Influences of soil properties and leaching on copper toxicity to barley root elongation
Abstract
The relationships developed between soil properties and phytotoxicity threshold values for copper require validation in a wide range of soils with different properties and climate characteristics before they can be applied for regulatory purposes in countries throughout the world. Seventeen soils, which are representative of the major soil types and properties in China, were spiked with Cu chloride. A subset of the Cu-spiked soils was leached with artificial rain water to compare toxicity with that in unleached soils. Barley root elongation tests were performed under controlled environmental conditions. The concentrations of added Cu causing a 50% inhibitory effect (EC50) ranged from 67 to 1,129 mg/kg in unleached soils and from 88 to 1,255 mg/kg in leached soil. Compared with the unleached toxicity thresholds, the leached EC10 (10% inhibition) and EC50 were higher by an average of 1.43- and 1.15-fold, respectively. Soil leaching significantly (p ≤ 0.05) decreased the toxicity of Cu in approximately 35% of the soils. In this study, no single soil property was found to explain over 35% of the variance in (log transformed) EC50. However, stepwise multiple regressions using soil pH, organic carbon (OC) content, and effective cation exchange capacity (eCEC) were found to explain over 80% of the variance in Cu toxicity across soils. The model developed for Chinese soils based on these factors was found to predict significantly (r2, 0.90) the phytotoxicity of Cu in European soils. These quantitative relationships between Cu toxicity and soil properties are helpful for developing soil-specific guidance on Cu toxicity thresholds. Environ. Toxicol. Chem. 2010;29:835–842. © 2009 SETAC
INTRODUCTION
Soil physicochemical properties (soil pH, cation exchange capacity [CEC], organic carbon [OC] content, etc.) are known to be important parameters in predicting the phytotoxicity of metals, such as copper, in soils 1-4. The toxicity of Cu in soils to barley measured using root elongation has been shown by Daoust et al. 3 to be strongly correlated with soil pH, organic matter, and clay content (r2 = 0.92). The toxicity of Cu to barley root elongation and tomato shoot growth in 18 European soils varying widely in soil properties was found to vary approximately 15-fold and 39-fold, respectively, and soil eCEC (measured at actual soil pH) was the best single predictor for toxicity values for both plant tests 4. Warne et al. 5 also reported the toxicity of Cu to wheat described by the median effective concentration values (EC50) to range from 240 to 6,680 mg Cu/kg in 14 Australian soils, with potential CEC (measured in alcoholic 1 M ammonium chloride at pH 8.5 for alkaline soils and pH 7.0 in 1 M ammonium chloride for all other soils) and pH as the most important soil physicochemical properties for the prediction of Cu phytotoxicity (r2adj, 0.91). Although it is known that soil physicochemical properties play an important role in modifying biotoxicity 1-7, few countries have considered their influence in soil quality guidelines 8-11 (see also http://www.thenbs.com/PublicationIndex/DocumentSummary.aspx?DocID = 266920; http://www.ccme.ca/publications/ceqg_rcqe.html?category; and http://www.nthb.cn/standard/standard05/20030411101228.html). With the large variation in soil types experienced in most countries, a single value for soil quality guideline is unlikely to provide the desired level of species protection at any given site and is likely to result in unnecessary or insufficient remediation or treatment depending on soil type.
Also, one of the major problems with laboratory ecotoxicity tests of metal salts is the effect of the counter-ion on metal partitioning and on the organism used. For example, Smit and Van Gestel 12 reported equilibration of the zinc contamination by percolating the soils with water before use in toxicity experiments strongly reduced the difference in Zn toxicity between laboratory-treated and aged soils. Stevens et al. 6 indicated that, in two of the five soils tested, leaching increased the EC50 values of lettuce (Lactuca sativa) significantly for Zn by 1.4- to 3.7-fold, and, in three of the five soils, leaching increased the EC50 values for lead by 1.6- to 3.0-fold. The shift in EC50 values resulting from soil leaching was possibly due to the direct concomitant toxicity of the counter-ion (NO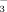 ) or an indirect effect of the salinity on metal speciation in soil solution, increasing its bioavailability. In addition, total Cu and Ni toxicity thresholds for soil microbial processes (nitrification potential, glucose-induced respiration, and maize residue C-mineralization) derived in freshly spiked soils were shown to be lower than threshold values measured in soils in which Cu and Ni had equilibrated and leaching had removed excess salts 13, 14.
