Phosphatidylserine enriched with polyunsaturated n-3 fatty acid supplementation for attention-deficit hyperactivity disorder in children and adolescents with epilepsy: A randomized placebo-controlled trial
Sylvain Rheims, Vania Herbillon, Anne de Saint-Martin, Stéphane Auvin, Sylvie N. Guyen The Tich, Julitta de Bellecize, Mathieu Milh, Alexis Arzimanoglou are member of the ERN EpiCARE.
Abstract
Background
Attention-deficit hyperactivity disorder (ADHD) is a frequent comorbidity in children with epilepsy, which management mostly relies on the usual treatments of ADHD, especially methylphenidate. Supplementation with polyunsaturated n-3 Fatty Acid (PUFA) has been proposed as an alternative therapeutic approach in ADHD without epilepsy but has never been evaluated in epilepsy-associated ADHD.
Methods
A multicenter double blind randomized placebo-controlled trial evaluating supplementation with PUFA, in eicosapentaenoic- and docosahexaenoic-acid form, conjugated to a phospholipid vector (PS-Omega3) in children aged >6 and <16-years old, and suffering from any type of epilepsy and ADHD (inattentive or combined type) according to DSM-V. After a 4-week baseline period, patients were allocated (1:1) either to placebo group or to PS-Omega 3 group and entered a 12 week-double-blind treatment period which was followed by a 12 week-open-label treatment period. The primary outcome was the reduction of the ADHD-rating scale IV attention-deficit subscore after 12 weeks of treatment.
Results
The study was stopped early because of lack of eligible participants and the expected sample size was not reached. Seventy-four patients were randomized, 44 in PS-Omega3, and 30 in the placebo group. The reduction after 12 weeks of treatment in the inattention subscore of the ADHD-IV scale was −1.57 in the PS-Omega3 group, and −2.90 in the placebo group (p = 0.33, α = 5%). Results were similar after 24 weeks of treatment and for all other ADHD-related secondary outcomes, with no difference between placebo and PS-Omega3.
Conclusion
Our study remaining underpowered, no formal conclusion about the effect of Ps-Omega3 could be drawn. However, our data strongly suggested that the PS-Omega 3 formulation used in the current study did not improve ADHD symptoms in children with epilepsy.
Plain Language Summary
Supplementation with polyunsaturated n-3 Fatty Acid (PUFA) has been proposed in ADHD but has never been evaluated in patients with both epilepsy and ADHD. To address this issue, we conducted a multicenter double blind randomized placebo-controlled trial evaluating supplementation with PUFA in children with epilepsy and ADHD. The study was stopped early because of lack of eligible participants, hampering formal conclusion. However, the evolution of the ADHD symptoms at 12 and 24 weeks did not differ between placebo and PUFA supplementation, strongly suggesting that PUFA did not improve ADHD symptoms in children with epilepsy.
Key points
- Supplementation with PUFA has been proposed in ADHD without epilepsy but has never been evaluated in epilepsy-associated ADHD.
- The study was stopped early because of lack of eligible participants.
- The evolution of the inattention subscore of the ADHD-IV scale at 12 and 24 weeks did not differ between placebo and Ps-Omega3.
- Though underpowered, our study data strongly suggested that PUFA did not improve ADHD symptoms in children with epilepsy.
1 INTRODUCTION
In children with epilepsy, the prevalence of attention-deficit hyperactivity disorder (ADHD) is 12% to 39% in newly diagnosed epileptic children1, 2 but can reach 70% in drug-resistant epilepsy.3 ADHD is an important contributor to cognitive difficulties commonly seen in children with epilepsy, leading to academic and neuropsychological difficulties more severe than those seen in children with epilepsy without ADHD.4, 5 ADHD symptoms are not associated with the underlying epilepsy syndrome, the severity of epilepsy, and/or the ongoing antiseizure medications (ASM).6 In addition, ADHD associated with childhood epilepsy differs from that observed in the general population, in that it is mainly represented by its attentional variant, suggesting that it represents a specific entity within the spectrum of the disease ADHD.2, 4
Currently, the therapeutic management of ADHD in children with epilepsy is based on the usual treatments of ADHD4, 5 though their efficacy in this specific population has not formally been evaluated in large double-blind controlled trials.4, 7 Because of its well-demonstrated efficacy in ADHD without epilepsy and of the lack of evidence suggesting a risk of drug-induced seizure aggravation,6 methylphenidate is thus the first-line drug in patients with ADHD and epilepsy.4 Data about the efficacy and the benefit/risk ratio of other molecules used in children with ADHD and epilepsy are more limited4, 8 with no evidence based on a randomized controlled trial.4, 7 It should however be noted, that up to 25% of patients with epilepsy and ADHD do not report clinically significant decrease of ADHD symptoms with methylphenidate,6 suggesting that development of alternative and/or complementary therapeutic strategies might be important.
