Cross-species sensitivity to a novel androgen receptor agonist of potential environmental concern, spironolactone
Abstract
Spironolactone is a pharmaceutical that in humans is used to treat conditions like hirsutism, various dermatologic afflictions, and female-pattern hair loss through antagonism of the androgen receptor. Although not routinely monitored in the environment, spironolactone has been detected downstream of a pharmaceutical manufacturer, indicating a potential for exposure of aquatic species. Furthermore, spironolactone has been reported to cause masculinization of female western mosquitofish, a response indicative of androgen receptor activation. Predictive methods to identify homologous proteins to the human and western mosquitofish androgen receptor suggest that vertebrates would be more susceptible to adverse effects mediated by chemicals like spironolactone that target the androgen receptor compared with invertebrate species that lack a relevant homolog. In addition, an adverse outcome pathway previously developed for activation of the androgen receptor suggests that androgen mimics can lead to reproductive toxicity in fish. To assess this, 21-d reproduction studies were conducted with 2 fish species, fathead minnow and Japanese medaka, and the invertebrate Daphnia magna. Spironolactone significantly reduced the fecundity of medaka and fathead minnows at 50 μg/L, whereas daphnia reproduction was not affected by concentrations as large as 500 μg/L. Phenotypic masculinization of females of both fish species was observed at 5 μg/L as evidenced by formation of tubercles in fathead minnows and papillary processes in Japanese medaka. Effects in fish occurred at concentrations below those reported in the environment. These results demonstrate how a priori knowledge of an adverse outcome pathway and the conservation of a key molecular target across vertebrates can be utilized to identify potential chemicals of concern in terms of monitoring and highlight potentially sensitive species and endpoints for testing. Environ Toxicol Chem 2013;32:2528–2541. © 2013 SETAC
INTRODUCTION
A strategic approach to identifying endocrine-disrupting chemicals ideally would utilize existing toxicological knowledge to prioritize and focus screening and environmental monitoring efforts. Pharmaceuticals represent a class of chemicals that are particularly amenable to such an exercise. The rigorous design and approval process for human drugs in the United States and elsewhere requires a compilation of extensive mammalian data related to the chemical's molecular target specificity, therapeutic mode of action, and potential toxicity or side effects. This a priori knowledge theoretically can be used to conduct screening-level assessments in terms of identifying those chemicals likely to pose the greatest risk to nontarget species.
Based on an evaluation of available information, we identified the pharmaceutical spironolactone as a possible endocrine-disrupting chemical, which may be of concern in aquatic environments. Recently, it was reported that spironolactone, a chemical that is not routinely monitored, was detected in a French river downstream of a pharmaceutical manufacturer at a concentration as high as 10 μg/L 1. Spironolactone (including key metabolites) has been reported to target the mineralocorticoid and androgen receptors in humans and is commonly prescribed for both human and veterinary uses 2-4. Initially, spironolactone was used as a diuretic to treat patients with chronic heart disease, but a notable side effect of the drug was the production of gynecomastia in men. Gynecomastia, the development of enlarged breast tissue, was observed to occur as early as 1 mo after initiation of spironolactone dosing 5. This side effect in humans occurs through spironolactone metabolite intermediates (e.g., 7α-thiomethylspironolactone) acting as androgen receptor antagonists, which, among other responses, decrease circulating testosterone and elevate 17β-estradiol 5-7. As a consequence of spironolactone activity as an antiandrogen, it is also currently used to treat a variety of skin conditions and female-pattern hair loss in humans 8, 9.
While an understanding of the biological activity of spironolactone in mammals was required prior to approval of the chemical for human therapeutic uses 7, little is known concerning its possible effects in nontarget species. Limited studies suggest that spironolactone has the potential to affect fish reproduction 10, 11. Raut et al. 11 reported phenotypic masculinization (anal fin elongation) and a decrease in hepatic vitellogenin mRNA in female western mosquitofish (Gambusia affinis) exposed to 4.2 μg spironolactone/L and 104.1 μg spironolactone/L, respectively. Although reproductive toxicity was not directly assessed in that study, the observed masculinization of females, which is indicative of androgen receptor activation, suggests that spironolactone could cause reproductive impairment. Studies with the synthetic steroid 17β-trenbolone, an environmental contaminant associated with livestock operations, have shown that activation of the androgen receptor in fish can be a key molecular initiating event in an adverse outcome pathway leading to impaired reproduction (Figure 1) 12, 13. Specifically, on perturbation of the androgen receptor in fathead minnow (Pimephales promelas), a number of events spanning multiple levels of biological organization occur, including androgen receptor–dependent somatic cell proliferation, leading to weight gain and nuptial tubercle and fat pad formation in females (indicative of androgen receptor activation), and reductions in testosterone and 17β-estradiol production, vitellogenesis, and deposition of vitellogenin in the developing oocytes, leading to reduced fecundity (Figure 1) 12, 14. Furthermore, in vitro studies with a mammalian cell line indicate that spironolactone possesses significant androgenic activity (V. Wilson, Research Triangle Park, NC, USA, personal communication). Together, the indication from in vitro and fish studies that spironolactone causes androgen receptor activation, along with an established adverse outcome pathway associating androgen receptor activation with impairment of reproduction, led us to predict that exposure to spironolactone has the potential to elicit adverse reproductive effects 12, 13.
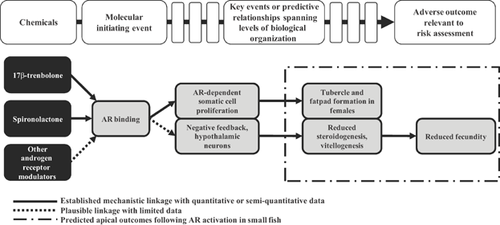
A key to application of the adverse outcome pathway concept to extrapolating potential biological effects across species is understanding phylogenetic conservation of molecular initiating events. Fortunately, there are rapidly evolving approaches to start to obtain this knowledge 15-17. We recently conducted an analysis based on protein sequence data comparing mammalian androgen receptor and estrogen receptor-α with homolog candidates spanning a diversity of taxa, quantitatively demonstrating the degree of conservation of these key nuclear hormone receptors across both vertebrate and invertebrate species 17. In conjunction with the adverse outcome pathway construct for androgen receptor activation and reproduction described above, knowledge of molecular target conservation can lead to informed selection of test organisms for toxicity studies, focusing resources on sensitive species most likely to drive risk-management decisions.
After consideration of available data for spironolactone, we prioritized it as a potential chemical warranting further testing because of: 1) the detection in the aquatic environment, 2) binding to the androgen receptor in humans and the protein sequence conservation of the androgen receptor across vertebrate species, 3) occurrence of data for mosquitofish suggesting androgen receptor agonism, and 4) adverse outcome pathway knowledge for small fish pertaining to activation of the androgen receptor. Based on this prioritization strategy, we conducted a comprehensive series of 21-d reproduction assays in 2 aquatic vertebrates, the fathead minnow and Japanese medaka (Oryzias latipes), and the invertebrate Daphnia magna, a cladoceran. We hypothesized from the available androgen receptor sequence data that spironolactone would have limited to no effect on invertebrates 17 but would impair reproduction in fish and elicit a suite of effects at multiple levels of biological organization similar to those defined for the androgen receptor activation–reproduction adverse outcome pathway based on 17β-trenbolone 12.