) or an indirect effect of the salinity on metal speciation in soil solution, increasing its bioavailability. In addition, total Cu and Ni toxicity thresholds for soil microbial processes (nitrification potential, glucose-induced respiration, and maize residue C-mineralization) derived in freshly spiked soils were shown to be lower than threshold values measured in soils in which Cu and Ni had equilibrated and leaching had removed excess salts 13, 14.
Recently, several models have been developed for European and Australian soil to predict, based on soil properties, the toxicity of Cu, Ni, and Zn 3-5, 15-19. However, it is unclear whether these quantitative relationships derived from European and Australian soils can be directly applied to soils in the Southeast Asian region. Southeast Asian soils are known to be dominated by variable charge surfaces and often are depleted in organic matter 20, whereas European soils are dominated by permanent charge minerals and have relatively high organic matter contents. Australian soils used for toxicity model development were also dominated by soils with permanent charge minerals 5, 19. Also, it is unclear whether the models developed with a different range of soil characteristics can be validated against each other. For laboratory ecotoxicity tests of metal salts, one of the major problems is the effect of the counter-ion on metal partitioning and on the organism used. For example, Smit and Van Gestel 19 reported that equilibration of the Zn contamination by percolating the soils with water before use in the toxicity experiment strongly reduced the difference in Zn toxicity between laboratory-treated and aged soils. Stevens et al. 2 indicated that using soils spiked with soluble metal salts was inappropriate for assessing risks from gradual accumulation of metals in soils over the long term, because toxicity was exaggerated by the salt effects on metal partitioning, and metal salt anions also might contribute to the toxic response. It was shown total Cu and Ni toxicity thresholds for soil microbial processes (nitrification potential, glucose-induced respiration, and maize residue C-mineralization) derived in freshly spiked soils were lower than threshold values measured in soils in which Cu and Ni had equilibrated and leaching had removed excess salts, and this difference could be attributed to decreasing Cu and Ni solubility with time and the effect of leaching on pH and ionic strength of the soil solution 20, 21. Hence, it was recommended that freshly spiked soils should be leached and equilibrated before toxicity testing to mimic more realistic exposure conditions.
Southeast Asia is a region of expanding urban populations and rapid industrialization. Use of metals in the region is growing rapidly, and government agencies, regulators, and scientists are trying to implement policies to ensure environmental protection. Effective policy development is hampered by a lack of sound local data building on recent scientific advances in the understanding of metal behavior and toxicity in soils, waters, and sediments. Current soil regulations concerning maximal permissible concentrations of metals in soils in China were developed in the 1980s, based on experiments in which soils were spiked with metal salts under laboratory conditions 22. These regulatory levels based on total metal concentrations are known to lead to several artefacts that often lead to overconservative toxicity threshold values.
The aims of the present study were to determine the influence of soil leaching on Cu toxicity (EC10 and EC50) on 17 Chinese soils using root elongation as the ecotoxicity endpoint and to develop quantitative relationships between soil physicochemical properties and ecotoxicity thresholds. Furthermore, we wished to examine whether the relationships with soil properties developed to predict Cu toxicity thresholds on Chinese soils could be applied to European temperate soils and vice versa.
MATERIALS AND METHODS
Soil properties and treatments
Seventeen soils with varying physical and chemical properties were selected from multiple locations in China (Table 1). The soils were selected to be representative of the major soil types, soil pH, and organic matter content of agricultural soils in China. Soils (0–20 cm) were air dried, sieved to <2 mm, and spiked with Cu (as CuCl2 in deionized water, 50 ml/kg) at eight dose rates (control plus seven Cu doses: 12.5–800 mg Cu/kg for soils with pH < 5, 25–1,600 mg Cu/kg for soils with pH from 5 to 7, and 37.5–2,400 mg Cu/kg for soils with pH > 7). After spiking, each soil treatment was thoroughly mixed on a plastic sheet by hand until the disappearance of wet lumps. The unspiked control samples were treated in a similar manner using deionized water only.