An association between ADHD and the polymorphism of the PUFA desaturase two gene,9 and numerous experimental studies suggest the positive impact of PUFAs on cognitive functions.10, 11 The efficacy of PUFA supplementation as an alternative therapeutic approach in ADHD without epilepsy due to abnormal serum PUFA profiles in children with ADHD remains unclear.12 The results of therapeutic trials are discordant, with no or limited improvement of the overall ADHD scores.13 Their detailed analysis reveals, however, that most well-conducted trials may have a potential efficacy on symptoms of inattention that are mainly seen in ADHD associated with epilepsy.14, 15 We hypothesized that in this specific pathological setting, PUFAs may be more effective than in isolated ADHD. In addition, one of the main limiting factors of these trials was the use of PUFA vectors with low brain tropism resulting in a very low efficiency in terms of PUFAs brain accretion. Thus, with the exception of one trial which used a phospholipid vector with a positive result,15 clinical trials in ADHD systematically used triglyceride vectors.12 However, it has been shown that the bioavailability of PUFAs is twice as high for phospholipid vectors compared to triglycerides,16 justifying that the use of phospholipid PUFA vectors is preferred to assess the real potential benefit of these therapeutic approaches.
Our objective was to assess the impact on ADHD of supplementation with PUFA via a supply of n-3 PUFA in the form eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) conjugated to a phospholipid vector specifically in children with epilepsy.
2 METHODS
2.1 Standard protocol approvals, registrations, and patient consents
The AGPI trial (ClinicalTrials.gov identifier: NCT02348073) was conducted in 12 Pediatric epilepsy and neurology units in France, between February 2015 and November 2019. The study was funded by the French Ministry of Health (PHRC National 2010) and sponsored by Hospices Civils de Lyon. The active product and the placebo capsules were provided by ENZYMOTEC. ENZYMOTEC was not involved in the study conduct or data analyses. The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice ICH-E6 Guideline CPMP/ICH/135/95, European Good Clinical Practice Directive 2005/28/EC, and Clinical Trial Directive 2001/20/EC and fulfilled the CONSORT guidelines (Appendix S2). Trial protocol, amendments, and informed consent were reviewed and approved by national ethics committee (Comité de Protection des Personnes) on 2014, September 24th and competent authority. Before participation, parents/legal guardian of all patients have signed a written informed consent. Children and adolescents also signed the inform consent form when they were able to do so.
2.2 Participants
- Children of either gender (boy/girl) aged 6 up to 15 years and 11 months suffering from epilepsy, as defined by the ILAE,17 regardless of syndrome classification or of seizure frequency
- Diagnosis of ADHD inattention or mixed type according to the DSM-V criteria
- Patients on a stable dose of ASM for at least 1 month prior to inclusion and for whom no change is considered a priori for the 3 months following the inclusion.
Patients suffering from an exclusively hyperactive form of ADHD according to DSM-V criteria, presenting intellectual deficiency defined by a score <70 on the verbal comprehension and perceptual reasoning of Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV), or another psychiatric comorbidity than ADHD according to the DSM-V criteria or diabetes were excluded. Were also excluded, patients who were treated with psychoactive drugs for ADHD within the previous month, or with dietary supplementation or ketogenic diet within the last 3 months or with an allergy to sea products or soy.
2.3 Study design
This was a prospective, multicenter, double blind, randomized, placebo-controlled (1:1) clinical trial (NCT02348073).
