Real-world effectiveness and tolerability of cenobamate in drug-resistant epilepsy: A retrospective analysis of the patients included into the Early Access Programs (EAP) in Germany, France, and United Kingdom
Abstract
Objective
Investigate real-world outcomes in drug-resistant epilepsy (DRE) patients treated with cenobamate as adjunctive treatment to other antiseizure medications (ASMs) within the Early Access Programs (EAP) in Germany, France, and the United Kingdom.
Methods
DRE adults with uncontrolled focal-onset seizures were included from 19 hospitals participating in the EAP in this retrospective study. Data were sourced from clinical records. Participants were evaluated at baseline, 1 months, and 3 months from cenobamate start, and 3, 6, and 12 months after maintenance. The primary effectiveness endpoint was the 50% responder rate, defined as the reduction in seizure frequency ≥50%.
Results
Data were collected from 298 patients who received at least one dose of cenobamate; efficacy was evaluated on 216 patients with seizure data available. At baseline, the median epilepsy duration was 22.2 years, and 41.9% of patients had previous epilepsy surgery, including vagus nerve stimulation, with a median of nine previously failed ASMs. The median number of seizures/month was 8.8. After 3 months of maintenance, the 50% responder rate (primary endpoint) was 49.3%; the median percentage seizure reduction from baseline was 49.1%. A total of 100%, ≥90%, and ≥75% seizures reduction were reported in 13.6%, 20.0%, and 33.6% of patients, respectively. Both the responder rate and the median percentage seizure reduction steadily increased during the observation period. At 6-month maintenance, the seizure-free rate was 24.2%. The retention rate assessed by Kaplan–Meier decreased from 96.6% at 1-month cenobamate start to 69.7% at 12-month maintenance. Adverse Drug Reactions (ADRs) to cenobamate occurred in 30.9% of patients, with asthenia, dizziness, and somnolence being the most frequent; the majority were mild-to-moderate and resolved during the observation period; three patients (1.0%) experienced a total of seven serious ADRs, all during titration.
Significance
In this study, cenobamate demonstrated to be an effective option for people with uncontrolled epilepsy even after multiple failed ASMs or failure of epilepsy surgery.
Plain Language Summary
This study involved patients with drug-resistant epilepsy, who had continued seizures despite using at least two antiseizure medications (ASMs). Patients received cenobamate (Ontozry) as epilepsy treatment during the Early Access Program (EAP) in France, Germany, and the United Kingdom. An EAP allows patients to receive promising new drugs under clinical supervision before they are commercially available. After 6 months from cenobamate start, 49.3% of patients had their seizures cut by half or more, and 13.6% became seizure-free. A total of 30.9% of patients had an undesirable reaction to cenobamate, mostly mild-to-moderate and resolved; the most frequent were asthenia, dizziness, and somnolence.
Key points
- Patients included in this EAP had a median history of 22 years of epilepsy, a median of 9 prior failed ASMs, and 41.9% had epilepsy surgery.
- The outcome in this drug-resistant population was consistent with findings from clinical trials and other RWE experiences: 49.3% of patients treated with cenobamate as adjunctive therapy achieved a 50% reduction in seizure frequency during the first 3 months of maintenance, with a seizure-free rate of 13.6%.
- The responder rate and seizure-free rates steadily increased over time, until the last observation period.
- Most of the CNB-related ADRs were mild-to-moderate and resolved/were resolving at the EAP end; they were consistent with the already known side effect profile of the drug: Only 1% of patients experienced SADRs, all during titration and none during maintenance, supporting the finding that most AEs occur early on and mostly resolve over time.
1 INTRODUCTION
With an estimated 5 million new cases each year, epilepsy accounts for a significant proportion of the world's brain health disease burden, with a prevalence of more than 50 million people worldwide.1-3 Although most patients with epilepsy achieve sustained seizure control, approximately a third do not achieve a year of seizure freedom, and when epilepsy is deemed drug refractory (a failure of two prior ASMs), their chance of 12-month seizure freedom drops to 4.1%.4 Focal-onset epilepsy, in contrast with generalized epilepsy, is much less likely to remit, more likely to relapse, and is more likely to be multidrug refractory.2, 5
Patients with uncontrolled seizures are at increased risk of morbidity, mortality, and a decreased quality of life. They are also more likely to have cognitive, psychiatric, emotional, and psychosocial difficulties.6, 7 The burden of drug-resistant epilepsy (DRE) has remained fairly stable, and the need for novel, more effective therapeutic options remains high.8, 9
Cenobamate (CNB) is a recently FDA-approved drug used to treat focal-onset seizures (FOS) in adult patients10; EMA authorized the drug in March 2021 for the adjunctive treatment of FOS with or without secondary generalization in adult patients with epilepsy who have not been adequately controlled despite treatment with at least 2 ASMs.11, 12 CNB is a novel tetrazole-derived carbamate with a dual mechanism of action, both of which are commonly associated with the generation and propagation of seizures. In fact, CNB acts as a positive allosteric modulator of the GABA A receptor, binding at a non-benzodiazepine site, and is effective in reducing repetitive neuronal firing by inhibition of voltage-gated sodium channels.13, 14
Two placebo-controlled studies with their respective open-label phases as well as an open-label study showed the efficacy and safety of CNB as adjunctive treatment to 1–3 ASMs in patients with focal epilepsy. CNB substantially improved seizure control, reflected by a significant seizure freedom rate.
