Epilepsies with onset during the first year of life: A prospective study on syndromes, etiologies, and outcomes
Abstract
Objective
Infantile seizures cause great concern for both doctors and parents. In addition to modern neuroimaging and genetics, clinical tools helpful in predicting the course of the disease are needed. We prospectively studied the incidence, electroclinical characteristics and etiologies of epilepsy syndromes with onset before the age of 12 months and looked for prognostic determinants of outcome by age 24 months.
Methods
From February 2017 through May 2019, we recruited all eligible infants diagnosed with epilepsy at our unit. Data on electroclinical studies, genetic investigations and drug response were gathered prospectively. The infants were given a structured neurological examination (Hammersmith Infantile Neurological examination [HINE] and Griffiths scales) at predetermined intervals until age 24 months at which age neurocognitive evaluation with Bayley scales was performed.
Results
Included were 60 infants (27 female). The mean onset age of epilepsy was 5.3 (±2.5 standard deviation) months. The incidence of epilepsy in the population-based cohort was 131 (95% confidence interval 99–172)/100 000. Epilepsy syndrome was identified in 80% and etiology in 58% of infants. Self-limited infantile epilepsy was the second most common syndrome (incidence 18/100 000) after infantile epileptic spasms syndrome. PRRT2 was the most common monogenic cause. At age 24 months, 37% of the infants had drug-resistant epilepsy (DRE) and half had a global developmental delay (GDD). Abnormal first HINE was the strongest predictor of GDD, followed by DRE and identified etiology. DRE was associated with structural etiology and GDD. Those with normal first HINE and good response to treatment had favorable outcomes, irrespective of the identified etiology.
Significance
Our results support a high incidence of self-limited epilepsy in infancy and PRRT2 as the genetic cause in the first year of life. Notwithstanding the advances in etiological discovery, we want to highlight the importance of clinical evaluation as standardized neurological examination with HINE proved a valuable tool in prognostication.
Plain Language Summary
One in every 700–800 babies develop epilepsy within the first year after birth. Our study identified an epilepsy syndrome in 80% and the cause of epilepsy in 60% of the participants. By age 2 years, over one-third of the children still experienced seizures, and almost half faced significant developmental delay. Structural brain abnormalities increased the likelihood of difficult epilepsy and developmental challenges. Babies whose epilepsy was caused by a gene defect varied widely in development and response to medications. Babies with normal neurological examination at first visit, especially if their seizures stopped quickly, had favorable development.
Key points
- Syndrome diagnosis can be reached in 80% of epilepsies with onset in the first year of life.
- Self-limited epilepsy of infancy is the second most common epilepsy syndrome after infantile epileptic spasms syndrome.
- At age 2 years, more than a third of the infants exhibit drug-resistant seizures, and just over half experience a global developmental delay.
- Abnormal neurological examination at seizure onset is a strong predictor of cognitive outcome.
1 INTRODUCTION
The incidence of childhood epilepsy peaks during the first year of life and its entire clinical picture is best studied in a prospective setting. Epilepsy starting in infancy carries insecurity about the future both regarding seizure and cognitive outcome. The course of the disease varies largely from self-limited syndromes to devastating epileptic encephalopathies. Obtaining a syndrome diagnosis, recognizing specific etiology, and identifying clinical features of prognostic value serve the clinician trying to predict the outcome.
Etiology as the most important determinant of prognosis has been proven in recent prospective studies.1, 2 In the past, identified etiology, especially structural causes,3 has been associated with worse outcomes. As genetic causes are common in epilepsy with onset during the first year of life, the meaning of identified etiology also becomes more nuanced. Discovering a causative variant in certain genes, such as PRRT2, predicts a positive outcome. Furthermore, the identification of specific etiology may open doors for personalized treatment.4
Despite the great advances in neuroimaging and genetic methods, etiology often remains unknown. Genetic testing is not universally available, and results are often pending. Hence, there is a need for additional tools for outcome prediction.
We aimed to study the incidence, etiology, and prognosis of epilepsies in infancy in a prospective patient cohort with a careful clinical follow-up. Additionally, we investigated how accurately we could predict cognitive outcomes at the early stages and identify those at high risk for treatment resistance and neurocognitive comorbidity.
2 METHODS
2.1 Recruitment
From 1.2.2017 to 31.5.2019, we prospectively recruited all infants diagnosed with epilepsy and seizure onset below age 12 months into this study. The included individuals presented with recurrent unprovoked seizures and were born in and residents of the Hospital District of Helsinki and Uusimaa (population-based [incidence] group). We excluded those with only provoked seizures or febrile seizures. The study cohort also contained infants from surrounding health districts who fulfilled the inclusion criteria referred to us for treatment. One of the authors (HJ, pediatric neurologist) took care of clinical follow-up and examined all the patients until a minimum age of 24 months. Clinical evaluation took place within a week of diagnosis, 1 month later, and at age 3, 7, 12, 18 and 24 months.
