47% when Na+ increased from 0.76 mmol L−1 to 1.76 mmol L−1, and 10.76 mmol L−1, respectively. Further analysis showed that the BAFss value of each PFAS in D. magna decreased linearly with the increase of Na+ concentration in the presence of soy peptone or bovine albumin (n = 4, r > 0.96, p < 0.05; Fig. 2).
Similar to Na+, the addition of Ca2+ to protein solutions also inhibited the PFAS bioaccumulation by D. magna. As shown in Figure 1, the body burden of all tested PFASs decreased gradually when Ca2+ concentration increased from 2.64 mmol L−1 to 3.64 mmol L−1. For example, when the Ca2+ concentration increased from 2.64 mmol L−1 to 2.89 mmol L−1, 3.14 mmol L−1, and 3.64 mmol L−1, the body burden of PFOS decreased from 1023 ± 53 ng g−1 to 921 ± 41 ng g−1, 780 ± 59 ng g−1, and 545 ± 57 ng g−1 for the systems in the presence of ng g−1 soy peptone, and a similar result was obtained for the systems in the presence of bovine albumin (Fig. 1). For soy peptone, the PFAS body burden and BAFss values decreased by 41% to approximately 48% when the concentration of Ca2+ increased from 2.64 mmol L−1 to 3.64 mmol L−1. For bovine albumin, they decreased by 46% to approximately 62%. As shown in Figure 2, BAFss value of each PFAS in D. magna decreased linearly with the increase of Ca2+ concentration (n = 4, r > 0.96, p < 0.05 except for PFDoA).
Comparison of the effect of Ca2+ and Na+ on PFAS bioaccumulation in the presence of protein
According to the results shown in Figure 3, Ca2+ was more efficient at inhibiting the PFAS bioaccumulation in D. magna than Na+ with the presence of protein in water. For instance, when Ca2+ concentration was 2.64 mmol L−1 and Na+ concentration increased from 0.76 mmol L−1 to 1.76 mmol L−1, the decreasing ratios of PFASs in D. magna caused by the increment of 1 mmol L−1 Na+ were 2% to approximately 5% in the presence of 10 mg L−1 soy peptone, and 3% to approximately 6% in the presence of 10 mg L−1 bovine albumin. When Na+ concentration was 0.76 mmol L−1 and Ca2+ concentration increased from 2.64 mmol L−1 to 3.64 mmol L−1, the decreasing ratios of PFASs in D. magna caused by the increment of 1 mmol L−1 Ca2+ were 41% to approximately 48% and 46% to approximately 58% in the presence of soy peptone and bovine albumin, respectively. According to the results shown in Figure 2, the decreasing rates of BAFss value of each PFAS caused by the increase of Ca2+ were much higher than that caused by Na+. For example, the decrease in BAFss (L kg−1) for PFASs was approximately 65 L kg−1 to 175 L kg−1and 5 L kg−1 to 17 L kg−1, caused by each increase in unit (mmol L−1) [Ca2+] and [Na+], respectively, in the presence of bovine albumin, and that was 57 L kg−1 to 185 L kg−1 and 5 L kg−1 to 16 L kg−1, respectively, in the presence of soy peptone.
Influencing mechanism of ions on PFAS bioaccumulation in the presence of protein
Effects of ions on the partition of PFASs between protein and water
Except for PFOS, the partition coefficients (Kp) of PFASs between protein and water increased with perfluoroalkyl chain length for all of the treatments, and the Kp value between bovine albumin and water was significantly higher than that between soy peptone and water for each of the PFAS (Table 2). The partition coefficients increased with rising Na+ and Ca2+ concentrations (Table 2), and the effect of Ca2+ on the binding of PFASs and protein was much greater than that of Na+. According to the relationship between logKp and log[Ca2+] as well as log[Na+] shown in Supplemental Data, Figure S3, the increase in logKp for PFASs between soy peptone and water was approximately 2.4 mmol L−1 to 5.4 mmol L−1 and 0.2 mmol L−1 to 0.7 mmol L−1 for each increase in log unit [Ca2+] and [Na+], respectively, and that between bovine albumin and water was approximately 4.2 mmol L−1 to 7.9 mmol L−1 and 0.4 mmol L−1 to 1.0 mmol L−1, respectively. These results indicate that the binding of PFASs to proteins was dependent, not only on the concentration, but also on the type of cations.