Most adverse events were rated as mild-to-moderate, and the majority were transient. The most common ones were dizziness, drowsiness, and headache. Long-term efficacy and tolerability have also been confirmed in open-label studies.15-18 The incidence of rare cases of hypersensitivity reactions (DRESS) seen in early trials has been significantly reduced by using the current recommended titration schedule, and no additional confirmed cases have occurred with long-term treatment with 100 000 patients treated until December 2023.19-21 Vigilance during dose titration is nevertheless still required, as less severe hypersensitivity reactions still occur.22
Real-world data are important to understand and confirm the efficacy and safety profile outside of the clinical trial setting.
In the European Union, CNB EAPs have been initiated from September 2020 in several countries for adult FOS patients. This retrospective study is aimed at analyzing the overall effectiveness and tolerability of CNB by collecting real-world data from FOS adult patients participating in EAPs in France, Germany, and the United Kingdom, according to the authorization received from local regulatory/ethic authorities.
2 METHODS
2.1 Study design
This was a multicenter, retrospective, observational study performed in France, Germany, and the United Kingdom to assess the effectiveness and tolerability of CNB in a real-world setting during EAPs. The study involved epileptologists from 19 hospitals. The study protocol was approved by the competent regulatory authorities and the ethics committee and followed the ethics code set out in the Declaration of Helsinki.
Adult patients diagnosed with drug-resistant focal epilepsy, participating in the EAP with CNB as adjunctive treatment from September 2020 to September 2022, were retrospectively screened for inclusion in the study. No arbitrary selection was applied, but patients with specific syndromes (e.g., Lennox–Gastaut and Dravet Syndrome) were excluded as per exclusion criteria. Reasons for exclusion were documented. Available data were collected after patient consent for processing of personal data according to the General Data Protection Regulation (GDPR) and applicable local regulations.
Patient's clinical data and any assessment performed according to clinical practice during the EAP were collected retrospectively. Duration of the follow-up was limited to the EAP duration: Once the EAP ended, the follow-up could not be collected even if the patients were still on CNB. The following time points were considered for efficacy evaluations: baseline, 1 and 3 months after start of CNB therapy and 3, 6, and 12 months after completion of the titration (maintenance phase).
2.2 Data collection
Data were collected from clinical records and were kept according to the usual clinical practice at each center by participating physicians. Baseline data included demographic and lifestyle profile, presence and severity of intellectual disability (according to investigator's judgment); comorbidities; epilepsy diagnosis and seizure type; history of epilepsy surgery; etiology; monthly (28 days) frequency of FOS (at time of CNB start); ASM history (all the ASMs used from epilepsy diagnosis and duration); concomitant ASMs; and other concomitant treatments.
2.2.1 Efficacy
The following information was collected from clinical charts at every visit: number of seizures CNB use: start date, treatment duration, reasons for discontinuation—if applicable, information on titration (duration, doses, etc.), last maintenance dose reached, and concomitant ASMs (duration, doses).
2.2.2 Tolerability
All AEs/ADRs that occurred from CNB initiation to observation end were collected: duration, relationship, seriousness of AEs/ADRs (non-serious AE/ADR or Serious AE/ADR [SAE/SADR]); and treatment discontinuation due to AE/ADRs (including reason for discontinuation, any action taken).
2.3 Evaluated outcomes
The primary efficacy endpoint was the 50% responder rate defined as a ≥50% reduction from baseline seizure frequency (all seizures) after 3 months of maintenance.
Secondary endpoints were calculated at all the time points and included: (a) Percentage of seizure-free patients (no seizures since the last visit), responder rates defined as ≥50%, ≥75%, and ≥90% reduction in seizure frequency from baseline; (b) Retention rate measured as the percentage of patients remaining in the study and on adjunctive therapy; (c) Percentage change in seizure frequency; (d) Average dose during maintenance; (e) Changes from baseline in the number of concomitant ASMs; (f) AEs and ADRs, including DRESS, rash/hypersensitivity that occurred during the EAP; and (g) Withdrawal due to ADRs.