MATERIALS AND METHODS
Androgen receptor conservation across species
A tiered approach was used to assess conservation of the androgen receptor across species, initially focusing on the human androgen receptor, which was reported to be modulated by spironolactone in vitro (V. Wilson, Research Triangle Park, NC, USA, personal communication) and was also identified as a molecular target for spironolactone in the DrugBank database 4. The second sequence similarity analysis used the knowledge that female western mosquitofish exposed to spironolactone developed a male secondary sex characteristic, likely via activation of the androgen receptor. Therefore, as a means to focus our sequence similarity analysis, we used the western mosquitofish androgen receptor as the query species/molecular target for the identification of homolog candidates. In each case, protein similarity was assessed using the method described by LaLone et al. 17, which quantitatively compares primary amino acid sequences and conserved domains across species. The National Center for Biotechnology Information protein accessions, AAA51729 and BAD52084, were identified for the human (Homo sapiens) and western mosquitofish androgen receptors, respectively 18.
Test material and organisms
The spironolactone metabolites canrenone and 7α-thiomethylspironolactone, both >95% pure, were purchased from Tocris Biosciences and Santa Cruz Biotechnology, respectively. Spironolactone was obtained from Sigma-Aldrich (S3378, >97% pure). Concentrated spironolactone stock solutions for the fish exposures were prepared by addition of neat spironolactone to 1 L of ultraviolet (UV)–treated, filtered Lake Superior water, which was stirred for 1 h, sonicated in a water bath at 40 °C for 3 h, and then stirred overnight. The concentrated stock was then diluted into 19 L of Lake Superior water in a glass carboy to desired target concentrations. For the D. magna 21-d exposure, spironolactone stock was prepared by addition of neat spironolactone to 25 mL of methanol and sonicated <1 min prior to use. The stock was then diluted in Lake Superior water to the desired concentrations (carrier solvent at 0.02%) for the exposure. Less than 24-h-old D. magna neonates and mature adult male and female fathead minnow and Japanese medaka (fathead minnow 9–10 mo, Japanese medaka 3–4 mo) were obtained from an on-site culture unit at the US Environmental Protection Agency Mid-Continent Ecology Division laboratory (Duluth, MN, USA). The female Japanese medaka selected for the present study had all previously produced egg clutches. The fathead minnow displayed prominent secondary sex characteristics (i.e., dorsal fat pads and tubercles on males and ovipositors on females). All animal exposures and laboratory practices utilizing animals were reviewed and approved by the Animal Care and Use Committee in accordance with the Animal Welfare Act and Interagency Research Animal Committee guidelines.
D. magna test
A 21-d chronic toxicity test with D. magna was conducted according to the Organisation for Economic Co-operation and Development guidelines, procedure 211 19. The study was initiated with third brood daphnid neonates (<24 h) of known parentage 20. Daphnia were held individually in polystyrene cups containing 25 mL of 0 μg spironolactone/L, 0.5 μg spironolactone/L, 5 μg spironolactone/L, 50 μg spironolactone/L, or 500 μg spironolactone/L diluted in Lake Superior water and exposed for 21 d using a daily static-renewal regime. Lake Superior water only and methanol diluted in Lake Superior water (0.02% carrier solvent) were used as controls. Stratified randomization techniques for treatment placement were implemented using predrilled Styrofoam test boards, with 10 replicates per test concentration. Temperature was maintained at 25 ± 1 °C using a water bath with a 16:8-h light:dark photoperiod. Test solutions were renewed by gently transferring daphnid adults to new exposure cups. Initially, neonates were fed 0.5 mL of a 50:50 dilution of green algae (Pseudokirchneriella subcapitata, 3.5 × 107 algal cells/mL):standard yeast-Cerophyll-trout chow, daily 20. Feeding was increased to 0.75 mL of food per day following the release of the first brood. Prior to use, the algae was centrifuged, washed, and resuspended in soft water to remove culture nutrients, as previously described 20. Spironolactone concentrations were measured prior to renewal and after 24-h exposures (Table 1). Water-quality measures were assessed over the duration of the test (mean ± standard deviation [SD], n): temperature, 24.1 °C ± 0.2 °C, n = 20; dissolved oxygen, 7.8 ± 0.8 mg/L, n = 12; pH, 7.8 ± 0.2, n = 12; hardness, 45.4 ± 1.9 mg/L as CaCO3, n = 2; alkalinity, 44.5 ± 1.9 mg/L as CaCO3, n = 2; and conductivity, 101.1 ± 1.6 S/cm, n = 2.
| Spironolactone (μg/L) (target concentration) | Exposure water concentration | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | |||||||||||||||
| 0a | 1 | 6 | 7 | 12 | 13 | 20 | Mean | 1 | 2 | 7 | 8 | 13 | 14 | 21 | Mean | |
| 0 | NDb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 0.5 | 0.74c (0.040) | 0.77 (0.015) | 0.65 (0.040) | 0.57 (0.020) | 0.64 (0.005) | 0.80 (0.010) | 0.71 (0.025) | 0.70 (0.022) | 0.55d (0.075) | 0.56 (0.000) | 0.21 (0.005) | 0.32 (0.005) | 0.28 (0.010) | 0.34 (0.005) | 0.19 (0.025) | 0.35 (0.040) |
| 5 | 5.3 (0.08) | 5.8 (0.07) | 4.4 (0.08) | 4.4 (0.19) | 6.0 (0.01) | 5.8 (0.24) | 5.5 (0.04) | 5.3 (0.18) | 4.1 (0.06) | 3.7 (0.10) | 1.3 (0.03) | 2.6 (0.10) | 2.2 (0.04) | 2.1 (0.08) | 2.3 (0.03) | 2.6 (0.26) |
| 50 | 51.4 (0.10) | 53.8 (0.20) | 46.4 (1.60) | 45.1 (2.60) | 54.7 (0.05) | 48.9 (2.05) | 43.1 (1.90) | 49.0 (1.22) | 22.1 (0.04) | 26.4 (0.05) | 6.5 (0.25) | 11.2 (0.40) | 16.3 (0.05) | 18.2 (0.05) | 12.7 (0.60) | 16.2 (1.74) |
| 500 | 495 (2.0) | 508 (16.5) | 452 (0.5) | 506 (13.0) | 520 (17.5) | 483 (32.0) | 489 (8.0) | 493 (7.2) | 271 (16.5) | 310 (3.5) | 69 (2.7) | 81 (0.2) | 124 (2.0) | 163 (2.0) | 135 (0.0) | 165 (23.6) |
- a Test day.
- b Detection limit for water was 0.02 ng/L.
- c Mean (standard error [SE]) of determinations from stocks measured in duplicate.
- d Mean (SE) of determinations from 10 combined cups and solution measured in duplicate.
- ND = not detected.
Survival of the parent and number of live neonates per female were recorded daily and used to determine time to first brood, number of broods per female, and mean brood size. Performance criteria for acceptance were met as control survival for both Lake Superior water and the methanol controls was greater than 80% and the average number of neonates produced per control group was ≥60 per female. Results are presented as the total number of viable offspring produced per adult daphnia per day. Data for adults that died during the experiment (non–treatment-related) were excluded from the analysis.
