Effectiveness and safety of mono- and add-on perampanel in pediatric patients with epilepsy: Experience from a single-center retrospective study
Abstract
Objective
To evaluate the effectiveness and safety of perampanel (PER) monotherapy (MT) or add-on therapy (AT) in Chinese children with epilepsy, as well as to evaluate the data from routine therapeutic drug monitoring (TDM) of PER for these pediatric patients.
Methods
This retrospective and observational study was carried out on children with epilepsy (n = 340) from 2020 to 2022 at the Children's Hospital of Nanjing Medical University. Outcome measures were the responder rate (50% or greater seizure reduction), long-term efficacy, and tolerability (number and types of adverse events) in MT and AT groups. Concentrations of plasma PER obtained from these patients, if available, were analyzed too.
Results
A total of 279 patients achieved at least 3 months of therapy, and 58.1% responded to PER therapy. 53 of the responders were seizure-free (32.7%). The retention rate dropped from 88.0% at 3 months to 40.6% at 12 months after treatment. Patients with MT achieved better seizure control than those with AT (P < 0.001). Intriguingly, PER exerted a very weak effect on patients who took more than 2 ASMs or were diagnosed with drug-resistant epilepsy. There were no significant differences in tolerability between the two groups. In addition, 179 patients were routinely monitored for PER, and the trough concentrations (C0) for these patients ranged from 30.0 to 992.0 ng/mL. However, no significant difference in C0 was observed between responders and nonresponders (333 ng/mL vs 325.5 ng/mL, P = 0.264).
Significance
This study provides effectiveness and safety data on Chinese children with epilepsy treated with PER either as MT or as AT. The efficacy of patients receiving MT was much better than cases administered with more than 2 ASMs or diagnosed with drug-resistant epilepsy. In addition, no association was found between the plasma PER concentration and efficacy or safety.
Plain Language Summary
The study reports the effects of perampanel on seizures and adverse effects in Chinese patients with epilepsy younger than 18 years. Seizures decreased in 58.1% of patients (responders); in a third of these responders, seizures stopped. After treatment was started, 88% of patients were still on perampanel at 3 months and 40.6% at 12 months. People who were treated with perampanel only were more likely to respond than those who received perampanel and other antiseizure treatments, although perampanel was tolerated equally well in these groups. Plasma perampanel concentration did not predict seizure response or adverse effects.
Key points
- PER monotherapy or add-on therapy seemed to be effective and safe in Chinese children with epilepsy.
- Forty-seven cases took PER monotherapy at least 3 months, and 89.4% of them achieved effective seizure control.
- The PER treatment led to a 37.5% responder rate among patients with drug-resistant epilepsy.
- The plasma PER levels were similar in responders and nonresponders.
1 INTRODUCTION
As the third-generation antiseizure medication (ASM), perampanel (PER) was specifically developed to act as a selective, noncompetitive antagonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ionotropic glutamate receptors in postsynaptic neurons.1-3 PER's unique mechanism of action has generated a lot of interest among researchers in related fields. Indeed, PER provides new opportunities and options for patients with drug-resistant epilepsy. Western countries have conducted many meaningful studies on this drug. For example, several double-blind, randomized, placebo-controlled trials revealed that participants receiving PER were more likely to achieve a 50% or greater reduction in seizure frequency (n = 2524; high-certainty evidence) with a responder rate of about 46.7%.4, 5 In addition, long-term clinical data from extension studies and post-marketing evaluations showed that the overall retention rate after 1-year follow-up was no more than 48%.6-8
However, this is not the case in China. There are only some small sample-size retrospective studies that examined the application of PER to Chinese pediatric patients. Indeed, there are about 10 million patients with epilepsy, two-thirds of whom are children.9, 10 In China, it has been over 2 years since PER was approved for treating focal onset seizures (with or without secondarily generalized seizures) in patients with epilepsy 4 years of age and older.11 The scarce research available on PER therapy only found that 44% of 38 cases with a 50% responder rate after 6 months of treatment and another study with 96 patients demonstrated the retention rate was 84.4% and 81.0% at 6 and 12 months.12, 13 Prospective or retrospective PER studies in a largescale of patients, especially for children are still lacking.
