Transformation and catalytic effects of sodium during coal pyrolysis
Lianfei Xu
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorCorresponding Author
Hui Liu
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Correspondence
Hui Liu and Qingxi Cao, School of Energy Science and Engineering, Harbin Institute of Technology, 92 West Dazhi Street, Harbin, China.
Email: [email protected]; [email protected]
Search for more papers by this authorDeng Zhao
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorCorresponding Author
Qingxi Cao
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Correspondence
Hui Liu and Qingxi Cao, School of Energy Science and Engineering, Harbin Institute of Technology, 92 West Dazhi Street, Harbin, China.
Email: [email protected]; [email protected]
Search for more papers by this authorJihui Gao
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorShaohua Wu
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorLianfei Xu
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorCorresponding Author
Hui Liu
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Correspondence
Hui Liu and Qingxi Cao, School of Energy Science and Engineering, Harbin Institute of Technology, 92 West Dazhi Street, Harbin, China.
Email: [email protected]; [email protected]
Search for more papers by this authorDeng Zhao
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorCorresponding Author
Qingxi Cao
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Correspondence
Hui Liu and Qingxi Cao, School of Energy Science and Engineering, Harbin Institute of Technology, 92 West Dazhi Street, Harbin, China.
Email: [email protected]; [email protected]
Search for more papers by this authorJihui Gao
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorShaohua Wu
School of Energy Science and Engineering, Harbin Institute of Technology, 92, West Dazhi Street, Harbin, China
Search for more papers by this authorSummary
The abundant sodium in Zhundong coal can act as an excellent catalyst for its thermal conversion. A horizontal fixed-bed reactor was selected to minimize the release of Na. Chemical form of Na in the coal was simplified by using acid-washed coal loaded with H2O-soluble and NH4Ac-soluble sodium. Most Na was retained in the char after pyrolysis of the coals at 500°C to 900°C. The H2O-soluble sodium in Na2SO4-chars remained unchanged below 700°C and then partly transformed to insoluble sodium; transformation of NH4Ac-soluble sodium to the H2O-soluble form dominated in the Na-chars. SEM-EDX analysis showed that the Na-char possessed a smoother surface than the Na2SO4-char. The Na/C ratio in the Na2SO4-char increased rapidly with temperature. Raman spectra of the chars showed that loaded Na2SO4 had little effect on its structure, but loaded NH4Ac-soluble sodium increased the amounts of polyaromatic (≥6) rings and active sites. Reactivity of the char was measured using TGA. Association analysis of these results revealed that insoluble sodium in the char showed excellent catalytic ability, but Na accumulated during oxidation did not. Both the char structure and the amount and occurrence modes of Na had strong effects on the char reactivity.
Supporting Information
| Filename | Description |
|---|---|
| er4160-sup-0001-Supporting_information.docxWord 2007 document , 879.7 KB |
Fig. S1. A two-stage gasification device.1 In the pyrolyser, solid residues from the cyclone separator heat the fresh coal from the storage bunker. The coal is then pyrolyzed without carrier gas. The char is transferred into the gasifier by shaking and gravity, and then reacts with gaseous agents in the gasifier. The gasification yields are separated by the cyclone separator. During the pyrolysis of fresh coal, the coal particles are stacked at bottom of the pyrolyser and there is no carrier gas flowing through the interstices between the coal particles. In other words, the reaction condition here is similar to that in a horizontal fixed-bed reactor. Figure S2. Contents of sodium and anions in raw coal on dry coal basis. The contents of NO3− and CH3COO− were much lower than that of any other anion. Figure S3. Curve-fitting of Raman spectrum of H-1-char-900 produced from pyrolysis of H-form-1 coal at 900°C. The G band represents aromatic ring breathing, rather than the
Figure S4. Scanning electron microscopy images of H-1-chars and H-2-chars derived from pyrolysis of H-form-1 and H-form-2 coals, respectively (× 10000). Figure S5. Raman spectra of chars prepared at 500, 700, and 900°C. (a) H-1- and Na2SO4-chars, (b) normalized Raman spectra of H-1- and Na2SO4-chars, and (c) normalized Raman spectra of H-2- and Na-chars. Table S1. Summary of Raman peak/band assignments |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Kuang M, Zhu Q, Ling Z, Ti S, Li Z. Improving gas/particle flow deflection and asymmetric combustion of a 600 MWe supercritical down-fired boiler by increasing its upper furnace height. Energy. 2017; 127: 581-593.
- 2Wei Y, Kuang M, Zhu Q, Ling Z, Ti S, Li Z. Alleviating gas/particle flow deflection and asymmetric combustion in a 600MWe supercritical down-fired boiler by expanding its furnace throat space. Appl Therm Eng. 2017; 123: 1201-1213.