| PFOA | PFNA | PFOS | PFDA | PFUnA | PFDoA | |
|---|---|---|---|---|---|---|
| Soy peptone | ||||||
| 0.76 mmol L–1 Na+, 2.64 mmol L–1 Ca2+, pH = 7.15 | 3.83 ± 0.016 | 4.10 ± 0.058 | 4.51 ± 0.012 | 4.61 ± 0.001 | 5.05 ± 0.026 | 5.19 ± 0.101 |
| 0.76 mmol L–1 Na+, 2.89 mmol L–1 Ca2+, pH = 7.08 | 4.10 ± 0.010 | 4.30 ± 0.012 | 4.87 ± 0.052 | 5.03 ± 0.011 | 5.22 ± 0.020 | 5.69 ± 0.013 |
| 0.76 mmol L–1 Na+, 3.64 mmol L–1 Ca2+, pH = 7.11 | 4.21 ± 0.013 | 4.71 ± 0.014 | 4.93 ± 0.021 | 5.22 ± 0.011 | 5.75 ± 0.017 | 6.01 ± 0.019 |
| 1.76 mmol L–1 Na+, 2.64 mmol L–1 Ca2+, pH = 7.13 | 3.88 ± 0.006 | 4.14 ± 0.012 | 4.55 ± 0.026 | 4.65 ± 0.020 | 5.11 ± 0.037 | 5.22 ± 0.028 |
| 5.76 mmol L–1 Na+, 2.64 mmol L–1 Ca2+, pH = 7.17 | 4.18 ± 0.022 | 4.36 ± 0.033 | 4.69 ± 0.010 | 5.06 ± 0.021 | 5.30 ± 0.032 | 5.78 ± 0.018 |
| Bovine albumin | ||||||
| 0.76 mmol L–1 Na+, 2.64 mmol L–1 Ca2+, pH = 7.15 | 4.47 ± 0.059 | 4.65 ± 0.058 | 4.92 ± 0.072 | 5.17 ± 0.243 | 5.30 ± 0.097 | 5.60 ± 0.070 |
| 0.76 mmol L–1 Na+, 2.89 mmol L–1 Ca2+, pH = 7.08 | 4.94 ± 0.020 | 5.20 ± 0.021 | 5.37 ± 0.023 | 5.74 ± 0.019 | 6.10 ± 0.011 | 6.21 ± 0.026 |
| 0.76 mmol L–1 Na+, 3.64 mmol L–1 Ca2+, pH = 7.11 | 5.14 ± 0.035 | 5.34 ± 0.019 | 5.65 ± 0.014 | 6.04 ± 0.018 | 6.45 ± 0.016 | 6.78 ± 0.037 |
| 1.76 mmol L–1 Na+, 2.64 mmol L–1 Ca2+, pH = 7.13 | 4.84 ± 0.033 | 5.08 ± 0.045 | 5.22 ± 0.028 | 5.62 ± 0.019 | 5.73 ± 0.024 | 5.92 ± 0.023 |
| 5.76 mmol L–1 Na+, 2.64 mmol L–1 Ca2+, pH = 7.17 | 5.02 ± 0.035 | 5.18 ± 0.029 | 5.26 ± 0.018 | 5.79 ± 0.019 | 6.17 ± 0.016 | 6.31 ± 0.017 |
- PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid; PFDA = perfluorodecanoic acid; PFUnA = perfluoroundecanoic acid; PFDoA = perfluorododecanoic acid.
Because protein is one kind of dissolved organic matter (DOM), we compared the results obtained in the present study with others about DOM. A similar result was reported for the binding of anthracene, one kind of polycyclic aromatic hydrocarbon (PAH), to 25 mg L−1 humic acid (pH = 7) that the partition coefficient increased with rising Na+ concentration 34. The increased partition coefficients of hydrophobic organic compounds (HOCs) between DOM and water with increasing ion strength have been found by other researchers. Gauthier et al. 35 found that the binding of pyrene, one kind of PAH, to humic acid increased with rising ionic strength when sodium chloride was used as the electrolyte. Jota and Hassett 36 reported an increasing binding of 2,2',5,5'-tetrachlorobiphenyl by humic acid when ionic strength was increased. Lee et al. 37 proposed a 3-stage variation model to describe DOM conformation change with increasing ionic strength, that is, change of DOM structural configuration, DOM aggregation, and salting-out effect. In addition, the salting-out effect of HOCs has been applied to explain the enhanced HOC sorption on humic substances after the addition of ions 38.