After inclusion, patients entered a baseline period of 4 weeks duration in order to prospectively determine the seizure frequency. They were then randomly allocated either to placebo group or to PS-Omega 3 group and entered a 12 week-double-blind treatment period which was followed by a 12 week-open-label treatment period. Final assessment was performed 8 weeks after the end of the treatment.
2.3.1 Intervention
PS-Omega 3 and placebo were matched by encapsulation masking odor and taste. Adherence to treatment was assessed by accountability of remaining capsules at weak 12 and week 24.
Ps-Omega
The study product was a supplementation of n-3 PUFA, composed of Lipirinen TM which contains EPA and DHA (EPA/DHA ratio of 2.5:1) associated with a phospholipid vector (phosphatidylserine). This product is marketed under the name Vayarin®. Each capsule of the study product contains 8.5 mg of DHA, 21.5 mg of EPA, and 75 mg of phosphatidylserine.
Patients were asked to take two capsules, twice daily, 20 to 30 min before breakfast and dinner, for 24 weeks. Capsules contained the supplementation of n-3 polyunsaturated fatty acids (PS-Omega 3 group) or the placebo (control group); and is marketed under the brand name Vayarin®, provided by Enzymotec. The placebo was made of cellulose and a small amount of fish powder.
Placebo
The placebo was made of cellulose and a small amount of fish powder to maintain the double- blind in odor and taste. The supplementation in n-3 PUFA in the placebo group may be considered as negligible. Placebo was administered as indistinguishable capsules, identical to the active product. The active product and the placebo capsules will be provided by ENZYMOTEC.
2.3.2 Randomization
The department of biostatistics, independently of the coordination center, generated the sequence of randomization using permuted blocks size of 4 or 6 with SAS software (proc plan). Randomization with a 1:1 allocation was centralized and concealed via secured internet server stratified by site, age (<12 and >12 years) and epileptic syndromes grouped into five classes according to the classification of the International League Against Epilepsy. The central pharmacy of Lyon prepared and provided undiscernible containers of sequentially numbered drugs to each central pharmacy of investigator's sites. Investigators enrolled participants at each site and randomized eligible patients using the web platform to assign the interventions. The central pharmacy of each site delivered the treatment to patients.
2.4 Outcomes
ADHD was assessed using the ADHD-rating scale IV and the Test Of Variables of Attention (TOVA). We had previously performed a partial validation of the French version of the ADHD-RS IV regarding construct validity, internal consistency (i.e., scale reliability), item reliability, and responsiveness in a group of French children with ADHD and epilepsy.18 The ADHD-rating scale IV scores were collected at inclusion, randomization visit, after 12 weeks of treatment and after 24 weeks of treatment. The TOVA was performed at the randomization visit, after 12 weeks of treatment, and after 24 weeks of treatment.
Seizure counts were recorded in patient diaries which were completed by parents and retrieved at each study visit. Patients' quality of life was assessed using the EFIQUACEE questionnaire, which was the unique quality of life scale for children suffering from epilepsy validated in French language.19, 20 This scale was completed by the parents at each study visit. In addition, psychiatric comorbidities were monitored at each visit using the children's depression inventory and the revised children's manifest anxiety scale. Dietary habits were monitored at each study visit with a dietary survey completed by the patients' parents. Long-chain n-3 PUFA intakes were then estimated using a web-based tool.21
2.4.1 Efficacy endpoints
Because, the attention variant of ADHD is predominant in patients with epilepsy,2, 4 the primary outcome was the ADHD-rating scale IV attention-deficit subscore in subjects assigned to supplementation of PS-Omega 3 in comparison with the placebo group after 12 weeks of treatment. Although the discriminative value of the subscale was validated,22 including for the French version,18 the level of pertinent clinical response specific to this subscore has not been formally established. Given the specific discriminative value of each subscores, a similar threshold determined for the total score, and corresponding to a 25% reduction seems acceptable.23, 24 However, in absence of consensus in the literature and in order to limit the risk of overestimation of the clinical relevance in the reduction of the inattentive subscore, we chose to set a threshold of 30% as a clinically relevant reduction.