The hospital district of Helsinki and Uusimaa has approximately 1.7 million inhabitants.5 The mean birth rate in the area from 2016 through 2019 was 16 373 per year and was slightly decreasing during this period (range 15 711–17 146 per year).6 From these figures, a denominator value of 38 203 was calculated as the estimated number of births over a period of 28 months equaling the length of the recruitment time. For the incidence figures, only the population-based group was considered.
2.2 Patient ascertainment
A pediatric neurologist is involved in the treatment of all infants with unprovoked seizures in our catchment area. All Infantile epileptic spasm syndrome (IESS) treatments are started at our center. Doctors were informed and repeatedly reminded about the study and asked to contact the investigator immediately if an eligible patient was referred to them. To ascertain the inclusion of all eligible patients from the Uusimaa Health district for the incidence figures, we checked all electroencephalography (EEG) referrals and reports of pediatric patients aged zero to 15 months from all the five EEG departments of the study catchment area during the recruitment period.
2.3 Definitions
The epilepsies and epilepsy syndromes were defined and classified according to the International League Against Epilepsy position papers7, 8 based on clinical data including seizure types, EEG or video EEG, etiological investigations, and course of illness. IESS included infants with hypsarrhythmia (HA), modified HA or multifocal spikes, and clinical spasms or typical ictal EEG recording showing very subtle clinical semiology previously described as subtle spasms.9 Self-limited epilepsies included both familial and non-familial cases. In the case of self-limited neonatal epilepsy with a first degree relative with similar phenotype (n = 1), etiology was considered genetic even without confirmative gene finding. The unknown etiology category included infants with normal metabolic studies, no pathogenic or likely pathogenic finding on genetic studies (described in detail below), and normal or unspecific neuroimaging. For example, infants with cerebral atrophy without malformation, subcortical white matter lesions without clear correspondence to known leukodystrophy and without genetic cause recognized were classified into unknown etiology. Drug resistant epilepsy (DRE) at 24 months was defined as ongoing seizures after two or more adequately trialed and tolerated antiseizure medications (ASMs) with a seizure-free period of <6 months. EEG was rated as normal or abnormal, the latter including both those with interictal epileptiform discharges (IED) and those with any background abnormality with or without IED. Seizure types were recorded and grouped into epileptic spasms at onset or during the first 6 months after onset, and to those with other seizure types. Occurrence of status epilepticus (SE) during the follow-up was noted. SE was defined as bilateral tonic–clonic seizure lasting over 5 min and focal seizure with impaired consciousness over 10 min.10
2.4 Genetic studies
At the time of the study at our unit, the choice of genetic studies in clinical practice varied depending on the phenotype and magnetic resonance imaging (MRI) findings. For developmental and epileptic encephalopathies (DEE) whole exome sequencing (WES) or commercial multigene epilepsy panel with over 450 genes was the first line investigation, followed by chromosomal microarray (CMA) if WES or the panel was negative. For other clinical phenotypes or where MRI suggested specific etiology, more limited panels were applied. Those with a suspicion of self-limited infantile epilepsy (SeLIE) or IESS patients with unknown etiology and quick response to treatment were not systematically tested. We offered on a research basis PRRT2-gene Sanger sequencing for those with a suspicion of SeLIE and WES for those with DEE or DRE and negative findings on multigene panel. Appendix S1 provides further information on the research-based WES analysis. For genetic diagnoses, we included data gathered until the preparation of the manuscript (June 2023).
2.5 Clinical follow-up, developmental and neurological examinations
One of the authors (HJ) assessed all participants during follow-up. At all visits, development was measured with the Griffiths Scales of Child Development, Third Edition (2015) and neurological examination was performed with the Hammersmith Infant Neurological Examination (HINE) or Hammersmith Neonatal Neurological examination (HNNE) for those <3 months (6 infants). We adopted the rating from Romeo and colleagues11 and graded the total HINE score, or HNNE optimality score,12 at the first visit as optimal or suboptimal. Cut-off score for optimal HNNE was at least 30.5 (out of maximum score 34) under age 3 months, and for optimal HINE from age 3 to 6 months at least 58, from 6 to 9 months at least 64, from 9 to 12 months at least 69, from 12 to 18 months at least 71 and from 18 months at least 74 (out of maximum total score 78). At the age of 24 months, neuropsychological evaluation was performed with Bayley Scales of Infant and Toddler Development, Third Edition (2005) (BSID-III). We classified the neurocognitive status at age 24 months or at last contact if the patient was deceased or had moved abroad earlier. Some of the final evaluations included in the outcome were postponed due to the COVID19 pandemic but not more than a maximum of 8 months. Neurodevelopment was categorized into two groups: 1. typical development or mild to moderate delay (BSID-III Cognitive and Language composite mean score over or equal to 70 or Griffiths over or equal to 70) and 2. global developmental delay (GDD) (BSID-III Cognitive and Language composite mean score below 70 or Griffiths below 70). For children without a language score, only the cognitive score was used. Griffiths general developmental quotient was accepted as the developmental measure for those who refused formal neuropsychological evaluation (n = 2) and for whom BSID-III was not applicable (n = 10) due to severe cognitive disability rendering formal testing impossible. For preterm infants, corrected age was applied to all neurocognitive norms, but we did not adjust the onset age of epilepsy to gestational age.