Fathead minnow and Japanese medaka tests
Continuous flow-through exposures were conducted by pumping appropriately diluted spironolactone stock solutions to 20-L glass tanks, containing 10 L of water. Test solutions were delivered at a flow of approximately 45 mL/min, without the use of carrier solvents, resulting in approximately 6 complete volume changes per day. Temperature was maintained at 25 ± 1 °C, with a photoperiod of 16:8 h light:dark. Fathead minnow were fed thawed adult brine shrimp (San Francisco Bay Brand) twice daily. Japanese medaka were fed 5 mL of newly hatched brine shrimp nauplii (Aquafauna Bio-Marine) twice daily. Toxicity tests with the 2 species were conducted simultaneously.
The 21-d small fish exposures to spironolactone followed a basic study design described previously 21. Briefly, 2 pairs of male and female fathead minnow or Japanese medaka divided by a transparent, porous, plastic screen were introduced to each 20-L aquarium. Each side of the divided fathead minnow tank contained 1 spawning substrate. During a 14-d acclimation phase, fish were held in the experimental system receiving UV-treated, filtered Lake Superior water (control), and the fecundity of each pair was monitored daily. Spironolactone exposures were conducted using only pairs that had successfully spawned during the acclimation. Four concentrations of spironolactone (0.05 μg/L, 0.5 μg/L, 5 μg/L, and 50 μg/L) and a control (Lake Superior water only) were delivered to 5 replicate tanks per treatment per fish species. Water quality assessed over the duration of the experiment was as follows (mean ± SD, n): temperature, 25.4 °C ± 0.2 °C, n = 20; dissolved oxygen, 6.8 ± 0.7 mg/L, n = 6; pH, 7.6 ± 0.2, n = 6; hardness, 46.4 ± 1.4 mg/L, n = 2 as CaCO3; alkalinity, 45.0 ± 0.9 mg/L as CaCO3, n =2; conductivity, 104.7 ± 3.1 S/cm, n = 2.
The total number of eggs spawned and the number of fertile eggs produced by each pair were recorded daily. After the 21-d exposure, adult fish were anesthetized in buffered MS-222 and wet weights were measured. Blood from fathead minnows was collected from the caudal vasculature (∼20 μL from females and ∼50 μL from males) and centrifuged in heparinized microhematocrit tubes to separate the plasma, which was then stored at −80 °C until extracted and used to measure vitellogenin protein and sex steroids. Dissection and collection of liver and gonad followed. Care was taken between sample collections to eliminate RNase contamination by cleaning dissection equipment with RNaseZap (Ambion). Total gonad weight, for determination of gonadosomatic index (GSI), was recorded prior to division into subsamples. For fathead minnow, an approximate 10-mg portion of the right ovary (16.2 ± 6.8 mg) or testis (16.1 ± 6.8 mg) was used for an ex vivo steroid production assay, and the remaining piece was preserved for RNA extraction. The left ovary or testis from the fathead minnows was collected and placed in a tissue cassette for histological examination. As a measure of secondary sex characteristics, tubercle size and number were scored according to procedures outlined in Jensen et al. 22 for the fathead minnow and papillary processes on the anal fins of Japanese medaka were quantified.
Steroid and vitellogenin protein measurements: fathead minnow
Ex vivo production of testosterone by testis and ovary tissue and 17β-estradiol by ovary tissue was determined using previously described methods 23, 24. A radioimmunoassay protocol described by Jensen et al. 22 was used to measure concentrations of testosterone and 17β-estradiol after a liquid:liquid extraction with diethyl ether of either plasma or ex vivo culture media samples. Measurements of the estrogen-inducible egg yolk precursor protein vitellogenin were determined from plasma samples using an enzyme-linked immunosorbent assay following the procedures described elsewhere 25, with the use of a fathead minnow polyclonal antibody and a purified fathead minnow vitellogenin protein standard.
Comparative vitellogenin gene measurements
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to quantify the relative abundance of mRNA transcripts for vitellogenin protein in each of the 3 species tested. TriReagent (Sigma) was used to extract total RNA from the liver of fathead minnow and the whole bodies of D. magna according to the manufacturer's protocol. Japanese medaka liver total RNA was extracted using RNeasy Protect Mini Kits (Qiagen) according to the manufacturer's protocol. Concentrations of RNA were measured using a Nanodrop ND 1000 spectrophotometer (Nanodrop Technologies). Purity of the RNA samples was assessed based on optical densities at 260 nm/280 nm and 260 nm/230 nm. Total RNA samples were diluted to 10 ng/μL in preparation for qRT-PCR analysis. Relative transcript abundance of vitellogenin expressed in liver tissue was determined using protocols previously described 26, 27. The primers used for measurement of fathead minnow vitellogenin and D. magna vitellogenin-2 were described previously by Biales et al. 28 and Hannas et al. 29, respectively. Primers and probes for Japanese medaka vitellogenin-1 were designed using Primer Express software (Applied Biosystems) and synthesized by Integrated DNA Technologies. The primer set for vitellogenin-1—forward (5′-AGGCAGTTTCTAAGGGCGAAC-3′)/reverse (5′-TGAATGGGCATAATCTTTGTGATT-3′)—was designed to produce a 108–base pair amplicon of the medaka vitellogenin-1 gene (National Center for Biotechnology Accession BAB79696), with the corresponding probe (5′-FAM-TTTGGGAAATGCAAGACACCCTA-Black Hole Quencher-3′) spanning an exon–exon boundary. The probe was purchased from Biosearch Technologies.
The fathead minnow and D. magna qRT-PCR assays were performed using Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems). For these qRT-PCRs, each 20-μL reaction contained 20 ng total RNA, 215 nM forward primer, and 215 nM reverse primer. The thermocycling program was set to 48 °C for 30 min to allow for reverse transcription, followed by 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 60 s for PCR amplification, and finally a dissociation stage of 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s.
The Japanese medaka qRT-PCR assay was performed using the Taqman EZ RT-PCR Kit (Applied Biosystems) according to the manufacturer's protocol. Each 30-μL reaction contained 40 ng total RNA, 500 nM forward primer, 500 nM reverse primer, and 333 nM probe. The thermocycling program was set to 50 °C for 2 min, 60 °C for 30 min, 95 °C for 5 min, and 45 cycles of 95 °C for 15 s and 60 °C for 60 s.
A standard curve was created for each gene of interest following a 10-fold serial dilution ranging from 200 copies to 20 000 000 copies for fathead minnow and D. magna qRT-PCRs and 1000 copies/ng total RNA to 100 000 000 copies/ng total RNA for the Japanese medaka vitellogenin-1 qRT-PCR. Complementary DNA standards were used for the fathead minnow assay. The RNA standards were used for the Japanese medaka and D. magna assays. For the Japanese medaka, characterized RNA was used to generate a standard curve to convert cycle threshold values to copies per nanogram of total RNA. The RNA was generated with a set of primers that produced an amplicon containing the vitellogenin-1 amplicon that was minimally larger from total cDNA from medaka livers. This amplicon was then converted to the appropriate RNA using a T7-MEGAscript kit (Ambion) and purified with the RNeasy Micro kit (Qiagen). Finally, the quantity and quality of the RNA product were determined on an Agilent 2100 Bioanalyzer. The standard curves developed to assess vitellogenin for all species were used to interpolate values from the standard curve, normalizing to the mass of total RNA in the reaction and expressed as copies of mRNA per nanogram of total RNA for each sample.