2.4 Statistical analysis
A descriptive analysis of the variables collected was performed. Categorical variables were described as absolute frequencies and percentages; continuous variables were presented with descriptive statistics. Baseline characteristics, prior/concomitant medications, and tolerability evaluations were presented in the Safety Set (SS), consisting of all patients who started CNB. The retention rate was reported in the SS using the Kaplan–Meier curve and estimate.
The efficacy analysis focused on seizure data and was conducted on the Full Analysis Set (FAS), including patients with at least one documented seizure assessment at or after the start of therapy.
Seizure outcomes were analyzed in the FAS, using the last observation carried forward (LOCF) approach for missing data within each post-baseline period. In addition, a sensitivity analysis, by visit, was performed in patients who completed the respective visits within the EAP (completers population).
Seizure rate per 28 days, percentage change from baseline in seizure rate, and 50%/75%/90%/100% seizure response were reported within post-baseline periods of follow-up planned in the study.
The percentages were calculated with the denominator as the number of patients available within each post-baseline period. All analyses were performed using SAS® software version 9.4.
3 RESULTS
3.1 Patient disposition
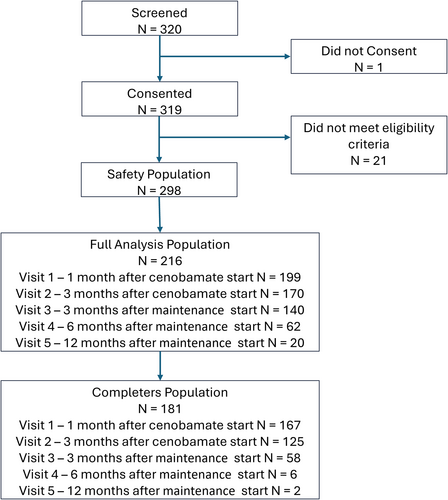
Overall, 320 patients were screened, and 319 consented to personal data processing. Twenty-one (21) patients screened were excluded from data collection since they did not meet eligibility criteria.
Two hundred and ninety-eight (298) patients were entered into the study database and received at least one dose of CNB (SS). At baseline, 216 patients were included in the FAS; 181 patients met the definition of the completers population.
The number of patients decreased throughout the observation study time points (1 month titration to 12 months maintenance) mostly due to later entry into the EAP: the median time of EAP end after CNB start was 4.34 months. When the EAP ended, out of 298 patients in the SS, 73 (24.5%) were in titration, 215 (72.1%) in maintenance, and 10 (3.4%) had completed the 3 + 12 month observation period. Overall, 38/298 (SS) patients discontinued CNB permanently before the EAP ended (12.7%).
Patient's disposition is summarized in Figure 1.
3.2 Baseline characteristics
Baseline characteristics are reported in Table 1. The mean age of patients (SS) was 37.0 years, and 56.7% were male. The mean (median) age at the time of the first epilepsy diagnosis was 24.1 (22.2) years old. Overall, 41.9% of patients had a history of epilepsy surgery (including VNS); the median number of failed ASMs (prior + concomitant ASMs) was 9 (Q1- Q3: 6–12).
| Characteristics | All patients (N = 298) |
|---|---|
| Gender | |
| Male, n (%) | 169 (56.7) |
| Mean age, years (range) | 37.0 (18–82) |
| Time to first epilepsy diagnosis at the time of CNB start (year) | |
| Mean (SD), [range] | 24.1 (12.6), [2–72] |
| Median (Q1; Q3) | 22.2 (14.9; 31.9) |
| Etiology, n (%) | |
| Structural | 165 (55.4) |
| Genetic | 12 (4.0) |
| Infectious | 10 (3.4) |
| Metabolic | 0 (0.0) |
| Immune | 8 (2.7) |
| Unknown | 103 (34.6) |
| Epilepsy type, n (%) | |
| Focal | 233 (78.2) |
| Generalized | 0 (0.0) |
| Combined generalized and focal | 51 (17.1) |
| Unknown | 14 (4.7) |
| Number of previous ASMs | |
| Mean (SD) | 7.2 (4.1) |
| Min; Max | 1; 25 |
| Median (Q1; Q3) | 7.0 (4.0; 10.00) |
| Number of concomitant ASMs | |
| Mean (SD) | 3.0 (1.1) |
| Min; Max | 1; 8 |
| Median (Q1; Q3) | 3 (2,4) |
| Failed ASM (prior + concomitant ASM), median (Q1; Q3) | 9 (6; 12) |
| Prior epilepsy surgery (VNS included), n (%) | |
| No | 171 (57.4) |
| Yes | 125 (41.9) |
| Unknown | 2 (0.7) |
| Patients needing caregiver support, n (%) | 94 (31.5) |
| Patients with intellectual disability, n (%) | 88 (29.5) |
| Mild | 35 (39.8) |
| Moderate | 21 (23.9) |
| Severe | 9 (10.2) |
| Profound | 4 (4.5) |
| Not known | 19 (21.6) |
| Patients with any psychiatric disorder n (%) | 80 (26.8) |
- Abbreviations: ASMs, antiseizure medications; Q1–Q3, First and third interquartile; SD, standard deviation; VNS, vagus nerve stimulation.