2.6 Statistics
We computed the Wilson method for 95% confidence interval (CI) of the incidence calculations. We summarized age at onset as mean and standard deviation (SD) and all other continuous variables as median and interquartile range (IQR). Comparison of continuous variables between groups was done by two-sided T-test and Kruskal-Wallis test. We performed univariate modeling for categorical variables associated with outcomes with Fisher's exact test. Differences between groups were analyzed as risk ratios (RR) with 95% CI. Significance was set at p < 0.05 (all two-sided), correction for multiple testing considered is reported in conjunction to the tests. Information from univariable associations was included in binary logistic regression modeling (Hosmer-Lemeshow goodness-of-fit) using the enter method. SPSS version 25 was used.
2.7 Ethics
This study was approved by the ethics committee at the Helsinki University Hospital and adhered to the tenets of the Declaration of Helsinki. We obtained informed written consent from the parents.
3 RESULTS
3.1 Participants
We recruited altogether 63 individuals (29 female) of whom 52 (24 female) belonged to the population-based group. Three participants were excluded and an additional two were lost to follow-up before age 24 months, due to death (n = 1) and move abroad (n = 1) (Figure 1).
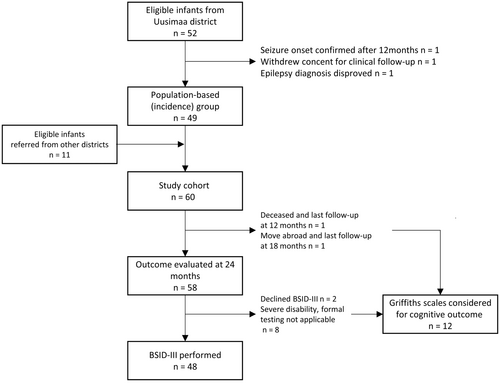
3.2 Incidence and mortality
The incidence of epilepsy during the first year of life in the original population-based cohort was 131 (95% CI 99–172)/100 000. From the EEG ascertainment, we identified six additional individuals. Including them increased the incidence to 147 (95% CI 113–190)/100 000. One child died (2% of the population-based group) before the age of 24 months.
3.3 Syndrome diagnoses and etiologies
For 48 (80%) infants, an epilepsy syndrome could be identified. Table 1 shows individual etiologies and outcomes within each syndrome group. IESS was the most common syndrome diagnosis. Self-limited (familial) infantile epilepsy was the second most common, and self-limited epilepsy syndromes were recognized in altogether 13 (22%) infants. Etiology was discovered in 35 (58%) participants. The structural and structural-genetic groups were combined in the statistical analyses. We identified a monogenic etiology in 16 individuals. A causative variant in PRRT2 was diagnosed in four and SCN1A in two individuals. Ten genes presented with a single pathogenic or likely pathogenic variant each. Details on genetic methods, specific variants, copy number variations and structural findings are listed in Table S1.