| No. | Site name | Site | pHa (1:5) | ECa (µS/cm) | eCECb (cmol+/kg) | OCc (%) | CaCO3 (%) | Total N (%) | Fe oxided (mg/kg) | Clay/silt/sande (<2 µm/2–20 µm/20 µm–2 mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Haikou, Hainan | 19°55′N, 111°29′E | 4.93 | 111 | 8.75 | 1.51 | 0.0 | 0.12 | 1,337 | 66/18/16 |
| S2 | Qiyang, Hunan | 26°45′N, 111°52′E | 5.31 | 74 | 7.47 | 0.87 | 0.0 | 0.09 | 1,146 | 46/35/19 |
| S3 | Guangzhou, Guangdong | 23°10′N, 113°18′E | 7.27 | 137 | 8.30 | 1.47 | 0.15 | 0.13 | 1,810 | 25/13/62 |
| S4 | Jiaxing, Zhejiang, | 30°77′N, 120°76′E | 6.70 | 159 | 19.3 | 1.42 | 0.0 | 0.15 | 6,211 | 41/42/17 |
| S5 | Hangzhou, Zhejiang | 30°26′N,120°25′E | 6.80 | 203 | 12.8 | 2.46 | 0.0 | 0.25 | 4,980 | 39/36/25 |
| S6 | Chongqing, Sichuan | 30°26′N, 106°26′E | 7.12 | 71 | 22.3 | 0.99 | 0.0 | 0.09 | 989 | 27/25/48 |
| S7 | Yangling, Shanxi | 34°19′N, 108°0′E | 8.83 | 83 | 8.46 | 0.62 | 8.92 | 0.08 | 707 | 16/14/70 |
| S8 | Zhengzhou, Henan | 34°47′N, 112°40′E | 8.86 | 109 | 8.50 | 1.57 | 0.15 | 0.07 | 581 | 28/41/31 |
| S9 | Zhangye, Gansu | 38°56′N, 100°27′E | 8.86 | 152 | 8.08 | 1.02 | 7.75 | 0.10 | 1,980 | 20/24/56 |
| S10 | Dezhou, Shandong, | 37°20′N, 116°29′E | 8.90 | 112 | 8.33 | 0.69 | 6.17 | 0.08 | 644 | 18/18/64 |
| S11 | Langfang, Hebei | 39°31′N, 116°44′E | 8.84 | 5.7 | 6.36 | 0.60 | 2.42 | 0.06 | 537 | 10/4/86 |
| S12 | Shijiazhuang, Hebei | 38°03′N, 114°26′E | 8.19 | 302 | 11.7 | 1.01 | 3.84 | 0.11 | 826 | 21/22/57 |
| S13 | Lingshan, Beijing | 39°55′N, 116°8′E | 7.48 | 93 | 22.7 | 4.28 | 4.27 | 0.37 | 1,697 | 20/21/59 |
| S14 | Hulunber, Neimeng | 46°03′N, 122°03′E | 7.66 | 888 | 22.7 | 2.66 | 0.27 | 0.25 | 2,477 | 37/16/47 |
| S15 | Urumchi, Xinjiang | 43°95′N, 87°46′E | 8.72 | 227 | 10.3 | 0.87 | 5.08 | 0.10 | 600 | 25/23/52 |
| S16 | Gongzhuling, Jilin | 42°40′N, 124°88′E | 7.82 | 147 | 28.8 | 2.17 | 0.27 | 0.20 | 1,447 | 45/26/29 |
| S17 | Hailun, Heilongjiang | 47°28′N, 126°57′E | 6.56 | 153 | 33.6 | 3.03 | 0.0 | 0.25 | 3,298 | 40/27/33 |
- a Measured in deionized water (soil:solution ratio 1:5).
- b Effective cation exchange capacity, determined using the unbuffered silver–thiourea method.
- c Determined by difference between total carbon and inorganic carbon content.
- d Oxalate extractable Fe.
- e Sedimentary method.
Soils were then split into unleached and leached treatments. The unleached soils were incubated for 2 d at 100% water-holding capacity, air dried at 25°C, sieved to <2 mm using plastic mesh, and stored for less than 2 months before the plant assay commenced. The soils were allowed to equilibrate overnight and leached with artificial rain water consisting of 5 × 10−4 M calcium chloride, 5 × 10−4 M calcium nitrate, 5 × 10−4 M magnesium chloride, 10−4 M sodium sulfate, and 10−4 M potassium chloride at pH 5.9 13. Soils were saturated with artificial rain water by placing the individual spiked soil treatments in a perforated pot (bottom covered with filter cloth, mesh size 140–150 µm) in a bucket containing the leaching solution. When the water level was above the soil surface, more solution was gently poured directly into the pots to increase the leaching volume to approximately two pore volumes and allowed to equilibrate overnight. Finally, the pots were taken out of the buckets and left to drain overnight. Similarly to the unleached soil, the leached soil treatments were then air dried at 25°C, sieved to <2 mm using a plastic mesh, and stored for less than 2 months before the plant assay commenced. Total Cu concentrations were measured in both leached and unleached soils as outlined below. The unleached and leached soils were incubated and equilibrated for 7 d at 70% of pF 1.9 before starting the root elongation assay.