The salting-out effect also may occur for PFASs; a comparison of the solubility of PFOS in fresh water (680 mg L−1) and ocean water (12.4 mg L−1) confirms that the salting-out effect is significant for PFASs 39. Therefore, the increase of Ca2+ and Na+ concentrations could decrease the solubility of PFASs and elevate their binding to proteins. In addition, previous studies showed that the binding of cations to the negatively charged carboxylic groups of DOM could reduce the charge of DOM 40, 41. Because protein has a net negative charge in solution 42, the binding of cations to the negatively charged groups of protein, such as carboxylic groups, could also reduce the charge of protein. The PFASs also charge negatively in water solutions, and so the charge reduction of protein may result in the decrease of the repulsive force between PFASs and protein. Na+ and Ca2+ can also form a bridge between PFASs and protein; this leads to the enhancement of binding between PFASs and proteins. Therefore, the partition coefficients of PFASs between protein and water increased with rising cation concentrations. Tang et al. 43 also reported that the presence of Ca2+ increased the adsorption of PFASs to mineral by binding to the mineral surface and thereby increasing the electrostatic attraction between PFASs and mineral. Because Ca2+ is a divalent cation and Na+ is a monovalent cation, Ca2+ would exert a stronger salting-out effect and decrease the charge of protein more pronouncedly than Na+, resulting in greater effect on the binding of PFASs with protein. In addition, as mentioned before, for all of the treatments with different concentrations of Ca2+ and Na+, the partition coefficients of PFASs were positively correlated with their perfluoroalkyl chain length (Table 2). Therefore, both hydrophobic interactions and electrostatic attraction are important processes for the binding of PFASs to proteins.
Effects of ions on the freely dissolved PFAS concentrations
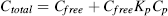 (3)
(3)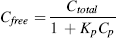 (4)
(4)CONCLUSIONS
The presence of protein (10 mg L−1 soy peptone or 10 mg L−1 bovine albumin) inhibited the bioaccumulation of PFASs by D. magna, and both Ca2+ and Na+ could inhibit the bioaccumulation of PFASs further, with the body burden of PFASs decreasing linearly with the increase of Ca2+ and Na+ concentrations. The impact of Ca2+ on PFAS bioaccumulation by D. magna was greater than that of Na+ in the water system containing bovine albumin or soy peptone.
The partition coefficients (Kp) of PFASs between proteins and water increased with rising Ca2+ and Na+ concentrations in aqueous solution, and the effect of Ca2+ was stronger than that of Na+. The increase in logKp for PFASs between soy peptone and water was approximately 2.4 mmol L−1 to 5.4 mmol L−1 and 0.2 mmol L−1 to 0.7 mmol L−1 for each increase in log unit [Ca2+] and [Na+], respectively. This suggests that both hydrophobic forces and electrostatic interactions play important roles in the association of PFASs with protein. The elevated partition coefficients of PFASs between protein and water caused by the increase of Ca2+ and Na+ concentrations led to the reduced concentrations of freely dissolved PFASs, resulting in the decreased body burden of PFASs in D. magna.
According to the present study, both Ca2+ and Na+ exerted significant influence on the PFAS bioavailability to and PFAS bioaccumulation by D. magna in the presence of proteins. Because protein compounds are ubiquitous in natural waters, and the variations of Ca2+, Na+, and other cation concentrations are significant, the present study suggests that both proteins and cations should be considered in the assessment of bioavailability and risk of PFASs in natural waters.
SUPPLEMENTAL DATA
Tables S1–S4.
Figures S1–S3 (225 KB).
Acknowledgment
The present study was supported by National Science Foundation for Distinguished Young Scholars, (No. 51325902), the Fund for Innovative Research Group of the National Natural Science Foundation of China (Grant No. 51421065), National Natural Science Foundation of China (No. 51279010), and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110003110030).