| Epilepsy syndrome | N (% of total) | N pop-based | Incidence per 100 000* | Etiology group N (N pop-based) | Specific etiologies (one each, unless N in parentheses) | DRE N (%) | GDD N (%) | Death at 24 months |
|---|---|---|---|---|---|---|---|---|
| Total | 60 (100) | 49 | 131 |
G 20 (16) S 10 (10) SG 5 (4) U 25 (19) |
22 (37) G 6 (15) S 5 (50) SG 5 (100) U 6 (24) |
31 (52) G 13 (65) S 8 (80) SG 4 (80) U 6 (24) |
1 | |
| Self-limited (familial) neonatal epilepsy | 2 (3) | 2 | 5 | G 2 | Familial (gene not found), KCNQ2 | 0 | 0 | |
| Self-limited (familial) infantile epilepsy | 10 (17) | 7 | 18 |
G 4 U 6 |
PRRT2 (4) |
G 0 U 0 |
G 0 U 0 |
|
| Myoclonic epilepsy of infancy | 1 (2) | 1 | U | 0 | 0 | |||
| Infantile epileptic spasms syndrome | 31 (52) | 25 | 65 |
G 9 S 9 SG 3 U 10** |
FCD, MCD (2), NF1 and FCD, perinatal asphyxia, perinatal stroke (3), PVL + IVH, TRAPPC4, trisomy 21 (5), TSC2, tumor, ZNHIT3 (PEHO-syndrome), 15q11 copy number gain (2), 17p13.3 microdeletion and MCD |
12 (39) G 1 (13) S 4 (44) SG 3 (100) U 3 (30)** |
22 (71) G 8 (100) S 7 (78) SG 2 (67) U 4 (40)** |
G 1 |
| Early DEE | 1 (2) | 1 | G | RNF13 | 1 (100) | 1 (100) | ||
| Dravet syndrome | 1 (2) | 1 | G | SCN1A | 1 (100) | 1 (100) | ||
| Other DEE | 1 (2) | 1 | U | 1 (100) | 1 (100) | |||
| Myoclonic-atonic epilepsy | 1 (2) | G | SCN1A | 1 (100) | 0 | |||
| Focal epilepsy, no syndrome identified | 11 (18) | 10 | 26 |
G 2 S 1 SG 2 U 6 |
ARX and MCD, ATP1A2, KMT2D, perinatal asphyxia, TSC1 |
G 1 (50) S 1 (100) SG 2 (100) U 2 (33) |
G 2 (100) S 1 (100) SG 2 (100) U 0 |
|
| Unclassified epilepsy | 1 (2) | 1 | U | 0 | 1 (100) |
- Note: *Incidence counted from population-based group for those syndromes with more than one individual, **3 individuals with suspected genetic etiology included in U (severe developmental delay and hypotonia prior to epilepsy [1], microcephalia, atrophy and subcortical white-matter lesions in MRI [1], atrophy in MRI and developmental delay prior to epilepsy [1]), their outcome: 2/3 DRE and 3/3 GDD.
- Abbreviations: DEE, Developmental and Epileptic Encephalopathy; DRE, ongoing seizures (seizure-free <6 months) after two or more trialed anti-seizure medicines; FCD, focal cortical dysplasia; G, genetic; GDD, global developmental delay, mean of Bayley scales III cognitive + language score < 70, if missing, Griffiths <70; MCD, malformation of cortical development; N pop-based, number of individuals in population-based cohort; PVL + IVH, periventricular leukomalacia and intraventricular hemorrhage connected with prematurity; S, structural; SG, structural-genetic; U, unknown.
3.4 Clinical presentation and etiological investigations
The cohort included eight preterm babies (gestational age <32 weeks in two, <34 weeks in three, <37 weeks in three). Seven participants had acute symptomatic seizures perinatally.
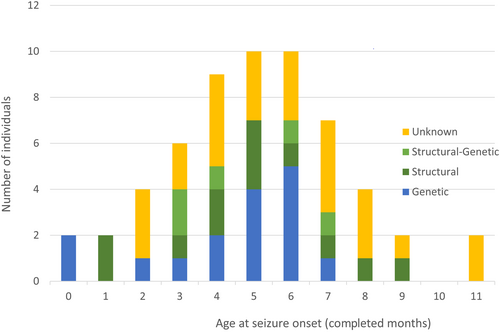
Seizures started at an average age of 5.3 (SD ± 2.5) months, with 75% of all cases having onset before the age of 7 months. The mean age at onset in infants with identified etiology was 4.6 (SD ± 2.3) months compared to 6.2 (SD ± 2.5) months in those with unknown etiology (p = 0.02, two-sided T-test). Onset age by etiology is depicted in Figure 2.
Etiology was established for 13 individuals before seizure onset by neuroimaging (n = 8) or karyotype studies (trisomy 21, n = 5). Genetic testing after recruitment to our study revealed the underlying cause of epilepsy for 15/47 (32%) and MRI for six (13%). All infants had brain MRIs. Epilepsy panels revealed the etiology in 7/21 (33%) and CMA in 3/24 (13%) investigations. In 23 WES (14 trio and nine single) five causative variants (22%) were found (all in trio). PRRT2-sequencing showed a pathogenic variant in 4/7 investigations.
EEG at the onset of epilepsy was performed for all 60 infants and was normal in 16, showing interictal epileptiform findings in 39 and abnormal background without epileptiform activity in five. An ictal recording during the first EEG was obtained for 31 (52%) participants. Initially, 30 infants presented with focal seizures, 27 with epileptic spasms or an EEG recording of a spasm series showing an ictal discharge compatible with subtle spasms, two with generalized seizures and one with an unclassified seizure type. Within the following months (median 3.4, IQR 2.8–4.2), five individuals with focal seizures developed epileptic spasms. They are included in the IESS group. SE presented as tonic–clonic in two and as focal SE with impaired consciousness in two patients.