Gonad histopathology
The excised fathead minnow gonads (left ovary or left testis) were fixed in Bouin's fixative for 72 h and then washed twice with 70% ethanol-lithium carbonate solution prior to processing into paraffin. Gonad samples were processed on a Sakura Tissue Tek VIP 1000 tissue processor (Sakura Finetek). One gonad sample was embedded from each fish. The tissue was oriented for longitudinal sectioning. Ten control males and females and 7 males and females from each spironolactone treatment were selected for evaluation, and a single slide was prepared for each fish. Tissue blocks were trimmed on the microtome to the widest region in the section; then, 3 sections at 50-μm intervals were collected, mounted on a glass slide, and stained with hematoxylin and eosin. All observed pathologies to the female and male gonads were scored for severity as described in Supplemental Data, Tables S3 and S4, respectively 30, 31.
In vitro cell assays
To explore one of the hypotheses generated from the fish tests, we used the MDA-kb2 human cell line, which is stably transfected with an androgen-responsive luciferase reporter gene, to assess both the androgenic and the antiandrogenic activities of canrenone. The method was slightly modified from that described previously 32, 33 in that methanol controls were included in the study design to reflect the use of 0.1% methanol as a carrier solvent for canrenone. Androgen receptor activation was assessed using canrenone concentrations of 0.5 μg/L, 5 μg/L, 50 μg/L, 100 μg/L, 200 μg/L, 500 μg/L, and 940 μg/L alone and, for assessing antiandrogenic activity, in combination with 11 nM of the androgen agonist 17β-trenbolone (reported median effective concentration [EC50] in the MDA-kb2 cells 33).
We also wanted to determine the activity of spironolactone and canrenone using a fish androgen receptor. To achieve this, transcriptional activation of the fathead minnow androgen receptor was measured using the adenovirus transduction method described previously 34. Briefly, CV-1 cells were transduced with both the fathead minnow androgen receptor and a reporter gene in 60-mM dishes, incubated for 24 h, dispersed into 96-well plates, dosed with designated chemicals, and incubated for 24 h; then, the plates were harvested and assessed for luminescence. Relative luminescence units were converted to fold-induction above the medium value, a background non-zero value that was set to unity. The highest chemical plus dihydrotestosterone-fold (to determine androgen receptor antagonism) or chemical alone (to determine androgen receptor activation) value was used to identify the top of the chemical response curve, and fold-induction data were normalized as percentage of the maximum response. Curves were plotted using logistic regression, and EC50 values were calculated using Prism software version 5.03 (GraphPad).
Chemical analysis: water
To confirm exposure regimes, spironolactone was routinely measured in water from the daphnia and fish tests. A set of field-collected water samples was also analyzed for spironolactone and its metabolites, 7α-thiomethylspironolactone and canrenone (see Field-collected grab and composite samples for field collection details). Water samples were directly analyzed using an Agilent model 1100 LC-MSD equipped with a photometric ionization interface. An aliquot of sample was injected onto a Zorbax SB-C18 (Agilent) column (2.1 × 150 mm) and eluted isocratically at a flow rate of 0.2 mL/min. The mobile phase consisted of 60% acetonitrile:0.1% formic acid along with a toluene dopant set at a flow rate of 0.05 mL/min. Spironolactone, 7α-thiomethylspironolactone, and canrenone concentrations were determined using masses 341, 389, and 341, respectively (selected-ion monitoring positive ion mode); and retention time was determined with an external standard method of quantitation. Quality-control samples such as procedural blanks, spiked recoveries, and duplicate analysis comprised 10% of the sample load. The detection limits for 7α-thiomethylspironolactone and canrenone were 0.1 μg/L and 0.03 μg/L, respectively. No spironolactone was detected in any control sample over the course of the experiments (detection limit = 0.2 μg/L).
Chemical analysis: tissues
Following exposure and dissection, fathead minnow were wrapped in solvent-rinsed aluminum foil, sealed in plastic zip-lock bags, and frozen at −20 °C until extraction. Each fish was cut into 2 or 3 sections, placed in a 50-mL round-bottom glass centrifuge tube, and weighed; then, 14 mL of acetonitrile was added. Samples were homogenized for 1 to 2 min with an Ultra-Turrax T25 tissue homogenizer (Janke & Kunkel) at 8000 rpm, then transferred to 50-mL conical polypropylene centrifuge tubes, and spun at 3010 g for 25 min at –10 °C. Supernatants were poured into 40-mL glass centrifuge tubes, dried under nitrogen gas to 10 mL (remaining supernatant), and then frozen at −20 °C for ≥1 h. Extracts were removed from the freezer, transferred to 15-mL centrifuge tubes, and further concentrated to 5 mL prior to freezing for an additional hour at −20 °C. Contents of tubes were transferred to vials, then aliquots diluted with high-purity water to yield a 25% acetonitrile concentration before liquid chromatographic-mass spectrometric analysis using methods similar to those used for water. A gradient method was used starting at 50% acetonitrile/formic acid and increasing to 90% acetonitrile/formic acid, with a flow rate of 0.200 mL/min and toluene dopant flow of 0.030 mL/min. The average percentage of recovery for spironolactone-spiked samples was 106 ± 12.5%.
Field-collected grab and composite samples
In conjunction with our toxicity testing, a small effort was initiated to assess the possible occurrence of spironolactone and its metabolites at several field sites where other work was being conducted by our group. Water was collected at 7 sites near wastewater-treatment plants in the St. Louis River estuary (Lake Superior, Duluth, MN, USA) and Maumee River (Lake Erie, Toledo, OH, USA) areas of concern (Supplemental Data, Table S5). Novel autosamplers with a timed pump/intake system and a storage container were used to generate composite samples. The composite samplers were suspended between a buoy and a cement block to midwater depth based on site (∼0.5–1.5 m) and programmed to collect 30-mL to 50-mL subsamples at 1-s intervals every 15 min over a 96-h period (total volume collected ∼11 L) into a 20-L low-density polyethylene collapsible cubitainer (Cole-Parmer Instrument, model 6100-40). Samples were stored on ice for approximately 2 h during transport, transferred to a muffled glass amber bottle, and stored at 4 °C until analysis. Grab samples were obtained using a peristaltic pump (Solinst; model 410) using site water–rinsed silicone tubing, from a depth of 1.5 m into a muffled amber glass 1-L bottle, placed on ice for approximately 2 h during transport, and stored at 4 °C until analysis.
Statistical analysis
Parametric data from the 21-d reproduction studies, including steroid and vitellogenin protein measurements, and vitellogenin expression data from the qRT-PCR assays were analyzed using analysis of variance with treatment and replicate as independent variables. When no significant replicate effect or treatment by replicate effect was identified from the analysis, replicate was excluded as a variable and a one-way analysis of variance was performed to assess differences across treatment groups. Duncan's multiple comparison test was used for post hoc analysis to examine differences between all treatment groups. Nonparametric data were analyzed using the Kruskal-Wallis test, followed by Dunn's post hoc analysis. When concentration values from the steroid radioimmunoassay data were below the detection limit, values of one-half the detection limit were used in the analysis. Differences were considered significant at p ≤ 0.05. The data collected from the MDA-kb2 studies were also analyzed using analysis of variance, followed by Dunnett's multiple comparison test as a post hoc analysis.