Barley root elongation assay
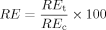
Soil analyses
Soil pH and electrical conductivity (EC) were determined in a water suspension of soil using a 1:5 (soil: solution) ratio 24. Total carbon and nitrogen were determined by high-temperature combustion in an atmosphere of oxygen using a Leco CNS-2000 (vario MAX CN elemental analyzer; Elementar Americas) 25. The inorganic carbon concentrations of soils were determined by measuring the liberated carbon dioxide contents following the addition of hydrochloric acid 26. Organic carbon concentrations were determined as the difference between total and inorganic carbon contents. The silver–thiourea method 27 was used to measure eCEC and exchangeable cations at the pH of the soil. Soil texture was analyzed using the method of Bowman and Hutka 28. Total Cu concentrations in soils were determined by digestion in aqua regia (1:3 [v/v] nitric acid and hydrochloric acid) at 140°C, and filtered digest solutions were analyzed by inductively coupled plasma–optical emission spectroscopy (Spectroflame Modula; Spectro) 29. Reference samples were included as a quality control (two replicates), and the recovery of certified reference soil samples was 101% on average with a mean standard deviation of 2.71%.
Data and statistical analysis
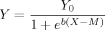 ()
()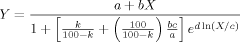 ()
()The adequacy of the predicted equations was checked by examining the distribution of the residuals and ensuring the minimum of calculated root mean squared error based on the difference in observed values and predicted values. Stepwise multiple linear regression analysis was employed in SPSS 12.0 for Windows® to examine the relationships between toxicity thresholds and soil properties 7. Relationships were deemed significant at p ≤ 0.05.
RESULTS
Dose–response curves and toxicity thresholds in unleached and leached soils
The dose–response curves for effects of Cu on barley root elongation in unleached and leached soils are shown in Figure 1, and predicted EC10 and EC50 values are presented in Table 2. The EC10 values ranged from 31 to 444 mg Cu/kg in unleached soils and from 38 to 715 mg Cu/kg in leached soils. EC50 values ranged from 67 to 1,129 mg Cu/kg in unleached soils and from 88 to 1,255 mg Cu/kg in leached soils.
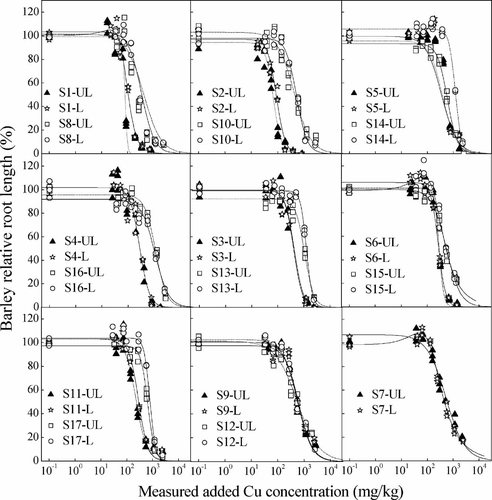
Dose–response curves of measured added Cu for barley root elongation in 17 unleached and leached Chinese soils. Symbols represent all replicated data points, and lines are the fitted log–logistic curves. UL and L represent unleached and leached soils, respectively.