3.5 Use of anti-seizure medications (ASM), other therapies and treatment response
Treatment for IESS commenced with first-line therapies (vigabatrin or hormonal treatment or both), infants with initial focal seizures developing into spasms were first treated with valproate (n = 2), phenobarbitone (n = 1) and vigabatrin (n = 2). Various ASMs were used for other seizure types and syndromes. Six children tried the ketogenic diet. One infant with desmoplastic infantile ganglioglioma had brain surgery at the age of 4 months.
Six months after the onset of epilepsy, 28 infants (47%) were treatment-resistant. At the end of the follow-up, 22 (37%) experienced DRE. Of the 38 infants in seizure remission, 22 were also off ASMs. By age 2 years, the number of ASMs tried per patient averaged 3 (median 2, range 1–7). Twenty individuals were on two or more ASMs.
3.6 Neurocognitive outcome
At the end of follow-up, 29 infants (48%) had age-appropriate development or mild to moderate delay whereas 31 (52%) had global delay. BSID-III cognitive index was available for 48 and language index for 42 participants, performed at a median age of 24.7 (IQR 24.3–26.2) months. The median BSID-III cognitive and language score was 79 (IQR 60–96). Those with structural and structural-genetic etiology had significantly lower scores compared to those with unknown etiology (median score for structural etiology group 61 [IQR 56–82] and unknown etiology 95 [IQR 80–106], Bonferroni corrected p = 0.02, Kruskal Wallis test). Comparisons between genetic versus unknown and structural versus genetic etiology groups were nonsignificant.
Nine participants (15%) had cerebral palsy (CP), six due to perinatal brain injury and three due to cerebral malformation.
The initial HINE score correlated with the BSID-III score (Spearman's correlation coefficient 0.66), but there was great individual variation without strong linear dependency. Figure S1 illustrates the initial HINE in relation to BSID-III with etiology indicated for each individual. Low initial HINE scores resulted in low cognitive scores whereas good initial scores did not ensure favorable development.
3.7 Outcome of infantile epileptic spasms syndrome
In the IESS group, seizure outcome did not differ from that of the whole cohort but, as expected, the cognitive outcome was worse. At the end of the follow-up, 71% of IESS patients had a global delay. Of 21 infants with IESS and identified etiology, 18 had a global delay, compared to 4/10 with unknown etiology (p = 0.02, Fisher's exact test). All nine individuals with confirmed genetic etiology had GDD despite good seizure control in the majority. All five infants with initial focal seizures exhibited both GDD and DRE. Of seven IESS infants without developmental concerns prior to spasms and with normal neuroimaging, only one had a global delay. For IESS the median treatment lag was 11 (IQR 3–22) days. The small size of the cohort does not allow evaluation of the effect of treatment lag on the outcome.
3.8 Predictors of outcome
The association of 12 binary variables representing patient history, electroclinical features, neurological examination, and etiology in regard to cognitive and seizure outcome is shown in Table 2. Abnormal first HINE and antecedent history of delayed development increased the risk of GDD. Additionally, epileptic spasms as seizure type, abnormal first EEG and identified etiology correlated with global delay, whereas there was a positive association with epilepsy in the first degree relative to favorable cognitive outcome. The strongest risk factor for poor seizure outcome was co-existing global delay. Structural etiology, abnormal first EEG, occurrence of SE, and seizure onset below age 4 months also associated with DRE.