Furthermore, pharmacokinetic variability has been incompletely studied in clinical trials of newly introduced ASMs. In general, evaluating the effectiveness and potential side effects and confirming pharmacokinetic interactions requires the essential use of therapeutic drug monitoring (TDM). This is especially true for treatment with ASMs.14 In our hospital, we utilize TDM to closely monitor the potential correlation between plasma PER concentration and clinical outcomes, striving to find some clinical evidence for personalized medication. Of note, we optimized previously a specific plasma reference range (180–610 ng/mL) on PER therapy for children with epilepsy.15 As the sample size was relatively small, honestly, we intend to perform a re-evaluative study on this finding in a new and larger cohort of pediatric patients.
Herein, the objectives of this study were three-fold: (1) to assess the clinical effects of PER monotherapy (MT) or add-on therapy (AT) in children with epilepsy; (2) to identify possible factors influencing the long-term effectiveness of PER; and (3) to explore the potential correlation between the exposure and efficacy.
2 MATERIALS AND METHODS
2.1 Study design
This was a retrospective and observational study in pediatrics with epilepsy conducted under normal clinical practice conditions at the Children's Hospital of Nanjing Medical University. The study included medical records of all patients who received PER as MT or AT for epilepsy and were followed at the Neurology Department between March 2020 and December 2022.
As defined by the current International League Against Epilepsy (ILAE) criteria,16 only patients younger than 18 years of age, who had been diagnosed with epilepsy and took PER MT or AT for >1 month were included in the study (Figure 1). Study participants with inaccurate epilepsy diagnoses and/or unreliable clinical records were excluded.
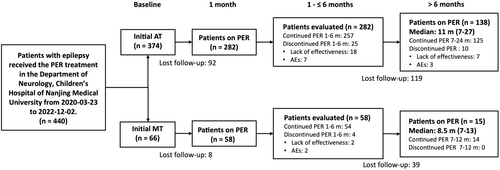
All patients in this study received once-daily oral administration of PER as MT or AT at bedtime. The dose of PER was titrated according to the individual patient response, aiming to balance efficacy and tolerability. Generally, the daily PER dose can be stratified according to the real body weight (BW). For children aged 4–12 years, weighing ≥30 kg, the starting dose was 2 mg/day. For children weighing ≥20 but <30 kg, the starting dose was 1 mg/day. For pediatric patients below the age of 4 years or weighing <20 kg, the starting dose was 0.5 mg/day. Thereafter, the dose if required was tailored to the maintenance level of 2–8 mg/day after 1–2 weeks based on the medical condition assessment.
The Ethics Committee of the Children's Hospital of Nanjing Medical University approved the study (Protocol number 202207141-1). Written consents were waivered due to the retrospective nature of the study.
2.2 Data collection
Information was collected about age, sex, BW, seizure type, and frequency, electroencephalography (EEG) findings, magnetic resonance imaging (MRI), duration of epilepsy, length of administration, number and types of ASMs in add-on, treatment response, reported adverse events (AEs), and reasons for therapy interruption. Ongoing non-pharmacological treatment options (e.g., ketogenic diet, vagus nerve stimulation) were recorded. Specific data on PER, including its initial and maximal dose and routine TDM, if possible, were also reviewed.
Seizure frequency was recorded for the past 3 months at baseline and at each follow-up period for 1, 3, 6, and 12 months, if available. AEs were recorded according to parents' and physicians' observations.
2.3 Clinical outcomes assessment
The assessment of the PER treatment efficacy consisted of two main components. The first part involved evaluating the effectiveness of PER treatment, which was assessed in individuals who had been under treatment for at least 3 months. Based on the percentage reduction in seizure frequency relative to baseline at the time of follow-up, patients were further categorized into subgroups as ‘responders’ (those with a ≥50% reduction in seizure frequency) and ‘nonresponders’ (those with a reduction in seizure frequency of <50%). The responder rate was determined by the proportion of responders, while the seizure-free rate was based on the proportion of patients who have remained seizure-free for at least a 6-month period.