| Soils | Unleached soil | Leached soil | Significancea | LF10d | LF50e | D10f (mg/kg) | D50g (mg/kg) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| EC10b (mg/kg) | EC50c (mg/kg) | EC10b (mg/kg) | EC50c (mg/kg) | EC10 | EC50 | |||||
| S1 | 64 (57–72)h | 79 (75–83) | 54 (43–66) | 105 (94–115) | NS | * | 0.84 | 1.33 | 11 | 26 |
| S2 | 31 (22–45) | 67 (60–76) | 38 (29–51) | 88 (78–100) | NS | * | 1.22 | 1.31 | 7 | 21 |
| S3 | 175 (141–218) | 404 (339–482) | 177 (134–234) | 425 (363–497) | NS | NS | 1.01 | 1.05 | 2 | 21 |
| S4 | 110 (76–161) | 277 (237–323) | 121 (85–172) | 297 (258–342) | NS | NS | 1.09 | 1.07 | 11 | 20 |
| S5 | 130 (96–178) | 401 (349–461) | 145 (100–210) | 445 (378–524) | NS | NS | 1.12 | 1.11 | 15 | 44 |
| S6 | 133 (116–152) | 269 (254–284) | 187 (162–216) | 290 (277–304) | * | NS | 1.40 | 1.08 | 54 | 21 |
| S7i | 82 (56–118) | 524 (443–619) | 154 (124–186) | 467 (409–525) | * | NS | 1.88 | 0.89 | 72 | 57 |
| S8 | 76 (46–126) | 410 (331–507) | 96 (70–132) | 338 (297–385) | NS | NS | 1.27 | 0.83 | 20 | 72 |
| S9 | 151 (115–197) | 578 (517–647) | 215 (108–322) | 627 (520–757) | NS | NS | 1.43 | 1.08 | 64 | 49 |
| S10 | 86 (55–136) | 421 (342–518) | 187 (142–245) | 513 (459–573) | * | NS | 2.17 | 1.22 | 101 | 92 |
| S11 | 80 (57–110) | 229 (198–254) | 112 (89–143) | 282 (256–310) | NS | * | 1.41 | 1.23 | 32 | 53 |
| S12 | 84 (54–128) | 307 (244–386) | 106 (76–148) | 327 (272–369) | NS | NS | 1.26 | 1.06 | 22 | 10 |
| S13 | 444 (331–595) | 1,073 (960–1,188) | 715 (628–815) | 1,255 (1,188–1,332) | * | * | 1.61 | 1.17 | 271 | 182 |
| S14 | 221 (179–274) | 589 (538–645) | 647 (544–770) | 1,192 (1,130–1,257) | * | * | 2.92 | 2.02 | 426 | 603 |
| S15i | 137 (101–187) | 545 (477–622) | 209 (166–254) | 534 (466–603) | NS | NS | 1.52 | 0.98 | 72 | 11 |
| S16 | 393 (293–526) | 1,129 (1,007–1,267) | 287 (173–477) | 1,060 (892–1260) | NS | NS | 0.73 | 0.94 | 106 | 69 |
| S17 | 325 (273–388) | 644 (603–687) | 446 (389–514) | 780 (722–842) | * | * | 1.37 | 1.21 | 121 | 136 |
| Mean | — | — | — | — | — | — | 1.43 | 1.15 | 83 | 87 |
- a Difference between unleached and leached EC10 (EC50) using t test: NS = not significant (p > 0.05); *significant (p ≤ 0.05).
- b Effective concentration (EC) of added Cu that caused a 10% reduction in the endpoint.
- c Effective concentration of added Cu that caused a 50% reduction in the endpoint.
- d LF10 = leached EC10 values/unleached EC10 values.
- e LF50 = leached EC50 values/unleached EC50 values.
- f Absolute difference between leached and unleached EC10 values.
- g Absolute difference between leached and unleached EC50 values.
- h Mean and ranges given in parentheses as ±95% confidence interval.
- i Hormesis in leached soils.
A significant (p ≤ 0.05) increase in barley root elongation (i.e., hormesis) with rate of Cu addition was observed in two of the leached soils (i.e., S7 and S15; Table 2, Fig. 1). The maximum hormesis response observed was a 10% and a 16% increase over the corresponding controls for the leached S7 and S15 soils, respectively. Previous work on hormesis of Cu for animal invertebrates or phytoplankton 32-36 showed that the maximum stimulatory responses were generally approximately 30 to 70% greater than the controls, which is far larger than observed in S7 and S15 soils. A hormetic effect for an essential element (i.e., Cu) is not surprising 37; hence, the toxicity thresholds for S7 and S15 soils were fitted by including hormesis in the dose–response curves.
The influence of leaching on Cu toxicity was found to be variable among the soils examined in the present study (Table 2). A significant difference was found between unleached and leached toxicity values (EC10 and EC50) for approximately 35% of the soils examined in the present study. Overall, leaching increased toxicity thresholds by an average factor of 1.43 for EC10 and 1.15 for EC50 values, respectively. However, the average absolute differences in toxicity thresholds between leached and unleached soils were 83 mg/kg for EC10 and 87 mg/kg for EC50 (Table 2).