RESULTS
Androgen receptor conservation
Using the approach and criteria described by LaLone et al. 17, cross-species comparisons of protein sequence and conserved domain information obtained from the National Center for Biotechnology Information protein database for the human androgen receptor and western mosquitofish androgen receptor confirmed that vertebrate species should have greater susceptibility than invertebrate species to the specific actions of chemicals, such as spironolactone, that target the androgen receptor (Supplemental Data, Tables S1 and S2). Examination of protein similarity to the human androgen receptor for western mosquitofish, fathead minnow, Japanese medaka, and Daphnia pulex (a representative daphnid species with a fully sequenced genome) indicated that the small fish have target proteins with considerably more similarity to the human androgen receptor than the cladoceran (Table 2). Specifically, the 3 small fish species contain proteins that are 31% to 32% similar to the human androgen receptor, whereas the identified protein (best pBLAST match based on bit score) for the invertebrate species is just 8% similar. With the knowledge that spironolactone also likely acts through the androgen receptor in the western mosquitofish—as evidenced by elongation of the anal fin in exposed females 11—we assessed protein target similarity using western mosquitofish androgen receptor as the query sequence. In this case, comparing across fish species, the sequence similarity analysis indicated that the Japanese medaka androgen receptor was most similar to the western mosquitofish (70%) and the fathead minnow androgen receptor slightly less so (48%; Table 2). In the present study, protein sequence similarity between the top pBLAST hit for the invertebrate D. pulex and the western mosquitofish androgen receptor was calculated to be 10%.
| Species, common name | Molecular target | Percentage similarity | Susceptibility prediction |
|---|---|---|---|
| First-tier sequence analysis | |||
| Target species | |||
| Homo sapiens, humans | Androgen receptor | 100% | Likely susceptible |
| Nontarget aquatic vertebrates of interest | |||
| Pimephales promelas, fathead minnow | Androgen receptor | 32% | ↓ |
| Oryzias latipes, Japanese medaka | Androgen receptor-β subtype | 32% | |
| Gambusia affinis, Western mosquitofish | Androgen receptor | 31% | |
| Nontarget aquatic invertebrate of interest | |||
| Daphnia, water flea | Hypothetical protein DAPPUDRAFT_46682 | 8% | Unlikely susceptible |
| Second-tier sequence analysis | |||
| Query species | |||
| Gambusia affinis, western mosquitofish | Androgen receptor | 100% | Likely susceptible |
| Aquatic vertebrates of interest | |||
| Oryzias latipes, Japanese medaka | Androgen receptor-β subtype | 70% | ↓ |
| Pimephales promelas, fathead minnow | Androgen receptor | 48% | |
| Aquatic invertebrate of interest | |||
| Daphnia, water flea | Hypothetical protein DAPPUDRAFT_46682 | 10% | Unlikely susceptible |
- a Metrics for assessing molecular target conservation and susceptibility prediction determined according to methods described by LaLone et al. 17.
Fathead minnow 21-d test
Measured chemical concentrations within tanks were slightly below nominal values on day 1 but remained relatively consistent when measured on days 5, 6, 12, 16, and 21 of the spironolactone exposure with fathead minnows (Table 3). The mean (standard error [SE], n = 36) measured concentrations in the 0.05 μg spironolactone/L, 0.5 μg spironolactone/L, 5 μg spironolactone/L, and 50 μg spironolactone/L treatment tanks were 0.04 (0.002) μg/L, 0.5 (0.01) μg/L, 2.6 (0.04) μg/L, and 43.7 (0.6) μg/L, respectively. Spironolactone was not detected in control Lake Superior water–only tanks at any sampling time.
| Spironolactone (μg/L) (target concentration) | Fathead minnow exposure water concentration | Japanese medaka exposure water concentration | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of exposure | Day of exposure | ||||||||||||||
| 1a | 5 | 6 | 12 | 16 | 21 | Mean | 1 | 3 | 7 | 10 | 15 | 17 | 21 | Mean | |
| 0 | NDb | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 0.05 | 0.03c (0.000) | 0.05(0.000) | 0.05 (0.002) | 0.04 (0.005) | 0.03 (0.003) | 0.02 (0.000) | 0.04 (0.002) | 0.06 (0.003) | 0.06 (0.002) | 0.05 (0.000) | 0.05 (0.002) | 0.03 (0.002) | 0.03 (0.002) | 0.03 (0.002) | 0.04 (0.002) |
| 0.5 | 0.5 (0.01) | 0.5 (0.01) | 0.6 (0.01) | 0.6 (0.02) | 0.5 (0.02) | 0.3 (0.01) | 0.5 (0.01) | 0.7 (0.01) | 0.5 (0.02) | 0.5 (0.004) | 0.7 (0.01) | 0.6 (0.003) | 0.5 (0.01) | 0.6 (0.01) | 0.6 (0.01) |
| 5 | 2.1 (0.02) | 2.0 (0.04) | 3.3 (0.04) | 3.1 (0.06) | 2.9 (0.03) | 2.0 (0.03) | 2.6 (0.04) | 6.5 (0.04) | 2.4 (0.02) | 2.4 (0.03) | 3.5 (0.03) | 3.2 (0.03) | 2.5 (0.02) | 2.6 (0.05) | 3.3 (0.03) |
| 50 | 27 (0.2) | 36 (0.5) | 56 (0.6) | 56 (0.8) | 45 (0.7) | 43 (0.6) | 44 (0.6) | 60 (0.5) | 28 (0.2) | 39 (0.4) | 57 (0.6) | 55 (1.0) | 44 (0.8) | 50 (0.3) | 48 (0.5) |
- a Test day.
- b Detection limit for water was 0.2 ng/L.
- c Mean (standard error) of determinations from 5 separate tanks.
- ND = not detected.
Fathead minnow fecundity was immediately impacted in the 50-μg spironolactone/L treatment group, and total cessation of spawning was also noted after 2 d of exposure to 5 μg spironolactone/L (Figure 2A1). Consistent with the cumulative fecundity results, the mean number of eggs per female per day and number of spawns per female were significantly reduced at the 5-μg spironolactone/L and 50-μg spironolactone/L test concentrations (Supplemental Data, Figure S1A and B). However, the number of eggs per spawn and the mean number of fertile eggs per spawn were statistically similar across treatment groups (p > 0.05; Supplemental Data, Figure S1C and D). No mortalities occurred throughout the duration of the 21-d test.
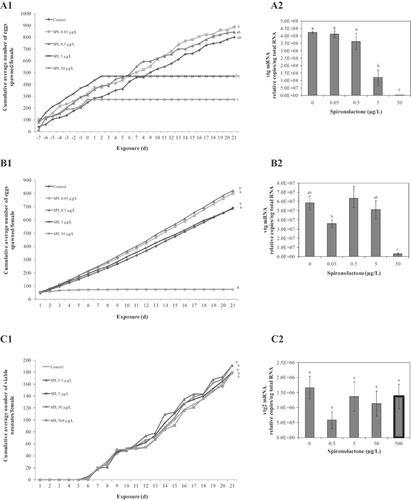
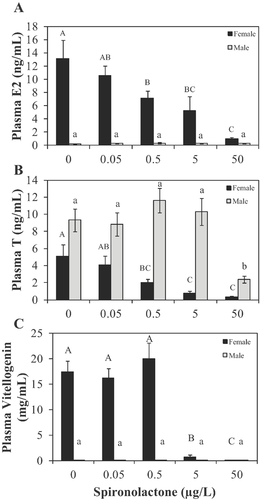
Female fathead minnow experienced a significant concentration-dependent decrease in both plasma 17β-estradiol and testosterone after 21 d of exposure to spironolactone (Figure 3A and B). Vitellogenin mRNA in liver and plasma vitellogenin protein concentrations were also significantly decreased in female fish exposed to the 2 highest concentrations (Figures 2A2 and 3C).