The most frequent prior medications were levetiracetam, 64.4% (n = 192), valproic acid, 51.0% (n = 152); lamotrigine, 48.7% (n = 145), lacosamide, and perampanel, both 48.3% (n = 144), zonisamide and topiramate, 47.0% (n = 140), carbamazepine, 42.9% (n = 128), oxcarbazepine, 40.3% (n = 120); the analgesics (gabapentin and pregabalin) were used by 30.9% (n = 92) of the SS; clobazam was the most used benzodiazepine, by 22.8% of patients (n = 68).
3.3 CNB dosage
In the FAS, the median (Q1; Q3) duration of titration was 70 (69.8; 71) days, the median (range) maintenance dose was 200 mg/day (25; 400); maintenance dose reached (in categories) is presented in Appendix S1. During the observation period, 28.4% of the SS achieved a maintenance dose <200 mg/day, 43.8% achieved 200 mg/day, and 27.8% achieved higher dosages (7.3% were on 250 mg/day, 16.1% on 300 mg/day, and 4.4% on 400 mg/day).
3.4 Retention rates
Retention rates at 1, 3, 6, and 12 months after CNB start were 96.6%, 93.3%, 84.7%, and 69.7%, respectively. A total of 93.3% of patients entering the maintenance phase completed it (Figure 2).

In total, during the whole observation period, 38/298 patients (12.7%) discontinued CNB, 18 patients during titration, and 20 patients during maintenance.
In 11/38 cases, the investigator reported that the decision to interrupt treatment was taken for “safety reasons,” in concomitance of the following ADRs: dizziness and fatigue (1 patient), hyponatremia and pancytopenia (1 patient), rash erythematous (1 patient), allergic reaction (1 patient) asthenia and vertigo (1 patient), nausea and dizziness (1 patient), aggression and headache (1 patient), dizziness (2 patients), ataxia, dizziness, and nausea (1 patient), and constipation and fatigue (1 patient). In 2/38 cases of CNB discontinuation, the AE “increase in seizure frequency” was reported.
In all the other cases (n = 27), the reason for discontinuation was not clearly described in the clinical chart: Anyway, in 18/27 cases, ADRs related to CNB occurred.
3.5 Effectiveness
The primary endpoint, 50% responder rate at 3-month maintenance, was achieved by 69/140 (49.3%) patients (FAS). This responder rate steadily increased from the first reported period to the last one.
In the completers population, it was achieved by 28/58 (48.3%) patients, indicating consistency with the results of the main population. The 50% responder rate by period is presented in Table 2 (Main and Sensitivity analyses).
| Visit | 50% responder rate, n/Na (%) | |
|---|---|---|
| Main analysis (FAS population) | Sensitivity analysis (completers population) | |
| Visit 1–1 month after cenobamate start | 66/199 (33.2) | 66/167 (39.5) |
| Visit 2–3 months after cenobamate start | 75/170 (44.1) | 58/125 (46.4) |
| Visit 3–3 months after maintenance start | 69/140 (49.3) | 28/58 (48.3) |
| Visit 4–6 months after maintenance start | 33/62 (53.2) | 10/16 (62.5) |
| Visit 5–12 months after maintenance start | 12/20 (60) | 1/2 (50) |
- a In the main analysis, the denominator N within each period indicates the total number of patients with available seizure data during the respective period. In the sensitivity analysis, the denominator N within each period indicates the total number of patients who complete the respective period.
At 3-month maintenance, ≥75%, ≥90%, and 100% responder rates were achieved by 33.6%, 20.0%, and 13.6% of patients, respectively. Responder rates by period are summarized in Figure 3.

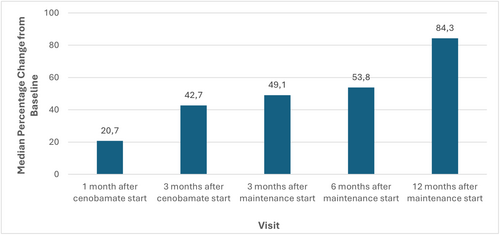
Figure 4 presents the percentage change in seizure rate from baseline by period. Baseline median seizure rate per 28 days was 8.8. At 3-month maintenance, the median seizure rate per 28 days was 4, and the median percentage change from baseline was −49.1%. The median percentage change from baseline progressively increased starting from 1 month after CNB start (20.7%) to 12-month maintenance (84.3%). The table with complete seizure data is in Appendix S2.