| Outcome → | GDD | DRE | ||||
|---|---|---|---|---|---|---|
| Predictor ↓ | n (%) with GDD in predictor subgroup | p-Value | Relative risk (RR) for GDD RR (95% CI) | n (%) with DRE in predictor subgroup | p-Value | RR (95% CI) for DRE |
| Gender | ||||||
| Female n = 27 | 12 (44%) | ns | 10 (37%) | ns | ||
| Male n = 33 | 19 (58%) | 11 (33%) | ||||
| First HINE | ||||||
| Suboptimal n = 30 | 26 (87%) | <0.001 a | 5.2 (2.3–11.7) | 14 (47%) | ns | |
| Optimal n = 30 | 5 (17%) | 8 (27%) | ||||
| Epilepsy in a first-degree relative | ||||||
| Present n = 5 | 0 (0%) | 0.02b | Not applicablec | 0 (0%) | ns | |
| Absent n = 55 | 31 (56%) | 22 (40%) | ||||
| Development prior to epilepsy | ||||||
| Delay n = 24 | 21 (88%) | <0.001 a | 3.2 (1.8–5.4) | 12 (50%) | ns | |
| Typical n = 36 | 10 (28%) | 10 (28%) | ||||
| Epilepsy onset age | ||||||
| <4 months n = 17 | 11 (65%) | ns | 10 (59%) | 0.04b | 2.1 (1.1–3.9) | |
| ≥4 months n = 43 | 20 (47%) | 12 (28%) | ||||
| Seizure type | ||||||
| Spasms n = 32 | 23 (72%) | 0.002 a | 2.5 (1.3–4.7) | 13 (41%) | ns | |
| No spasms n = 28 | 8 (29%) | 9 (32%) | ||||
| Status epilepticus | ||||||
| Present n = 4 | 1 (25%) | ns | 4 (100%) | 0.02b | 3.1 (2.1–4.6) | |
| Absent n = 56 | 28 (50%) | 18 (32%) | ||||
| First EEG | ||||||
| Abnormal n = 44 | 29 (67%) | <0.001 a | 20 (46%) | 0.03b | ||
| Normal n = 16 | 2 (13%) | 5.3 (1.4–19.6) | 2 (13%) | 3.6 (1.0–13.8) | ||
| Etiology | ||||||
| Identified n = 35 | 25 (71%) | 0.001 a | 3.0 (1.4–6.2) | 16 (46%) | ns | |
| Unknown n = 25 | 6 (24%) | 6 (24%) | ||||
| Etiology | ||||||
| Structural n = 15 | 12 (80%) | 0.02b | 1.9 (1.2–2.9) | 10 (67%) | 0.01b | 2.5 (1.4–4.6) |
| Not structural n = 45 | 19 (42%) | 12 (27%) | ||||
| GDD n = 31 | 18 (58%) | <0.001 a | 4.2 (1.6–11.0) | |||
| No GDD n = 29 | 4 (14%) | |||||
| DRE n = 22 | 18 (82%) | <0.001 a | 4.2 (1.6–11.0) | |||
| No DRE n = 38 | 13 (34%) | |||||
- Note: Probabilities from group comparisons with Fisher's exact test (two-sided).
- Abbreviations: CI, confidence interval; DRE, ongoing seizures (seizure free <6 months) after two or more trialed antiseizure medications; GDD, global developmental delay, mean of Bayley scales III cognitive + language score < 70, if missing, Griffiths scales <70; HINE, Hammersmith Infant Neurological Examination; ns, not significant (p > 0.05).
- a Significant with Bonferroni corrected p < 0.0023, corrected for multiple testing ×22 (bold).
- b Significant with p < 0.05.
- c Cannot be counted as zero cases with GDD and 1st degree relative, RR for typical development 2.3 (1.7–3.1).
As we found a prominent association between DRE and global delay, we proceeded to examine the impact of the etiology behind this connection. In infants without identified etiology, DRE still showed a significant association with GDD (p = 0.02, Fischer's exact test, RR 6.3, 95% CI 1.5–26.4). Those with optimal initial HINE and unknown etiology demonstrated a particularly good outcome, namely, 17/18 had favorable development and only two experienced DRE. Even those with normal developmental history at seizure onset and unknown etiology showed similar trends. Of 20 such infants, only three developed GDD and three had DRE.
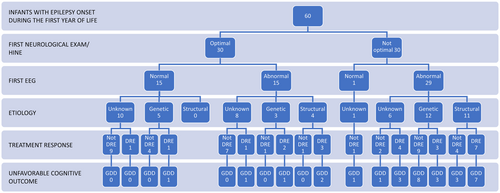
Figure 3 portrays the path from initial HINE and EEG which is the information available rapidly after seizure onset to outcome (DRE and GDD). Stratification by etiology is also displayed in the chart.
3.9 Multivariate modeling
From the univariate analysis, we included significant variables in binary logistic regression model. In the GDD model, etiology was coded as ‘identified’ versus ‘unknown’ and in the DRE model as ‘structural’ versus ‘not structural’. By sequentially testing different combinations, we searched for primary determinants of seizure and cognitive outcome (i.e., significant variables) but also for a model that most accurately classified the individuals with a balanced sensitivity and specificity.
Abnormal ‘first HINE’ and co-existing ‘DRE’ proved significant determinants (GDD1 model, Table 3) of GDD. With the variable ‘prior developmental delay’, also ‘identified etiology’ reached statistical significance (GDD2 model, Table 3). Multivariate models for seizure outcome yielded only moderate accuracy, area under curve and relatively low discrimination (DRE1 and DRE2 model, Table 3). Only co-existing GDD showed significant impact but including structural etiology resulted in a better performance of the model.