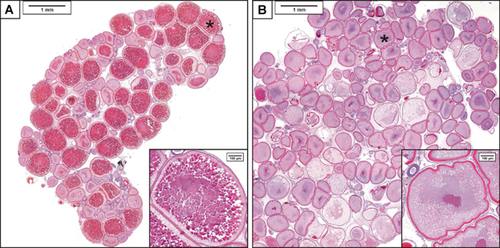
Exposure to spironolactone had marked effects on ovarian histology, eliciting invagination of the vitelline membrane and decreased deposition of vitellogenin protein in the developing oocytes (Figure 4). The decrease in yolk formation, which ranged from moderate to severe, was observed in 6 of the 7 samples in the 50-μg/L exposure concentration group (Supplemental Data, Table S3). The remaining sample in that group had a stage 1 ovary comprised mostly of early-stage oocytes, suggesting the fish had not spawned for an extended duration. Minimal to moderate increases in oocyte atresia were also observed in the ovaries of 6 samples from the 5-μg/L and all 7 samples from the 50-μg/L concentrations (Figure 4 and Supplemental Data, Table S3).
Impacts of spironolactone on males were also observed. For example, circulating testosterone was significantly decreased in males exposed to 50 µg spironolactone/L for 21 d (Figure 3B). Plasma 17β-estradiol concentrations in males were not impacted, nor was there any evidence of vitellogenin protein induction (Figure 3B and C).
Effects on the testis of exposed males included an increase in interstitial cell hyperplasia/hypertrophy. The prevalence of interstitial cell hyperplasia/hypertrophy was low, with 2 of the 7 fish in the 5-µg/L and 50-µg/L groups presenting minimal severity scores (Supplemental Data, Table S4).
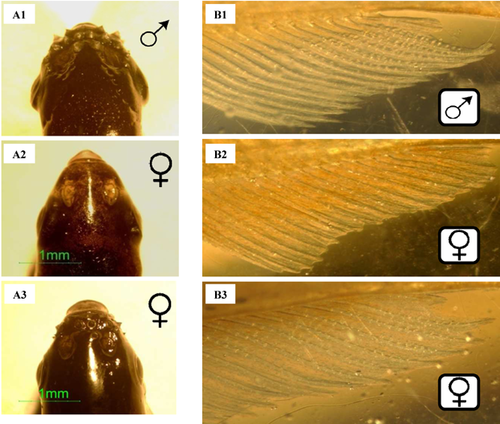
Significant morphological changes were identified in fathead minnow exposed to 50 µg spironolactone/L. Body mass was greater in both males and females (Table 4). Also, GSI values were significantly elevated in males and females exposed to 50 µg spironolactone/L and 5 µg spironolactone/L, respectively (Table 4). Tubercles, a key male secondary sexual characteristic, were identified in females exposed to spironolactone at a concentration as low as 0.5 µg/L, with statistically significant effects at 5 µg spironolactone/L and 50 µg spironolactone/L (Table 4 and Figure 5A). Male tubercle scores were unchanged from those of control fish after exposure to spironolactone (Table 4).
| Fathead minnow morphology | Japanese medaka morphology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement | Sex | Spironolactone concentration (nominal, μg/L) | Spironolactone concentration (nominal, μg/L) | ||||||||
| 0 | 0.05 | 0.5 | 5 | 50 | 0 | 0.05 | 0.5 | 5 | 50 | ||
| Body mass (g) | M | 3.4a,b (0.2) | 3.3a (0.2) | 3.7a (0.2) | 3.8a,b (0.1) | 4.0b (0.2) | 0.3a (0.03) | 0.3a,b (0.02) | 0.3a,b (0.01) | 0.3a (0.01) | 0.4b (0.2) |
| F | 1.5a (0.1) | 1.7a (0.1) | 1.7a (0.1) | 2.1a (0.1) | 2.3b (0.1) | 0.4a (0.02) | 0.5a,b (0.03) | 0.5a,b (0.03) | 0.4a (0.03) | 0.5b (0.02) | |
| GSI (%) | M | 1.3a (0.1) | 1.1a (0.1) | 1.3a (0.1) | 1.8a,b (0.1) | 3.2b (1.3) | 1.7a (0.2) | 1.7a (0.2) | 1.5a (0.1) | 1.6a (0.2) | 2.3b (0.3) |
| F | 13.1a (0.9) | 13.4a (0.7) | 15.0a,b (1.2) | 19.3b (2.5) | 10.7a (1.9) | 17.7a (3.2) | 19.4a (3.1) | 20.8a (3.0) | 21.4a (2.7) | 25.9a (3.8) | |
| Tubercle score | Papillary process count | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Secondary sex characteristic score | M | 25.7a (2.7) | 21.2a (1.3) | 29.2a (1.6) | 24.3a (2.7) | 26.2a (2.4) | 77.9a (4.6) | 89.0a,b (7.8) | 86.4a,b (5.8) | 82.6a,b (4.8) | 96.9b (4.9) |
| F | 0a | 0a | 0.9a (0.3) | 10.5b (1.4) | 11.4b (1.5) | 0a | 0a | 0a | 52.3b (3.9) | 65.0c (4.8) | |
- a Mean (± standard error, n = 10), fish mass, gonadal somatic index (GSI), tubercle score for male and female fathead minnows, and papillary process count from male and female Japanese medaka.
- b Letters represent the statistical relationship across treatments per measurement within species using Duncan's multiple comparisons test.
- M = male; F = female.
The mean tissue residue concentration (± SE) collected from dissected fathead minnow males and females exposed for 21 d to 50 µg spironolactone/L was 64.3 ± 9.2 ng/g (n = 8), whereas that of the metabolite canrenone was 33.7 ± 2.6 ng/g (n = 8). All control fish tissue residues were below the limit of detection (n = 6), which was 9 ng/g to 23 ng/g (depending on fish wt).
Japanese medaka 21-d reproduction assay
Consistent with measured tank concentrations for the fathead minnow exposure, measurements taken on days 1, 3, 7, 10, 15, 17, and 21 from the Japanese medaka tanks remained near nominal during the 21-d test. Mean (SE, n = 36) concentrations in the 0.05-µg spironolactone/L, 0.5-µg spironolactone/L, 5-µg spironolactone/L, and 50-µg spironolactone/L treatment tanks were 0.04 (0.002) µg/L, 0.6 (0.007) µg/L, 3.3 (0.03) µg/L, and 47.5 (0.5) µg/L, respectively (Table 3). Again, spironolactone was not detected in any control Lake Superior water tanks.
Complete cessation of spawning in Japanese medaka occurred within 8 d of exposure to 50 µg spironolactone/L (Figure 2B1). Fecundity measures remained similar to control for the other spironolactone treatments. The mean number of eggs per female per day, the number of spawns per female, and the number of eggs per spawn also were significantly reduced at 50 µg spironolactone/L (Supplemental Data, Figure S2A–C). The mean number of fertile eggs per spawn was consistent across treatment groups (p > 0.05; Supplemental Data, Figure S2D). No treatment-related mortalities occurred during the 21-d test. Vitellogenin mRNA was significantly decreased in female Japanese medaka exposed to the highest test concentration of spironolactone (Figure 2B2).