3.5.1 Effectiveness and cenobamate dosage
A subgroup analysis based on maintenance dose (<200 mg or ≥200 mg) was conducted, indicating the general consistency of efficacy results in the subgroups: The 50% responder rate during 3 months after maintenance start was achieved in 46.2% in the patients' subgroup with <200 mg maintenance dose and in 52.6% in the patients' subgroup with ≥200 mg maintenance dose. The percent reduction in seizure frequency from baseline, in the two subgroups, was 44.1% and 54.2%, respectively.
3.6 Concomitant ASMs during the study
In the SS (N = 298), the median number of concomitant ASMs was 3.0 (range: 1.0; 8.0), 66.1% of patients were on adjunctive treatment with more than two, and 25.5% with more than three concomitant ASMs.
The most used concomitant ASMs were clobazam (32.5%), lamotrigine (32.9%), followed by lacosamide (29.2%), perampanel (24.8%), brivaracetam (23.5%), levetiracetam (22.1%), zonisamide (18.8%), and valproic acid (16.1%). The mean number of concomitant ASMs shows a slight decrease over time during the observation period (except for the 12-month maintenance time point, where the number of patients is extremely low): in the SS, the change from baseline was 0.0 at 1 month from CNB start (n = 268), −0.1 at 3 months after CNB start (n = 195); −0.2 at 3-month maintenance (n = 117); and −0.5 at 6-month maintenance (n = 40). At 12 months, it was −0.1 (n = 11).
3.7 Tolerability
Overall, 314 Adverse Events (AEs) in 137 (46.0%) patients were reported in clinical charts. Severity was mild, moderate, and severe for 51.0%, 44.9%, and 4.1% of cases, respectively. Outcomes were “recovered/resolved” in 53.2%, “recovering/resolving” in 15.9%, “unknown” in 10.2%, and “unrecovered/unresolved” in 20.7% of cases. Concomitant treatments were increased/reduced in dosage in 24.84% of cases and discontinued in 4.46% of cases.
Fourteen (14) events occurred in eight patients (2.7%) were classified as serious AEs (SAEs, see below).
ADRs CNB-related were 188 (59.9% of all AEs) and occurred in 92 patients (30.9%); the most frequent (≥5%) were fatigue/asthenia (12.1%), dizziness (6.0%), and somnolence (5.7%).
Results from post hoc analysis indicated that, based on investigators judgment, 18.6% of the ADRs CNB-related were of sodium-channel blocker nature (ataxia, diplopia, dizziness, unsteadiness, and balance disorders), and 9.6% were of GABAergic nature (somnolence, drowsiness, and sedation). Following ADRs to CNB, concomitant medications were reduced in dosage in 13.8% of cases, discontinued in 3.7% of cases, and a new treatment was prescribed in 1.8% of cases. At EAP end, 56.4% of the CNB-related ADRs were recovered, 15.4% were recovering, 14.4% were not recovered; for the remaining 13.8%, the outcome was unknown.
Overall, seven SADRs related to CNB occurred in three patients (1.0%) during titration; the SAE classification was due to hospitalization or prolongation of hospitalization: One patient experienced acute urinary retention, somnolence, and general physical health deterioration; one patient experienced disturbance in attention; and one patient experienced loss of personal independence in daily activities, balance disorders, and somnolence. All events were graded severe in intensity except the AE “moderate” disturbance in attention. In all cases, concomitant treatments were reduced; for 2/3 patients, the CNB dosage was also reduced (from 300 mg/day to 250 mg/day and from 200 mg/day to 100 mg/day, respectively).
Four (4) SADRs related to other drugs occurred in two patients. Nystagmus, tremor, and gait disturbance, all moderate, occurring in one patient, were judged as possibly related to lamotrigine leading to dose reduction; the SADR “lower limb edema,”, mild in intensity, occurred in one patient was judged to be due to drug interactions, CNB dosage was decreased for safety reasons.
Seven (7) ADRs of special interest were considered related to CNB, all mild or moderate in intensity: dermatitis allergic + conjunctivitis, pruritus, rash, rosacea, rash erythematous, and hypersensitivity occurred in seven patients during the titration phase. In 5/7 cases, CNB was discontinued; in one case, CNB was down-titrated to 12.5 mg/day, and the patient was still on treatment at the EAP end. In one other case, no action was taken.
No differences in other classes of events between titration and the maintenance phase were observed, even if the frequency was lower in the maintenance phase. During titration, 128 ADRs occurred in 66 (22.1%) patients; 50.8% of ADRs were mild and 7.8% severe. Seven (7) SADRs occurred in three patients. During maintenance, 60 ADRs occurred in 31 patients (15.0%). Events were mild for 48.3% of patients; 1.7% were severe.