Multiple linear regression models to predict Cu toxicity in soils
The significant (p ≤ 0.05) linear regression models to predict Cu toxicity thresholds (EC10 and EC50 values) in relation to soil properties are presented in Table 3. In the present study, logarithm of eCEC was found to be the best single factor in predicting Cu toxicity in unleached EC10 (r2 = 0.58), leached EC10 (r2 = 0.46), and leached EC50 (r2 = 0.32) values; and soil pH was found to be the best single factor in predicting unleached EC50 (r2 = 0.35) values. From the results of single regression analysis, no single factor was found to explain >35% of variance in logarithm of EC50 values in leached and unleached soils. However, when two factors (soil pH and log OC or log eCEC) were introduced into the regression models, the predictability of regression models was improved significantly, with r2 > 0.65 for log EC10 and with r2 ≥ 0.75 for log EC50; and, when incorporating three factors (soil pH, log OC, along with log eCEC), the multiple linear regression models were further improved, with r2 ≥ 0.81 for log EC10 and r2 ≥ 0.88 for log EC50 across soils. These results suggest that soil pH, OC, and eCEC are simultaneously responsible for predicting Cu toxicity in Chinese soils. Other soil factors, such as clay content, calcium carbonate (CaCO3) content, and iron oxide concentrations did not significantly improve the models, so they were excluded from regression equations.
| Regression equation | r2 | p | |||
|---|---|---|---|---|---|
| Unleached soil (n = 17) | — | — | — | — | |
| 1 | log EC10 = 1.993 + 0.849 log OC | 0.52 | 0.001 | — | — |
| 2 | log EC10 = 1.028 + 0.977 log eCEC | 0.58 | <0.001 | — | — |
| 3 | log EC10 = 1.134 + 0.110 soil pH + 1.044 log OC | 0.70 | 0.011 | <0.001 | — |
| 4 | log EC10 = 0.540 + 0.106 soil pH + 0.629 log OC + 0.617 log eCEC | 0.81 | 0.005 | 0.011 | 0.015 |
| 5 | log EC50 = 1.785 + 0.717 log eCEC | 0.26 | 0.037 | — | — |
| 6 | log EC50 = 1.388 + 0.157 soil pH | 0.35 | 0.012 | — | — |
| 7 | log EC50 = 0.025 + 0.197 soil pH + 0.956 log eCEC | 0.79 | <0.001 | <0.001 | — |
| 8 | log EC50 = 0.725 + 0.227 soil pH + 0.964 log OC | 0.83 | <0.001 | <0.001 | — |
| 9 | log EC50 = 0.241+ 0.224 soil pH + 0.627 log OC + 0.502 log eCEC | 0.89 | <0.001 | 0.004 | 0.017 |
| — | Leached soil (n = 17) | — | — | — | — |
| 10 | log EC10 = 2.133 + 0.777 log OC | 0.34 | 0.014 | — | — |
| 11 | log EC10 = 1.165 + 0.972 log eCEC | 0.46 | 0.003 | — | — |
| 12 | log EC10 = 0.853 + 0.164 soil pH + 1.068 log OC | 0.66 | 0.003 | <0.001 | — |
| 13 | log EC10 = −0.029 + 0.134 soil pH + 1.134 log eCEC | 0.69 | 0.006 | <0.001 | — |
| 14 | log EC10 = 1.177 + 0.159 soil pH + 0.597 log OC + 0.702 log eCEC | 0.83 | 0.001 | 0.038 | 0.022 |
| 15 | log EC50 = 1.632 + 0.131 soil pH | 0.26 | 0.037 | — | — |
| 16 | log EC50 = 2.541 + 0.649 log OC | 0.26 | 0.037 | — | — |
| 17 | log EC50 = 1.763 + 0.783 log eCEC | 0.32 | 0.017 | — | — |
| 18 | log EC50 = 0.214 + 0.174 soil pH + 0.994 log eCEC | 0.75 | <0.001 | <0.001 | — |
| 19 | log EC50 = 0.933 + 0.206 soil pH + 1.014 log OC | 0.81 | <0.001 | <0.001 | — |
| 20 | log EC50 = 0.447 + 0.202 soil pH + 0.675 log OC + 0.505 log eCEC | 0.88 | <0.001 | 0.003 | 0.022 |
- r2 = coefficient of determination (percentage of variance accounted for by the regression model); p = significant level; eCEC = effective cation exchange capacity; OC = organic carbon content; EC50 = median effective concentration value; EC10 = 10% of effective concentration value.