The secondary outcomes were the ADHD-rating scale IV total score, the TOVA total score and the EFIQUACEE quality of life score as well as the number of subjects with a reduction in the frequency of seizures ≥50%, in the PS-Omega 3 group compared with the placebo group at 12 and 24 weeks.
2.4.2 Safety assessment
PS-Omega 3 supplementation safety was assessed by interviews, telephone follow-ups, clinical examinations and collection of adverse events during the study. Data on harms were collected on the case report form adverse event sheet in a free text format. Investigators collected data on harms and ascertained the intensity at each visit, and the sponsor monitored safety data. No between group comparisons was planned on data on harms.
2.5 Statistics
2.5.1 Sample size
As there is no reference score in children with ADHD and epilepsy, the reference value for the sample size calculation was based on three clinical trials in ADHD among general population.23, 25, 26 According to the proposed hypothesis of a reduction in the ADHD-rating scale IV inattentive subscore of 3 points (15%) in the placebo group and six points (30%) in the active group, with a common standard deviation of eight points, 113 subjects are required in each group, that is, a total of 226 subjects, to reject the null hypothesis in 80% of cases, for a significance level of 5% (two-tailed). A proportion of 20% of the patients being expected to withdraw the study for adverse events or other reasons, 272 patients will be randomized. No interim analysis was planned.
2.5.2 Analysis
The analysis was conducted according to the intention to treat principle. Participant's characteristics were described using the following descriptive statistics: number, number (%) of missing data, mean, frequencies, standard deviation, median, first and third quartiles and minimum and maximum. The analysis of the primary endpoint concerned the changes in the score on all measurements taken between randomization and the 12-week visit using a mixed regression model of the score, taking into account the time of measurement, the study arm and its interaction with time. No subgroup analysis was planned.
All other analyses used R, version 3.0.1. All tests were two-tailed, and p < 0.05 was considered for statistical significance.
3 RESULTS
3.1 Patient allocation and characteristics
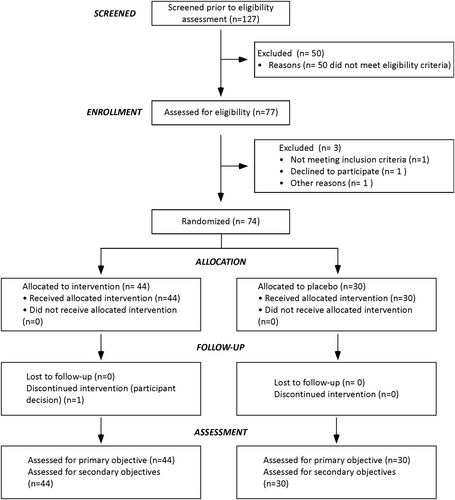
Seventy-four patients were randomized. Among them, 44 (35 boys and 9 girls) were allocated to the PS-Omega 3 group and 30 (22 boys and 8 girls) to the PCB group. The consort diagram of the study population is shown in Figure 1. The study lasts 4-year and 9 months with 4-year recruitment, from March 2015 to December 2017, and a 36-week follow-up period per patient. The study was stopped early on September 2018 because the pool of available participants was exhausted.
As shown in Table 1, baseline patients' demographics, their cognitive status or dietary habits in terms of long-chain n-3 PUFA intakes, as well as epilepsy and ADHD characteristics were similar in both groups. Only five patients (11%) in the PS-Omega 3 group and 2 (7%) in the PCB group fulfilled criteria for drug-resistant epilepsy. In contrast, 35 of patients (79%) in PS-Omega 3 and 19 (63%) in PCB did not report seizure over the 3 months preceding study inclusion (79% in PS-Omega 3 and 63% in PCB), including nine (21%) and six (20%) who were off of ASM. Regarding ADHD, 19 patients in the PS-Omega 3 group (43%) and 17 (57%) in the PCB group suffered ADHD inattention type according to the DSM-V criteria. Ten patients in the PS-Omega 3 group (23%) and 8 (27%) in the PCB group had previously been treated with methylphenidate.