4 DISCUSSION
This study evaluated the response to CNB in a highly drug-resistant epilepsy population obtained in a real-world setting related to the EAPs in France, Germany, and the United Kingdom.
Data from CNB EAP from other countries were published; for example, Villanueva et al.23 included 170 highly refractory epileptic patients from the Spanish EAP. The mean (median) seizures/month value at baseline was 27.6 (11.3), similar to the 24.3 (8.8) of this study. A total of 99.4% (169/170) had failed at least five ASMs and had so-called absolute drug resistance24; An increasing seizure reduction and seizure-free rate during therapy was reported, and the proportion of patients achieving seizure freedom from baseline increased from 7.8% to 16.9% between 3 and 6 months of treatment; this is consistent with this study, where the proportion of seizure-free patients increased from 8.0% to 11.8% at 1 and 3 months from CNB start, to 13.6% and 24.2% at 3 and 6-month maintenance. Data from the Italian EAP (236 subjects) are also in line: the seizure-free rates were 9.8%, 12.2%, and 16.3% at 3, 6, and 9 months from CNB start.25 Sustained seizure-free presurgical patients treated with CNB may have the option of postponing or canceling surgery, on a case-by-case basis, as suggested by a recent expert panel.26 However, the group also reports that CNB could be a useful treatment where surgery has failed. In this study, 41.9% of patients had previous surgery.
Pena-Ceballos et al.27 collected data from 57 patients with focal epilepsy treated with CNB for at least 3 months within the EAP in Ireland; the median of previously failed ASMs was 9; they defined their population as patients with ultra-refractory epilepsy (failure of ≥6 treatments for epilepsy). Patients had a higher median of seizures/month than this study (60 = highly active epilepsy): Most patients experienced meaningful seizure outcomes over a median treatment period of 11 months, 5.3% achieved seizure freedom, 42.1% reached a 75%–99% seizure frequency reduction, and 28.1% a 50%–74% seizure frequency reduction.
In the present study, long-term observations are limited to a low number of subjects (n = 20 at 12 month-maintenance); nevertheless, the seizure-free rate is 11.8% at the end of titration and increases to 45% at 12-month maintenance. Similarly, the responder rate has a constant improvement trend, from 44.1% at the end of titration up to 60.0% at 12-month maintenance, suggesting that a favorable outcome can be obtained by long-term treatment, despite the initial response.
The cohort exposed to CNB in this study is likely to be different from those included in randomized clinical trials, where patients with greater concomitant ASM use or psychiatric comorbidities are routinely excluded. The demonstrated effectiveness outcomes resulting from this analysis are, however, consistent with outcomes from clinical trials. From a Phase II randomized study reported by Krauss et al.15 in 437 patients assigned to either placebo (n = 108), CNB 100 mg (n = 108), 200 mg (n = 110), or 400 mg (n = 111), the responder rates (≥ 50% reduction) during the maintenance phase were 25% for the placebo group, 40% for the 100 mg dose group, 56% for the 200 mg group, and 64% for the 400 mg group.
Consistently, at 3-month maintenance, we observed 49.3% of patients achieving ≥ 50% reduction in seizure frequency. When we analyzed the response in two dosage subgroups (<200 mg or ≥200 mg), the responder rates were 46.2% for the <200 mg subgroup and 52.6% for the ≥200 mg subgroup, slightly suggesting the need for a higher dose of CNB in these severe patients to achieve better results as previously shown in clinical trials.28, 29
The median CNB dosage (200 mg/day) during maintenance in this real-world data collection was consistent with previous studies.15-19 Nevertheless, it should be noted that about 28% of patients reached dosages >200 mg/day. The trend to use higher dosages in the long term is supported by data emerging from the real world and clinical trials suggesting that many patients experienced clinically relevant seizure reductions at 100 or 200 mg/day CNB,30 but that higher dosages were needed to achieve seizure freedom, particularly in the long term.27, 31, 32 In the above-reported trial by Krauss et al., during the 12-month maintenance, the seizure-free rate was 1% for the placebo group, 4% (p = 0.3688) for the CNB 100 mg group, 11% (p = 0.0022) for the CNB 200 mg group, and 21% (p < 0.0001) for the CNB 400 mg group (p values vs. placebo).15
This is consistent with the fact that the responder rate in this study steadily increased from the first reported period to the last one: The seizure-free rate increased from 13.6% at 3 months after maintenance start to 24.2% at 6 months to 45% at 12 months, although the sample size decreased significantly over time due to the late entrance of patients in the EAP. It should be noted that in a recent retrospective study in Spain,29 the mean CNB daily dose in the PP population was 207.8 ± 25.7 mg at 3 months, 267.2 ± 53.9 mg at 6 months, and 326.2 ± 80 mg at 12 months. The seizure-free percentages at 3, 6, and 12 months were 21.9%, 44.8%, and 33.3%, respectively.