DISCUSSION
Influence of soil leaching on Cu toxicity
In the present study, soil leaching was found to decrease significantly (p ≤ 0.05) the toxicity (EC10 and EC50 values) of Cu in approximately 35% of the soils examined. Usually, effects of concentrations of counter-ion (chloride) in soil solution on root growth or on metal partitioning would be expected to be greater at higher Cu doses. From the average differences (83 and 87 mg/kg) of leached EC10 (or EC50) and unleached EC10 (or EC50) in Table 2, it was found that, in general, the effect of leaching on EC50 values was slightly greater than that on EC10. The analysis of soil solutions (data not shown) showed that, depending on soil type, leaching removed water-soluble Cu, chloride, calcium, and sodium and increased soil solution pH by up to 0.75 units, likely because most of the soils had net negative charge. Also, in the present study, more Cu (2.23–4.80% of total Cu) was found to be leached from soils with pH ≥7.5 and OC contents ≤1.5% than from soils with pH ≥7.5 and OC content >2.0% (0.02–0.39% of total Cu). These results are similar to those previously published by Oorts et al. 13, who observed more Cu (up to 50% of the added Cu) loss from an acidic sandy soil after leaching than alkaline and high-OC soils (less than 10% at the highest Cu doses). Almost all leached EC50 values were less that 1.5-fold greater than unleached EC50 values, except for the S14 soil that had a high initial electrical conductivity (888 µS/cm).
Leaching increased EC50 values for barley root elongation in the present study by a median factor of 1.08, which was lower than that (1.3) for the soil microbial assays (potential nitrification rate, glucose-induced respiration, and maize residue C-mineralization) performed by Oorts et al. 13. Generally speaking, the effect of leaching on increasing EC50 values was not significant for all Chinese soils except for S14 soil with LF50 2.02. However, the influence of leaching on Cu toxicity thresholds could not be neglected for the high-saline soils.
Predicting Cu toxicity in soils
Multiple linear regression models developed using soil pH, OC, and eCEC to predict unleached and leached EC50 values (r2 ≥ 0.88) for Cu were in good agreement with measured toxicity thresholds (Fig. 2). The predicted EC50 values were found fully to lie within a twofold range of the measured values. The finding in the present study of the importance of soil pH, OC, and eCEC in predicting Cu toxicity is consistent with the findings previously published by Daoust et al. 3, Broos et al. 19, and Oorts et al. 13. In the study by Daoust et al. 3, it was reported that increases in soil pH and organic matter content contributed to a decrease in Cu toxicity for barley root elongation (r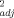 = 0.91) using 10 artificial soils. Broos et al. 19 found that Cu toxicity to substrate-induced nitrification in 12 Australian soils was affected first by soil pH (r
= 0.91) using 10 artificial soils. Broos et al. 19 found that Cu toxicity to substrate-induced nitrification in 12 Australian soils was affected first by soil pH (r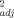 = 0.73) and equally second by potential CEC (r
= 0.73) and equally second by potential CEC (r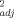 = 0.63) and clay content (r
= 0.63) and clay content (r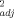 = 0.63). Oorts et al. 13 showed that OC (r2 = 0.57) and pH (r2 = 0.52) were the two most important soil properties in predicting Cu toxicity on glucose-induced respiration and maize residue mineralization in 19 European soils. It is widely recognized that soil pH is one of the most important soil properties that determines the partitioning of trace metals in soils 38, 39 and that partitioning of Cu is associated closely with organic matter contents in soils 40.
= 0.63). Oorts et al. 13 showed that OC (r2 = 0.57) and pH (r2 = 0.52) were the two most important soil properties in predicting Cu toxicity on glucose-induced respiration and maize residue mineralization in 19 European soils. It is widely recognized that soil pH is one of the most important soil properties that determines the partitioning of trace metals in soils 38, 39 and that partitioning of Cu is associated closely with organic matter contents in soils 40.
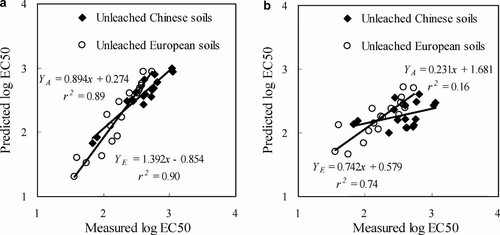
Measured toxicity thresholds versus predicted toxicity thresholds from barley root elongation tests derived from regression Equation 9 (log EC50 = 0.241 + 0.224 soil pH + 0.627 log OC + 0.502 log eCEC) in the present study (a) and the regression Eqn. (log EC50 = 0.803 + 0.542 log eCEC + 0.279 log Fe oxide) of Rooney et al. (b). The YE represents Rooney's predicted thresholds, and YA represents unleached predicted thresholds in this paper. EC50 = median effective concentration; OC = organic carbon; eCEC = effective cation exchange capacity;
Comparison of Cu phytotoxicity models
The models developed for Chinese soils were compared with those published previously by Rooney et al. 4 for European soils (Fig. 2). Because Rooney et al. 4 assessed Cu phytotoxicity in unleached soils, we compared the relevant models for unleached soils from the present study (Eqn. 9 in Table 3).