| PS-omega 3 | PCB | |
|---|---|---|
| Total, n | 44 | 30 |
| Age, mean ± SD | 10.3 ± 2.7 | 9.6 ± 2.1 |
| Gender (boys), n (%) | 35 (79) | 22 (73) |
| Epilepsy | ||
| Syndrome, n (%) | ||
| Non idiopathic focal epilepsy | 10 (23) | 10 (33) |
| Idiopathic focal epilepsy | 10 (23) | 6 (20) |
| Childhood absence epilepsy | 10 (23) | 5 (17) |
| Other forms of genetically determined generalized epilepsies | 7 (16) | 3 (10) |
| Others | 7 (16) | 6 (20) |
| Age at epilepsy onset, mean ± SD | 4.5 ± 2.9 | 5.2 ± 2.8 |
| Patients without seizures in the 3 months prior to inclusion, n (%) | 35 (79) | 19 (63) |
| Patients fulfilling criteria of drug-resistant epilepsy, n (%) | 5 (11) | 2 (7) |
| Number of ongoing AED, n (%) | ||
| 0 | 9 (21) | 6 (20) |
| 1 | 26 (59) | 14 (47) |
| 2 | 7 (16) | 9 (30) |
| 3 | 2 (5) | 1 (3) |
| School performances, n (%) | ||
| History of grade repetition | 4 (13) | 15 (34) |
| Parental estimation of school performances | ||
| Very good/good | 10 (23) | 13 (43) |
| Intermediate | 25 (57) | 11 (37) |
| Insufficient/very insufficient | 9 (20) | 6 (20) |
| Cognitive functioning (WISC-IV) | ||
| Verbal comprehension, mean score ± SD | 101 ± 17.5 | 90 ± 28.5 |
| Perceptual reasoning, mean score ± SD | 93 ± 17.3 | 81.8 ± 25.7 |
| ADHD | ||
| Type, n (%) | ||
| ADHD-I | 19 (43) | 17 (57) |
| ADHD-C | 25 (57) | 13 (43) |
| Age at ADHD onset, mean ± SD | 5.6 ± 2.1 | 5.9 ± 2.3 |
| Past therapy with methylphenidate, n (%) | 10 (23) | 8 (27) |
| Dietary habits/Long-chain n-3 PUFA intakes (g/day) | ||
| Total PUFAs | 20.72 ± 18.49 | 14.95 ± 8.81 |
| Eicosapentaenoic acid | 0.119 ± 0.10 | 0.064 ± 0.07 |
| Docosahexaenoic acid | 0.194 ± 0.16 | 0.127 ± 0.15 |
3.2 Efficacy outcomes
As show in Table 2, mean ADHD-rating scale IV inattentive subscore, total score, TOVA score and EFIQUACEE score were similar at the randomization visit in the two groups. The mean adherence to treatment at the end of randomized period was 93.86% in the PCB and 86.00% in the PS-Omega 3 group.
| PS-OMEGA 3 | PCB | |||||
|---|---|---|---|---|---|---|
| Rando. | 12-weeks | 24 weeks | Rando. | 12-weeks | 24 weeks | |
| ADHD-rating scale IV; Inattentive subscore | ||||||
| Mean ± SD (range) | 16.5 ± 5.0 (5–27) | 14.9 ± 5.5 (3–27) | 13.5 ± 5.6 (0–24) | 17.7 ± 4.6 (9–27) | 14.8 ± 5.2 (6–27) | 12.8 ± 5.1 (1–25) |
| Evolution from randomization | – | −1.57 ± 5.00 (−11 to 7) | −3.10 ± 5.93 (−15 to 15) | – | −2.90 ± 5.27 (−17 to 9) | −4.87 ± 5.75 (−19 to 8) |
| ADHD-rating scale IV, total score | ||||||
| Mean ± SD (range) | 28.5 ± 10.3 (6–51) | 25.8 ± 10.3 (3–46) | 23.5 ± 10.9 (3–47) | 31.2 ± 9.42 (15–50) | 25.9 ± 10.1 (10–53) | 23.5 ± 10.9 (4–52) |
| Evolution from randomization | – | −2.64 ± 7.96 (−22 to 21) | −5.31 ± 10.15 (−24 to 34) | – | −5.33 ± 8.36 (−27 to 11) | −7.77 ± 10.63 (−10 to 16) |
| TOVA score | ||||||
| Mean ± SD (range) | −2.92 ± 8.15 (−50–3.92) | −1.94 ± 3.21 (−9.36 to 3.88) | −1.36 ± 2.97 (−9.44 to 4.57) | −1.85 ± 2.54 (−7.67 to 2.56) | −2.21 ± 3.52 (−8.41 to 3.80) | −2.57 ± 4.02 (−11.24 to 5.82) |