| Outcome/model | Predictor variables | Wald statis tic | p | Odds ratio (OR) | 95% CI OR | Performance of the model | |||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | AUC | Sensitivity | Specificity | ||||||
| GDD1 | DRE | 6.8 | 0.009 | 20.7 | 2.1–202.8 | 87% | 0.94 | 90% | 83% |
| Identified etiology | ns | ||||||||
| First HINE sub-optimal | 12.9 | <0.001 | 60.0 | 6.4–651.7 | |||||
| GDD2 | DRE | 7.1 | 0.008 | 8.8 | 1.8–43.2 | 85% | 0.91 | 84% | 83% |
| Identified etiology | 4.3 | 0.03 | 5.5 | 1.2–26.0 | |||||
| DD prior epi onset | 10.7 | 0.001 | 15.0 | 3.0–75.7 | |||||
| DRE1 | GDD | 4.6 | 0.03 | 4.4 | 1.1–16.9 | 77% | 0.83 | 64% | 84% |
| Structural etiology | ns | ||||||||
| Epi onset <4 months | ns | ||||||||
| EEG abnormal | ns | ||||||||
| DRE2 | Structural etiology | 4.3 | 0.04 | 4.3 | 1.1–17.2 | 72% | 0.77 | 68% | 74% |
| Epi onset <4 months | ns | ||||||||
| EEG abnormal | ns | ||||||||
- Note: Multivariate modeling performed with binary logistic regression, enter method, Hosmer-Lemeshow goodness-of-fit. Choice of variables was informed by univariate correlations (Table 2), omitting those with only few positive cases (SE and epilepsy in 1st degree relative) and nonsignificant variables which did not enhance model performance. First HINE and DD prior to epilepsy were not entered into same model due to collinearity (Spearman's correlation coefficient = 0.82). Onset age was additionally tested as a continuous variable but remained nonsignificant.
- Abbreviations: Accuracy, ratio of all correct predictions; AUC, area under curve; CI, confidence interval; DD, developmental delay; DRE, ongoing seizures (seizure-free <6 months) after two or more trialed anti-seizure medicines; Epi, epilepsy; GDD, global delay, mean of Bayley III cognitive + language score <70, if missing, Griffiths <70; HINE, Hammersmith infantile neurological examination; ns, not significant (p > 0.05); SE, status epilepticus.
4 DISCUSSION
We found an incidence of 131 (95% CI 99–172)/100 000 for epilepsy starting before age 12 months in our population-based prospective study, confirming the high incidence of epilepsy in the first year of life. Similarly, we report further data on some of the most common epilepsies in this age group: the incidence of self-limited infantile epilepsy 18 (95% CI 9–38)/100 000 and PRRT2-gene as the most common monogenic cause of SeLIE.
Our figures are in line with the recent large prospective studies for early-onset epilepsy reporting the first year of life incidence around 136–139/100 000,13, 14 higher than some of the earlier prospective and large retrospective cohorts with an incidence around 80–120/100 000.3, 15-18 Including also those recognized from the EEG referrals, we reached an incidence similar to that of a Norwegian registry study 144/100 000.19 The incidences of most syndromes also concur with recent prospective studies, like the few SeLIE-incidences reported around 14–20/100 000.3, 13 Conversely, our IESS incidence of 65 (95% CI 44–96)/100 000 exceeds the previous figures. In general, the incidence of IESS in the Nordic countries tends to be high14, 20, 21 but ours is even higher. This may be explained partly by chance due to our short recruitment time. The possibility of patient selection with more difficult cases referred to our study does not seem likely as we did not observe an overrepresentation of “benign” cases in those identified via EEG referrals.
The proportion of identified etiology (61% of the population-based group) and the distribution of etiologies (33% genetic, 18% structural in the population-based group) are comparable to earlier cohorts.2, 14 The outcome of infantile epilepsy is often unfavorable, shown by global delay in just over half and pharmacoresistance in more than one third of the individuals in the present study. Developmental outcome was similar to the recent Swedish and Scottish studies which both reported 49% with global developmental delay or intellectual disability at the end of follow-up.1, 13 Similarly, both previous research and our study agree on treatment resistance existing in around one third of cases.2, 22, 23 On the other hand, outcome was worse than in a previous study from our center.3 One obvious reason is the high number of IESS in our population.
Structural and structural-genetic etiology was associated with poor outcomes both regarding seizures and cognition. For genetic etiology, both the developmental and epilepsy outcomes varied depending on the specific gene and its syndromic presentation. Treatment resistance, in addition to etiology, contributed to an increased risk of unfavorable outcomes. Pharmaco-responsiveness was evident quite early as the majority (32/38, 84%) of those with good responses showed lasting remission by 6 months from the onset of seizures.