In that study, the authors focused on co-ASM use in a highly drug-resistant population treated with CNB, concluding that optimization of co-ASM management during CNB treatment allowed high seizure freedom rates despite meaningful reductions in co-medication. They reported a reduction in seizure frequency/month from baseline to the last visit (p < 0.0001). Between baseline and the study end, the mean number of co-ASMs in the PP population was reduced from 2.9 to 1.6 (p < 0.0001). The percentage of patients with ≥3 co-ASMs was reduced from 61.8% at baseline to 14.3% at 12 months; one patient was receiving CNB as monotherapy at the last visit.
In the present study, a trend in co-ASM reduction could be observed over the time after CNB initiation.
The retention rate (RR) calculated with the KM (about 93% at 3 months, 85% at 6 months, and 70% at 12 months) was similar to RR from the above real-world study as well as the Italian EAP, where the RRs were 94%, 88%, and 77% and 94%, 91%, and 78.8% at 3, 6, and 12 months of follow-up, respectively [31; 25].
It should be noted that, in total, during the whole observation period, only 12.7% (38/298) of SS discontinued CNB treatment: 18 during titration and 20 during maintenance.
Due to the retrospective nature, it was not always possible to establish the correlation between AEs and CNB discontinuation. However, in 11/38 cases of discontinuation, the reason was clearly stated in the clinical chart as a safety reason.
Seven (7) cutaneous reactions (2.3% of total CNB-related ADRs) that occurred during the titration phase were mild or moderate in intensity. In 6/7 cases, CNB was discontinued/reduced in dosage, and all of them resolved, with one exception (rosacea), for which the outcome was unknown at the end of the observation period.
These data are consistent with data published from other clinical practice series33-36 and with the outcome from the large open-label safety study with CNB, where the start-low, go-slow titration approach used resulted in no further cases of DRESS.17 These authors reported mild or moderate skin and subcutaneous tissue disorders in 189 patients (14.1%) and a safety profile consistent with the AEs reported in this study, with somnolence, dizziness, and fatigue as the AEs with the higher frequency. Since cenobamate's launch in 2020, more than 100 000 patients have been treated worldwide, with no new confirmed cases of DRESS reported so far.21
During titration, the ADR rate was higher in the 12.5 mg dosage, suggesting that they occur soon after CNB start. Except for cutaneous disorders above, occurring only during titration, no differences in the class of events between titration and maintenance were observed, but notably, the frequency of all events was lower during maintenance, with no relevant relationship with dosage in the frequency of events and no SADRs occurred.
In conclusion, the tolerability profile of CNB from this real-world study confirms the findings from clinical trials37 and other RWE studies; the nature and frequency of ADRs reported are in line with the product SPC and emerging real-world evidence. Allergic reactions only occurred in 7/298 patients with a rate of 2.3% (in the SPC described as “uncommon,” i.e., <1%); moreover, this rate was consistent with the cutaneous event rate in a similar real-world study on EAP from Villanueva et al. (2.9%).
The unique unlisted SADR was the report of urinary retention in a patient with a neurological bladder.
4.1 Limitations
This is a retrospective study that relies on clinical records for seizure data. Seizure counts in retrospective studies are not as accurate as in prospective trials, and the lack of data affected the efficacy endpoints, as discussed above. Since the study aimed to collect data only during the EAP and not beyond, a low rate of the 298 patients included completed the 3 + 12-month long-term observation, and the number of patients observed decreased significantly during time, depending on the EAP conclusion period, which varied between the three countries.
4.2 Clinical relevance
A key strength of this study is that it mirrors the real-world practice in a highly refractory population for whom compassionate use was required. Many of the patients in this study would not have been eligible for clinical trials due to their high number of concomitant ASMs and associated psychiatric disorders.
As far as we know, this is the largest real-world study that has been performed to date and includes a long follow-up in a subset of the population, thereby adding useful information for physicians based on clinical practice.
4.3 Overall conclusion
Our study evidenced that CNB can be considered generally well tolerated and effective in highly refractory focal or combined generalized and focal epilepsy.
Patients included in this EAP had a median history of 22 years of epilepsy, a median of 9 prior failed ASMs, and 41.9% had epilepsy surgery. Nevertheless, in this difficult-to-treat patient population, 49.3% of patients treated with CNB as adjunctive therapy achieved a 50% reduction in seizure frequency during the first 3 months of maintenance, with a seizure-free rate of 13.6%. This responder rate steadily increased over time, till the last observation period: at 6 and 12 months of maintenance, the responder rates in terms of 100% and 50% seizure reduction were 24.2%, 53.2%, 45%, and 60%, respectively.