| Evolution from randomization | – | 1.25 ± 7.03 (−6.54 to 41.09) | 1.77 ± 7.89 (−4.51 to 46.41) | – | −0.25 ± 2.97 (−8.00 to 5.91) | −0.67 ± 3.56 (−8.78 to 8.00) |
| Quality of life score (EFIQUACEE) | ||||||
| Mean ± SD (range) | 15.9 ± 2.9 (8–22) | 16.6 ± 2.8 (11–23) | 16.6 ± 2.6 (9–21) | 16.0 ± 2.2 (13–20) | 15.9 ± 2.8 (10–21) | 16.4 ± 3.1 (7–21) |
| Evolution from randomization | – | 0.72 ± 1.87 (−3 to 5) | 0.72 ± 1.87 (−3 to 5) | – | −0.10 ± 2.07 (−4 to 4) | 0.43 ± 2.51 (−10 to 6) |
3.2.1 Primary endpoint
The reduction at 12 weeks in the ADHD-rating scale IV attention-deficit subscore was −1.57 in the PS-Omega3 group, and −2.90 in the PCB group. This difference (−1.33 in favor of placebo) was not statistically significant (p = 0.33, α = 5%; Table 2).
3.2.2 Secondary endpoints
The reduction at 12 weeks in the total score of the ADHD-rating scale IV was −2.64 in the PS-Omega 3 group and −5.33 in the PCB group (difference −2.69, p = 0.15, α = 5%). The results remained similar after 24 weeks of treatment, without significant difference between PS-Omega 3 and PCB, both for the attention-deficit subscore and for the total score. Similarly, the evolution in the TOVA score or of the EFIQUACEE quality of life score did not differed between the PS-Omega3 group and the PCB group neither after 12 weeks of treatment nor after was 1.25 in the PS-Omega3 group (Table 2).
Only 16 patients reported seizures during the prospective baseline, six in the PS-Omega 3 and 10 in the PCB group. Among them, a reduction in the seizure's frequency was reported after 24 weeks of treatment for five in the PS-Omega 3 group and in six the PCB group. Among the patients who had not reported seizure during the baseline period (n = 58), three patients allocated to PS-Omega 3 and two to the PCB had at least one seizure during the follow-up.
3.3 Safety assessment
No patient withdraw from the trial due adverse event. Only one mild intensity nausea that resolved without sequelae was considered related to the PS-Omega 3. All other adverse events were considered unrelated to treatments. The most reported adverse events were infections (N = 18 [23.4%], 10 PS-Omega3 vs. 8 PCB), gastrointestinal disorders (N = 12 [15.6%] 9 PS-Omega3 vs. 3 PCB), and headache (N = 11 [11.1%] 8 PS-Omega3 vs 3 PCB). Finally, six serious adverse events were reported all unrelated to the study treatment.
4 DISCUSSION
In this multicenter randomized controlled double-blind trial, we aimed to evaluate whether a supplementation in n-3 PUFA could improve ADHD in children and adolescents with epilepsy. Unfortunately, because of difficulties in recruiting eligible participants, we were unable to reach the expected sample size and our study remained underpowered. Accordingly, the effect of PUFA could not be estimated precisely enough and no formal conclusion could be drawn. However, our data strongly suggested that the PS-Omega 3 formulation used in the current study did not improve ADHD symptoms in children with epilepsy.