In multivariate analyses, the result of neurological examination with HINE or the history of developmental delay prior to seizure onset and the co-existing DRE were the strongest determinants of GDD outcome. Models performed worse in predicting seizure outcomes with only co-existing GDD increasing the odds of DRE significantly. Similar to some studies, earlier seizure onset age did not independently affect DRE outcome when etiology was controlled for.2, 3
The HINE and HNNE are standardized, widely used clinical neurological examinations evaluating cranial nerve function, posture, movements, tone, and reflexes.12, 24, 25 Both examinations reliably detect motor impairment with high predictive power,26 and newer reports also state age-specific cut-off scores for cognitive delay without CP.11, 27 In our study, rating the first HINE as optimal or suboptimal demonstrated a more accurate and objective prediction than just parental report of developmental history. Additionally, those with normal first HINE and drug-responsive seizures had a favorable outcome, irrespective of the identified etiology.
In clinical practice, MRI reveals clear structural etiology quickly after the onset of seizures, but the results of the genetic studies are often pending. Standardized neurological examination can be performed without delay and repeated as needed. It offers an additional tool for prediction.
Early identification of etiology allows targeted treatment for some patients,28, 29 a more accurate prediction of outcome and appropriate support for the families.30 However, as resources for genetic investigations vary globally, withholding early testing in infants with normal neurological examination and quick response to treatment may be cost-effective, unless there is a clinical suspicion of a syndrome for which specific treatments are available, such as Dravet syndrome. If etiology remains unsolved, seizures prove difficult to control or developmental concerns arise, repeated neuroimaging and further genetic studies should be performed.
We managed to collect a prospective cohort with quite a high ascertainment rate representative of the diversity of infant epilepsies. The major strength of our study is the thorough, hands-on, follow-up for all infants allowing high syndrome diagnosis rate and precise description of the clinical characteristics for all participants. Our study also has several limitations. First, the short recruitment period and relatively large recruitment area add uncertainty to our results, e.g., some incidence figures are affected by chance demonstrated by four patients with Down syndrome (16% of all IESS patients) in the population-based group. We detected IESS in four infants by follow-up of EEG prior to the recognition of clinical spasms, but early detection and start of ASM should not increase prevalence. Another major weakness is the short follow-up period, as both cognitive and seizure outcome at this early age are uncertain; mild ID may not yet be detected, and epilepsy relapses often occur after several years.31 Third, the cognitive (BSID-III) cut-off is quite coarse, the use of American norms might underestimate the problems 32 and higher risk cut-offs have been suggested.33 Fourth, studies have reported higher incidence of epilepsy in connection with social deprivation, which was not considered in our patient population.2, 34 Fifth, uniform genetic testing of all infants would have increased the reliability of our results and probably diminished the group of unknown etiology. Lastly, some of the factors earlier connected to increased likelihood of treatment resistance, e.g., history of SE,1 background slowing on initial EEG22 or longer treatment lag,35 could not be adequately analyzed in our cohort due to its limited size. Larger numbers would allow more etiology-specific categorization especially within genetic causes leading to greater accuracy in outcome prediction. Future research will hopefully reveal more precise predictors of refractoriness as poor seizure control independently contributed to adverse cognitive outcome.
5 CONCLUSIONS
Our study confirms the relatively high incidence of self-limited infantile epilepsy and supports the finding of previous study13 of PRRT2 as the most common gene causing epilepsy in infancy. Structural etiology is associated with a high probability for poor outcome while genetic etiologies are very heterogeneous regarding both developmental and seizure outcome. Notwithstanding the advances especially in genetic investigations, clinical examination remains an important tool in prognostication, here shown by the strong association of the HINE results with cognitive outcomes.
AUTHOR CONTRIBUTIONS
HJ, TL and EG designed the study. HJ gathered, prepared, and analyzed the data, and drafted the original manuscript. HJ, TL and EG reviewed and edited the original manuscript. TJ analyzed the PRRT2 sequencing data, MJ analyzed the research-based WES data and AEL coordinated the genetic research studies. SS performed research-based neurocognitive examinations. All authors reviewed, edited, and approved the final submitted version of the manuscript.
ACKNOWLEDGMENTS
Our sincere thanks go to the families who participated in the study. We wish to thank study nurse Hanna Hassinen for help with participant management, neuropsychologist Mirva Lauerma for help with the neurocognitive evaluations and Henri Lehtinen for help with their interpretation, laboratory technician Paula Hakala for laboratory assistance, BSc Katri Aksentjeff for assistance in the WES data analysis and PhD Carolina Courage for interpretation of the WES data.
FUNDING INFORMATION
The study was funded by Foundation for Pediatric Research, Pediatric Research center HUS Helsinki University Hospital and Folkhälsan Research Foundation.
CONFLICT OF INTEREST STATEMENT
HJ has received speaker‘s honaria for educational events from Jazz Pharmaceuticals, Nutricia and Eisai. The remaining authors report no conflict of interests. We confirm that we have read the Journal‘s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.