- 3Xu L, Liu H, Fang H, Gao J, Wu S. Effects of various inorganic sodium salts present in Zhundong coal on the char characteristics. Fuel. 2017; 203: 120-127.
- 4Song G, Song W, Qi X, Lu Q. Transformation characteristics of sodium of Zhundong coal combustion/gasification in circulating fluidized bed. Energy Fuel. 2016; 30(4): 3473-3478.
- 5Gao M, Lv P, Yang Z, Bai Y, Li F, Xie K. Effects of Ca/Na compounds on coal gasification reactivity and char characteristics in H2O/CO2 mixtures. Fuel. 2017; 206: 107-116.
- 6Tsai Y-T, Yang Y, Wang C, Shu C-M, Deng J. Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Intern J Energy Res. 2018; 42(3): 1158-1171.
- 7Wei X, Huang J, Liu T, Fang Y, Wang Y. Transformation of alkali metals during pyrolysis and gasification of a lignite. Energy Fuel. 2008; 22(3): 1840-1844.
- 8Sugawara K, Enda Y, Inoue H, Sugawara T, Shirai M. Dynamic behavior of trace elements during pyrolysis of coals. Fuel. 2002; 81(11–12): 1439-1443.
- 9Wang Ca JX, Wang Y, Yan Y, Cui J, Liu Y, Che D. Release and transformation of sodium during pyrolysis of Zhundong coals. Energy Fuel. 2015; 29(1): 78-85.
- 10Li C-Z, Sathe C, Kershaw JR, Pang Y. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Fuel. 2000; 79(3–4): 427-438.
- 11Li CZ. Some recent advances in the understanding of the pyrolysis and gasification behaviour of Victorian brown coal. Fuel. 2007; 86(12–13): 1664-1683.
- 12Zhang L-x, Kudo S, Tsubouchi N, Hayashi J-i, Ohtsuka Y, Norinaga K. Catalytic effects of Na and Ca from inexpensive materials on in-situ steam gasification of char from rapid pyrolysis of low rank coal in a drop-tube reactor. Fuel Process Technol 2013; 113(0): 1–7.
- 13Marchand DJ, Schneider E, Williams BP, et al. Physical and chemical changes of coal during catalytic fluidized bed gasification. Fuel Process Technol. 2015; 130: 292-298.
- 14Li Y, Zhou C, Li N, et al. Production of high H2/CO syngas by steam gasification of shengli lignite: Catalytic effect of inherent minerals. Energy Fuel. 2015; 29(8): 4738-4746.
- 15Sheth A, Yeboah YD, Godavarty A, Xu Y, Agrawal PK. Catalytic gasification of coal using eutectic salts: Reaction kinetics with binary and ternary eutectic catalysts. Fuel. 2003; 82(3): 305-317.
- 16Beigom GK, Mostafa G, Mostafa R, et al. Development and application of vanadium oxide/polyaniline composite as a novel cathode catalyst in microbial fuel cell. Intern J Energy Res. 2014; 38(1): 70-77.
- 17Sheng C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel. 2007; 86(15): 2316-2324.
- 18Eom S, Ahn S, Rhie Y, et al. Influence of devolatilized gases composition from raw coal fuel in the lab scale DCFC (direct carbon fuel cell) system. Energy. 2014; 74: 734-740.
- 19Asadullah M, Zhang S, Min Z, Yimsiri P, Li C-Z. Effects of biomass char structure on its gasification reactivity. Bioresour Technol. 2010; 101(20): 7935-7943.
- 20Li X, Hayashi J-i, Li C-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel. 2006; 85(12–13): 1700-1707.
- 21Chabalala VP, Wagner N, Potgieter-Vermaak S. Investigation into the evolution of char structure using Raman spectroscopy in conjunction with coal petrography; part 1. Fuel Process Technol. 2011; 92(4): 750-756.



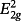 fundamental vibration, in highly disordered carbon material.2 The D band represents medium- to large-sized (≥ 6) aromatic ring systems in amorphous carbon structures.2 The GR, VL, and VR bands between the G and D bands represent relatively small aromatics with three to five fused benzene rings.3 The SL and S bands represent sp3-rich structures (Table S1).
fundamental vibration, in highly disordered carbon material.2 The D band represents medium- to large-sized (≥ 6) aromatic ring systems in amorphous carbon structures.2 The GR, VL, and VR bands between the G and D bands represent relatively small aromatics with three to five fused benzene rings.3 The SL and S bands represent sp3-rich structures (Table S1).