Both body mass and GSI were greater in male Japanese medaka exposed to 50 µg spironolactone/L compared to males from other treatment groups (Table 4). Female Japanese medaka exposed to 50 µg spironolactone/L also had greater body weight compared with other groups, but no significant differences were detected for GSI (Table 4).
Males exposed to 50 µg spironolactone/L exhibited an increased number of papillary processes on their anal fins. This common secondary sex characteristic of male Japanese medaka was also noted on the anal fins of females exposed to either 5 µg spironolactone/L or 50 µg spironolactone/L, where the prevalence of these features was significantly increased compared with controls (p < 0.002; Figure 5B).
D. magna 21-d test
Chemical concentrations were near nominal throughout the duration of the 21-d test (Table 1), measured on days 0, 1, 6, 12, 13, and 20. After a 24-h period, there was an average loss of chemical in the exposure cups ranging between 33% and 50%, across all treatment groups. The cumulative mean number of viable neonates per D. magna female was similar across all treatment groups during the 21-d exposure to spironolactone (Figure 2C1). Even at the highest concentration tested (500 µg spironolactone/L), no adverse reproductive effects were observed. There also were no significant differences between treatment groups when examining the average number of broods per female, neonates per brood, or days to first brood (Supplemental Data, Figure S3A–C). There were no significant differences in vitellogenin-2 mRNA abundance in whole-body RNA extracts prepared from female daphnids exposed to spironolactone for 21 d (Figure 2C2), nor was body length (head to the base of spike) impacted (Supplemental Data, Figure S3D).
MDA-kb2 cell assays
Canrenone produced slight antiandrogenic activity (500 µg/L and 1000 µg/L) and androgenic activity (1000 µg/L) in the MDA-kb2 cell assay (Supplemental Data, Figure S4A and B).
Fathead minnow transcriptional activation assays
Both spironolactone and canrenone were capable of producing androgenic and antiandrogenic effects in the in vitro fathead minnow androgen receptor activation assay. The EC50 values were similar for both spironolactone and canrenone at 1.7 µg/L and 0.9 µg/L, respectively, when the assay was run in the androgen receptor agonist mode; and when conducted in the antagonist mode, the EC50 values were 33 708 µg/L and 17 662 µg/L, respectively (Supplemental Data, Figure S5A and B).
Analytical measures of field-collected samples
Neither spironolactone nor its major metabolites, 7α-thiomethylspironolactone and canrenone, were detected in surface water samples (grab and composite) collected from 7 sites located near municipal wastewater-treatment plants in Duluth, Minnesota, USA, and Toledo, Ohio, USA (Supplemental Data, Table S5).
DISCUSSION
Pharmaceuticals in the environment are almost never detected unless they are targeted through specific analytical measurements. For monitoring purposes, this highlights the importance of effectively identifying chemicals that have the greatest potential to cause harm to nontarget organisms. Three considerations are commonly employed to identify compounds for monitoring in the aquatic environment: 1) existence of established analytical techniques, 2) occurrence of undesirable/adverse effects based on empirical testing, and/or 3) high chemical production volume and usage (as a surrogate for an exposure prediction). As the number of anthropogenic chemicals detected in the environment has increased, the need for predictive tools that can be used to help inform and optimize monitoring and testing based on the potential for adverse effects has been increasingly recognized 35. The goal of the present study was to demonstrate the predictive utility of the adverse outcome pathway framework as an effects-based approach for prioritizing compounds likely to cause toxicity to aquatic organisms. We hypothesized that chemicals that act through a common molecular initiating event (i.e., androgen receptor activation in the present study) would result in consistent and predictable adverse effects, which could be evaluated through the characterization of reproductive toxicity produced by perturbation of the androgen receptor. Furthermore, through our understanding of molecular target conservation, we hypothesized that vertebrate species would have greater intrinsic susceptibility to effects mediated by chemicals that modulate the human androgen receptor than invertebrates. From these complementary approaches, the human pharmaceutical spironolactone was identified as a potential endocrine-disrupting chemical of concern in the aquatic environment. After conducting cross-species reproduction studies, which confirmed our hypotheses, we collected surface water samples near wastewater-treatment plants to start to assess the possible environmental relevance of spironolactone and its key metabolites.
Cross-chemical and -species consistency of an adverse outcome pathway for androgen receptor activation and reproductive effects
Previous work with 17β-trenbolone conducted by Ankley et al. 12, 13 established an adverse outcome pathway linking activation of the androgen receptor in fathead minnow to reproductive toxicity. Based on this, we predicted that fathead minnow exposed to other known androgen receptor agonists such as spironolactone would lead to the same adverse reproductive and masculinization effects delineated in the adverse outcome pathway for androgen receptor activation constructed with 17β-trenbolone (Figure 1). The effects elicited in fathead minnow following 21-d exposure to spironolactone in the present study provide further evidence that chemicals that target the same molecular initiating event trigger predictable responses (Figure 1). Results obtained from the exposure of Japanese medaka and fathead minnow to spironolactone, showing female masculinization and substantial reproductive toxicity, along with the previously published western mosquitofish study (describing masculinization of females measured by anal fin elongation 11), demonstrate the consistency and predictive quality of the established androgen receptor activation–reproduction adverse outcome pathway across species within the same organism class.
The consistent adverse effects observed between phylogenetically similar species is in part because of the common molecular initiating event. From our quantitative measure of molecular target similarity across species compared to the human androgen receptor, and further to the western mosquitofish, it was predicted that the small fish vertebrates used as models in the present study would be similarly susceptible to spironolactone and that an invertebrate that lacked a homolog would likely be less susceptible. Our results from the 21-d exposures provide evidence of the practicality of this molecular target relevance evaluation for identification of those taxa that are most likely to experience adverse effects associated with exposure. Two key endpoints were assessed in all 3 test species: reproductive success and vitellogenin protein gene expression. Modulation of expression and production of vitellogenin protein, the precursor protein for egg yolk in oviparous organisms, has long been an established biomarker for endocrine-disrupting chemicals in fish. Increases in vitellogenin protein in males and decreases of vitellogenin protein in females have been linked to exposure to estrogenic and androgenic chemicals, respectively 36, 37. However, it is unclear whether fluctuations in vitellogenin expression can be used as indicators of invertebrate endocrine disruption 38.
Others have hypothesized that induction or suppression of vitellogenin (Vtg2) mRNA in D. magna may be related to the action of the chemical on the ecdysteroid receptor, which plays a major role in embryonic development 29. Though it is known that both ecdysteroid and vitellogenin increase during the course of ovary maturation, little evidence exists indicating that ecdysteroids stimulate vitellogenesis in invertebrates 39, 40. The androgenic steroid testosterone has been shown to act as an antiecdysteroid and to interfere with ecdysteroid-related processes involved in the developmental arrest of daphnid embryos 41. Therefore, it could be hypothesized that other androgen modulators (e.g., spironolactone) may have similar effects on ecdysteroids that could be captured through examination of vitellogenin transcript abundance in invertebrates.
The established adverse outcome pathway for androgen receptor activation in fathead minnow, with the key event of reduced vitellogenesis in females, and the associated sequence similarity analysis led to the prediction that both fathead minnow and Japanese medaka would exhibit inhibition of vitellogenin expression on exposure to spironolactone, whereas D. magna may not. When cross-species comparisons were made for both endpoints, the vertebrates were substantially (several orders of magnitude) more sensitive to spironolactone than the invertebrate. In fact, even at the highest test concentration of 500 µg spironolactone/L, D. magna experienced no observable adverse effects.