It was found that the models developed in this study using soil pH, log OC content, and log eCEC for Chinese soils could be applied to predict accurately the phytotoxicity of Cu in the European soils (Fig. 2a). However, the Cu toxicity models developed using eCEC and Fe oxide (e.g., log EC50 = 0.803 + 0.542 log eCEC + 0.279 log Fe oxide) by Rooney et al. 4 for European soils were found to poorly predict the phytotoxicity of Cu in Chinese soils (Fig. 2b), although the method of eCEC measurement in the paper of Rooney et al. 4 was the same as that in the present study; both were based on the silver–thiourea method 27. Hence, the data from Rooney et al. 4 were reanalyzed, and a better regression equation between toxicity thresholds (EC50) and soil pH and OC content was obtained for European soils (log EC50 = 0.803 + 0.216 soil pH + 0.633 log OC, r2 = 0.93). When this equation was used to predict the toxicity thresholds for Chinese soils in this present study, a comparatively good prediction for log EC50 values was also produced, with an r2 of 0.78. These results indicated that soil pH and OC content are two important factors in predicting toxicity thresholds in both Chinese and European soils. The importance of eCEC in predicting EC50 values for the European soils was probably because the variation of eCEC in the European soils in the study of Rooney et al. 4 came mainly from the variances of soil clay, OC content, and soil pH (r2 = 0.90). However, for Chinese soils in the present study, the eCEC could not be predicted well by soil pH, clay, and OC content, although it was correlated only with OC content (r2 = 0.46) because of the differences in clay mineralogy in the range of Chinese soils. Hence, the regression model based on soil eCEC and iron oxide developed using the European soils mostly was not applicable to Chinese soils. Furthermore, there were two European soils with high OC content (Rhydtalog and Zegveld, 12.9 and 23.3%), which were far beyond those in Chinese soils used in the present study. To ensure the accuracy in validating the mutual regression models derived from European and Chinese soils, the two high-OC soils were excluded from regression models, so the ranges of soil properties for European 4 and Chinese soils reported in the present study are approximately consistent. It was revealed that soil pH, OC content, and eCEC were the three most significant parameters explaining EC50 values based on the 16 European soils (log EC50 = 0.881 + 0.174 soil pH + 0.674 log OC + 0.172 log eCEC, r2 = 0.96). When the equation with three parameters (pH, log OC, and log eCEC) derived from European soils 4 was used to predict the log EC50 values for Chinese soils, the predictability for toxicity thresholds was improved from an r2 of 0.78 to an r2 of 0.87, compared with results from the equation with two parameters (pH and log OC). Broos et al. 19 also reported regression models based on the soil microbial toxicity assays for the European and Australian soils were quite similar after omitting three high-OC soils. Therefore, it was concluded that soil pH and OC content are two very important factors in controlling Cu toxicity to barley root elongation in European and Chinese soils, and eCEC should also be considered because of the significant improvement for regression models for Chinese soils (Table 3) and European soils. Finally, an equation based on soil pH, OC content, and eCEC combining Chinese and European soils together was established to predict toxicity thresholds (log EC50 = 0.708 + 0.205 soil pH + 0.748 log OC + 0.169 log eCEC), with a high r2 of 0.92. Thus, the regression model could be applied to predict the phytotoxicity of Cu in both Chinese and European soils accurately by considering soil pH, OC content, and eCEC.
CONCLUSIONS
Toxicity of Cu to barley root elongation across a wide range of Chinese soils varied 17- to 14-fold in unleached and leached soils, respectively, indicating that soil physicochemical properties strongly influenced phytotoxicity. Soil leaching decreased Cu toxicity significantly in approximately 35% of the soils examined and was found to be greatest in saline S14 soil. Freshly spiked soils should be leached and equilibrated before toxicity testing to mimic more realistic field exposure conditions. Multiple linear regression analysis showed that soil pH, OC, and eCEC were the three most useful predictors of Cu toxicity to barley root elongation and could explain >80% of the variation in phytotoxicity across soils. These empirically based models using simple, easily measured (and already mapped) soil properties have the potential to improve risk assessments significantly for Cu in soils using soil-specific ecotoxicity thresholds.
Acknowledgements
The authors thank the Natural Science Foundation of China (projects 20677077 and 40620120436), the International Copper Association, Rio Tinto, and the Nickel Producers Environmental Research Association for financial support. The authors also thank the national long-term soil experimental stations in China for soil collection and Cathy Fiebiger for technical assistance.