Additionally, tolerability data was encouraging as most of the AEs were mild-to-moderate and were consistent with the already known tolerability profile of the drug. In total, during the whole observation period, 38/298 patients (12.7%) discontinued CNB; only 1% of patients experienced SADRs, all during titration and none during maintenance, supporting the finding that most AEs occur early on and resolve over time.
AUTHOR CONTRIBUTIONS
Sylvain Rheims, Bernhard J. Steinhoff, Edouard Hirsch, Felix Rosenow, Arnaud Biraben, and Rhys Thomas were all investigators responsible for data acquisition. Thangavelu Karthinathan analyzed the data and created tables and figures. All authors discussed the results, revised the first draft, and contributed to the final manuscript.
ACKNOWLEDGMENTS
The authors acknowledge all the investigators who participated in the CENOR study: Adam Strzelczyk, MD; Sophie Von Brauchitsch, MD; Laurent Maximilian Willems, MD; Hans-Beatus Straub, MD; Anna-Lena Friedo, MD; Benedikt Greshake, MD; Susanne Knake, MD; Holger Lerche, MD; Sigrid Schuh-Hofer, MD; Stephan Lauxmann, MD; Christian Brandt, MD; Thomas Mayer, MD; Peter Hopp, MD; Martin Hirsch, MD; Hajo M. Hamer, MD; Caroline Reindl, MD; Susanne Knake, MD; Ilka Immisch, MD; Rainer Surges, MD; Michael Rademacher, MD; Randi Von Wrede, MD; Fabrice Bartolomei, MD; Sandrine Aubert-Conil, MD; Stanislas Lagarde, MD; Sophie Dupont, MD; Adrien Benard, MD; Maliia Dragos-Mihai, MD; Maria Paola Valenti-Hirsch, MD; Nathalie Chastan, MD; Philippe Derambure, MD; Louis Maillard, MD; Louise Tyvaert, MD; Jacques Jonas, MD; Irina Klemina, MD; and Alyaa Eissa, MD. The authors acknowledge study nurses, study coordinators, and all the members of the CENOR study team involved in data collection. The authors also acknowledge the statistical programming support received from Deepti Kannan, Medastats LLC. The authors also acknowledge Patrizia Mascagni, Hippocrates Research Srl, for her assistance with medical writing.
FUNDING INFORMATION
This study has been funded by Angelini Pharma.
CONFLICT OF INTEREST STATEMENT
Sylvain Rheims: Received speaker and/or consultant fees from Angelini Pharma, UCB Pharma, EISAI, Livona, Zogenix, and JAZZ Pharma. Bernhard J. Steinhoff: Advisory and consulting honoraria: Angelini, Jazz/GW Pharmaceuticals, Precisis, Roche Diagnostics, UCB. Speaker's honoraria: Angelini, Desitin, Eisai, Jazz/GW Pharmaceuticals, Medscape, Tabuk, Teva, UCB, and Zogenix. Research support: Eisai, European Union, Janssen-Cilag, Jazz/GW Pharmaceuticals, SK Life Sciences, UCB, and Zogenix. Felix Rosenow: Honoraria for scientific advice and as a speaker: Angelini Pharma, Eisai Pharma, Jazz Pharmaceuticals, Roche Pharma, Stoke Therapeutics, Takeda, and UCB Pharma. Research support: European Union (EU-FP7), German Research Foundation (DFG), Federal State of Hesse, Germany, Detlev Wrobel Fonds for Epilepsy Research, Reiss-Stiftung, Dr. Senckenbergische Stiftung, Kassel-Stiftung, Ernst Max von Grunelius-Stiftung, Chaja-Stiftung, Desitin Arzneimittel, Dr. Schär Deutschland GmbH, Nutricia Milupa GmbH, and Vitaflo Deutschland GmbH. Edouard Hirsch: Advisory and consulting honoraria: Angelini, Jazz/GW Pharmaceuticals, UCB. Speaker's honoraria: Angelini, Desitin, Jazz/GW Pharmaceuticals, and UCB. Rhys Thomas: Honoraria from Angelini, Bial, Biocodex, Eisai, Jazz, LivaNova, Paladin Labs, Neuraxpharm, Sanofi, Takeda, and UCB Pharma. Meeting support from Angelini, Bial, and UCB Pharma. Unrestricted funding support from Angelini. Joint working partnership with UCB Pharma. Arnaud Biraben: Advisory and consulting honoraria: Angelini and Jazz. Speaker's honoraria: Angelini and Jazz pharmaceutical. Alessandro Lovera, Paola Lipone, Alessandro Comandini, Caroline Benoist, Elena Alvarez Baron, John Paul Leach, and Agnese Cattaneo are employees of Angelini Pharma Spa. Thangavelu Karthinathan was contracted by Angelini Pharma to analyze the data.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data is available upon reasonable request to the corresponding author.