Overall, our data are in line with the results of double-blind studies which evaluated PUFA supplementation in ADHD without epilepsy. A recent meta-analysis of 31 studies conducted with children and Adolescents with ADHD thus reported an effect of PUFA neither on ADHD core symptoms rated by parents nor on quality of life.13 It has been discussed that part of these disappointing results might have been related to the low transmission of PUFA to the brain tissue. Because experimental data had shown that providing EPA and DHA directly as lysophosphatidylcholine (LysoPC) or its precursor effectively doubles the brain accretion during oral supplementation,16 we chose to use a PUFA n-3 formulation with EPA and DHA conjugated to a phospholipid vector, phosphatidylserine type. Once administered, the phosphatidylserine is decarboxylated and methylated into a phosphatidylcholine in the enterocytes; circulation in the plasma is then possible via phosphatidylcholine or lysophosphatidylcholine bound to albumin.27, 28 The dose of n3-PUFA previously used in ADHD ranged between 60 mg and 4 g per day. However, most of the trials used a daily dose of 650 and 800 mg in children aged 6 to 12 years, but without clear dose-effect relationship.13 Here, we used a low-dose regimen, with 86 mg/day EPA and 34 mg/day DHA. Considering both the optimization of the incorporation of n-3 PUFAs in brain that we were targeting by using a phospholipid vector and the positive results observed in ADHD without epilepsy using a similar formulation,15 the risk that the lack of efficacy of PS-Omega 3 that we observed in the present trial was only dose-related appeared low. However, we could not exclude this hypothesis.
Two studies evaluated the effect of PUFA supplementation on seizures,29, 30 with inconclusive results.7 Our study was not designed to evaluate the effect of PS-Omega 3 on seizure frequency. Accordingly, patients with well-controlled epilepsy were eligible and only 16 patients reported seizures during the prospective baseline period, hampering any conclusion on the relation between supplementation with PS-Omega 3 and evolution of epilepsy.
There are no other ongoing trials registered in clinicaltrial.gov or published with PUFA in ADHD children with epilepsy for a meta-analysis including our results. Because of the potential low efficacy of PUFA for ADHD, we believe that it is not worth exploring with more RCTs the potential benefit of PUFA for ADHD in children with epilepsy. There is growing evidence that psychosocial treatments for youth with ADHD and epilepsy might be promising, and the management of children with epilepsy and ADHD should consider psychosocial treatments before or along the use of stimulants medications.
AUTHOR CONTRIBUTION
S. Rheims was the scientific coordinator of the AGPI study. S. Rheims, V. Herbillon, S. Gaillard, L. Bezin, J. Bodennec, and B. Kassai designed the study. S. Rheims and S. Gaillard ensured the national coordination of the study. S. Rheims, S. Gaillard C. Mercier, and B. Kassai reviewed and analyzed the data. C. Mercier and P. Roy performed the statistical analyses. N. Villeuve, F. Villega, C. Cances, P. Castelnau, S. Napuri, A. de Saint-Martin, S. Auvin, S. N Guyen The Tich, P. Berquin, J. de Bellecize, M. Milh, and A. Arzimanoglou ensured the coordination of the study in their respective center, including the completion and the accuracy of the clinical data required for the study. All authors contributed to the preparation of the manuscript.
ACKNOWLEDGMENTS
We thank Pr Philippe Ryvlin for his help in the design of the study. We also thank Valerie Laudy and Clarisse Saunier for their help in the coordination of the study.
CONFLICT OF INTEREST STATEMENT
SR received speaker and/or consulting fees from UCB Pharma, Angelini Pharma, EISAI, Livanova, JAZZ Pharma, Medtronic, Zogenix. AA, in agreement with his Institution, has served as principal investigator or member of DMCs in clinical trials for Eisai, UCB, GW Pharma; received consulting fees from Jazz, Zogenix, Eisai, Takeda, Biocodex, Encoded Therapeutics; unrestricted research grants from, UCB, Caixa Foundation and Jazz and academic research grants from EJP-RD and the EU. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
FUNDING INFORMATION
The study was funded by the French Ministry of Health (PHRC national 2011-AGPI). The active product and the placebo capsules were provided by ENZYMOTEC. ENZYMOTEC was not involved in the study conduct or data analyses.
Open Research
DATA AVAILABILITY STATEMENT
All anonymized data will be shared by request from any qualified investigator.