In addition to observing differences between vertebrate and invertebrate sensitivity to spironolactone, the 2 fish species displayed a moderate difference in sensitivity to the androgen receptor agonist. When examining vitellogenin expression and masculinization effects, fathead minnow (lowest adverse effects concentration of 5 µg/L and 0.5 µg/L for the respective end points) displayed greater sensitivity to spironolactone than Japanese medaka (lowest adverse effects concentration of 50 µg/L and 5 µg/L spironolactone for the respective endpoints). This suggests that although the androgen receptor activation–reproduction adverse outcome pathway is consistent for fish, other physiological differences, perhaps related to pharmacokinetics and metabolism, will dictate absolute potency in any given species.
Biological activity of spironolactone in fish versus humans
Although the present study provides evidence that in fish spironolactone acts as an androgen receptor agonist, one therapeutic benefit of spironolactone for humans is because of its action as an androgen receptor antagonist 11. While the protein sequence similarity–based approach to evaluating molecular target relevance can identify species likely to be intrinsically susceptible to a chemical that perturbs a specific molecular target, it does not account for other aspects of chemical exposure that may modulate toxicity. In this instance, complementary information, such as mammalian pharmacokinetic data, can inform the seemingly contradictory nature of the interaction of spironolactone with the androgen receptor across species. In mammals, spironolactone is rapidly dethioacetylated to canrenone via a number of active metabolite intermediates 42. Evidence suggests that the metabolite intermediate 7α-thiomethylspironolactone is most likely responsible for the therapeutic antiandrogenic effect of spironolactone in humans 6. Consistent with these data, androgenic and antiandrogenic activities were identified for spironolactone using the MDA-kb2 cell line (human breast cancer cell line transfected with a luciferase reporter gene for the androgen receptor) and the fathead minnow androgen receptor cell assay, with EC50 values indicating that spironolactone is more potent as an androgen receptor agonist than antagonist (Supplemental Data, Figure S5, and V. Wilson, Research Triangle Park, North Carolina, USA, personal communication). Furthermore, our results from the MDA-kb2 and fathead minnow transcriptional activation cell assays identify canrenone as a weak androgen receptor modulator (both an androgen receptor agonist and antagonist), which is in line with other reports indicating that the metabolite intermediates of spironolactone lead to antiandrogenic effects in humans 43. Consistent with this, the human half-life of elimination for spironolactone was reported to be 1.4 h, whereas its metabolite intermediates and canrenone have a half-life ≥14 h 6.
Both spironolactone and canrenone were detected in the tissues of male and female fathead minnow exposed to spironolactone, with concentrations of the parent compound approximately twice those of the metabolite. These data provide evidence that the metabolic pathway converting spironolactone to canrenone may be similar between humans and fish and suggest 2 possible hypotheses that could explain the differences in the biological activities of spironolactone in fish versus humans. First, there is an important difference associated with route of exposure. The fish are exposed continuously via the water, without first-pass hepatic metabolism. In contrast, human therapeutic exposure involves oral dosing, which is discontinuous and involves rapid first-pass metabolism of the parent compound to intermediates 7. Therefore, even though evidence suggests that fish also metabolize spironolactone, the continuous exposure makes it more likely for the parent compound to exert its effects in fish than in mammals exposed under an oral dosing regimen. A second possible hypothesis for the differential effects of spironolactone in mammals versus fish could be that spironolactone is less efficiently/more slowly metabolized in the fish. It therefore is conceivable that the active metabolite intermediates that lead to the therapeutic (i.e., antiandrogenic) effects in humans cannot reach a high enough concentration in fish to cause effective antagonism of the androgen receptor. Either hypothesis suggests that spironolactone metabolism in fish versus humans may be the major determinant of whether an androgenic effect (via the parent compound) or an antiandrogenic effect (via intermediate spironolactone metabolites) is observed. This highlights the importance of knowledge of excreted metabolites when assessing and/or prioritizing the effects of chemicals actually entering the environment. A major source for this type of information related to the active metabolites of drugs can be found in the drug labels approved by the US Food and Drug Administration 44.
A contaminant of environmental concern
Spironolactone has been detected in the aquatic environment along with canrenone at relatively high concentrations (both ∼10 µg/L) downstream of a pharmaceutical manufacturer 1. However, in the case of wastewater-treatment plants, human excrement is the major source of pharmaceutical contamination. In our preliminary analyses, neither spironolactone nor canrenone were detected in any of the field-collected samples taken from the vicinity of municipal discharge sites in Duluth, Minnesota, or Toledo, Ohio. This is, perhaps, not surprising as spironolactone is rapidly metabolized to canrenone, which can be bound to plasma proteins in humans, lessening the likelihood of occurrence in excrement 45. Nonetheless, when comparing the effect concentrations observed in our 21-d spironolactone studies with fathead minnow and Japanese medaka to those reported by Gilbert 1, spironolactone would be highlighted as a chemical with the potential to cause adverse effects in the aquatic environment. Significantly, Sanchez et al. 46 reported masculinization of female gudgeon populations downstream of a pharmaceutical manufacturer, in the same French river (Dore River) where the comparatively high spironolactone and canrenone concentrations were detected 1. Although current data are lacking for the effects of canrenone on aquatic organisms, the knowledge that this metabolite also acts as both an androgen receptor agonist and an antagonist in the MDA-kb2 and fathead minnow transcriptional activation assays (albeit weakly) indicates that both spironolactone and its metabolites likely warrant additional monitoring in the environment, particularly in situations where surface water could receive significant pharmaceutical wastes (e.g., from drug production, hospitals).
CONCLUSION
Results from the present study provide further support for the adverse outcome pathway, initially derived from 17β-trenbolone, relating activation of the androgen receptor to impacts on reproduction in fathead minnow. The present study also further defines the taxonomic domain of applicability of the androgen receptor activation–reproduction adverse outcome pathway through combining knowledge of molecular target conservation. Overall, this effort refines a prioritization strategy for the identification of chemicals that act on similar molecular initiating events and elicit predictable adverse effects in a subset of species that can be used for focused ecotoxicity testing and/or environmental monitoring purposes 17. Through this approach spironolactone was evaluated and recognized as an endocrine-disrupting chemical capable of producing reproductive toxicity in fish at environmentally relevant concentrations. As such, we recommend spironolactone and canrenone as appropriate targets for monitoring in the aquatic environment.
SUPPLEMENTAL DATA
Tables S1–S5.
Figures S1–S5. (500 KB DOCX).
Acknowledgment
We thank K. Lott and J. Jensen for their assistance in maintaining the fathead minnow, Japanese medaka, and daphnid cultures. Also, we thank H. Helgen for his continued computer programming support associated with the homology assessments. We acknowledge J. Nichols and L.E. Gray, Jr., for their advice concerning the metabolism of spironolactone in humans versus fish and interpretation of the in vitro transcriptional activation data, respectively. J. Tietge provided helpful review comments on an earlier version of the article.
Disclaimer
This article has been reviewed in accordance with the requirements of the US Environmental Protection Agency (USEPA) Office of Research and Development; however, the recommendations made herein do not represent USEPA policy. Mention of products or trade names does not indicate endorsement by the USEPA.