The second part focused on the retention rate, i.e., long-term efficacy, defined as the probability of those patients retaining their efficacy after a 1-month seizure control, was assessed using Kaplan–Meier survival curves for patients who achieved seizure-free in the first month of therapy. Moreover, the factors influencing the retention rate were investigated by Cox regression analysis.
Tolerability was assessed by recording the type and number of AEs and the reason for drug discontinuation.
2.4 Routine therapeutic monitoring of PER
Pediatricians suggest implementing TDM of PER during the dose titration process. Thereafter, patients are recommended to undergo TDM of PER every 3 months, and the most recent concentration data for each patient were included in the statistical analysis. Blood samples were collected at a steady-state concentration of PER which was defined as the patient taking the same PER dosing schedule for at least 21 days.17, 18 The bioanalysis was performed on an LC–MS/MS system. In brief, the LC–MS/MS system consisted of a Triple Quad™ 4500MD mass spectrometer (AB Sciex Pte. Ltd, Singapore) interfaced via a Turbo V™ ion source with a Jasper™ liquid chromatography system (AB Sciex Pte. Ltd, Singapore), which comprised a binary pumps (Sciex Dx™), an online degasser (Sciex Dx™), an autosampler (Sciex Dx™), and a column oven (Sciex Dx™). A published method was used for monitoring PER, which was developed and validated in our laboratory.18
2.5 Statistical analysis
Demographic data and clinical characteristics were described as the frequency for categorical variables, means, and standard deviations for normally distributed continuous variables, and median with an interquartile range for nonnormally distributed continuous variables, respectively. Continuous variables were compared using the Mann–Whitney U test. Pearson's chi-square test and Fisher's exact test were used to compare categorical variables.
Retention rate on PER was estimated using Kaplan–Meier survival curves, and the log-rank test was performed to evaluate the effect of other variables on this parameter. Both univariate and additive multivariate Cox proportional hazard regression models were used to identify independent predictors for retention rate. After univariate analysis, factors with statistical differences were further included in the multivariate Cox regression analysis. A P value < 0.05 was considered statistically significant.
All analyses were performed with GraphPad Prism 9 (GraphPad Software, La Jolla, CA, United States) and R software (R Project for Statistical Computing, v4.2.2).
3 RESULTS
3.1 Patients' characteristics
A total of 340 patients were included in this study (Figure 1). Their ages ranged from 1–18 years (mean: 8.4 ± 3.8), and their median weight was 29.5 kg (range: 9–92 kg). 46.5% of the patients were female (n = 158). More than half of the patients had focal epilepsy (54.7%) and 28.2% (n = 96) were diagnosed with drug-resistant epilepsy. 282 patients received AT, and 39.4% of them were administered with enzyme-inducing ASMs (EIASMs, i.e., carbamazepine, oxcarbazepine, phenobarbital, and topiramate).
3.2 Efficacy outcomes
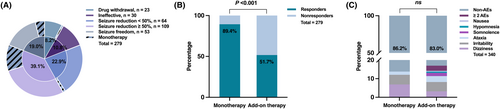
PER treatment was ineffective for 23% of patients and half quit. 22 patients simultaneously with ketogenic diet therapy or vagus nerve stimulation, 72.7% of them were diagnosed with pharmacoresistant epilepsy, and 86.4% of them were still ineffective in treatment.
In all, 279 patients received treatment with PER for at least 3 months. Most patients achieved seizure control, whereas 22.9% only achieved seizure reduction <50% (Figure 2A). The responder rate for these patients was 58.1% (n = 162), including 84 females (Table 1). In responders, the median treatment time was 6.3 months, which was similar to nonresponders (median: 6.5 months, P = 0.939). However, nonresponders have a longer disease history than responders (median: 33.1 vs 26.2 months, P = 0.003).
| Variables | Responders | Nonresponders | Total | P-value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| All patients | 162 (58.1) | 117 (41.9) | 279 | <0.001* |
| Age, y | ||||
| Mean (±SD) | 8.4 (3.6) | 8.3 (4.0) | 8.4 (3.8) | 0.824 |
| Sex | ||||
| Female | 84 (51.9) | 54 (46.2) | 138 (49.5) | 0.396 |
| Weight, kg | ||||
| Median (range) | 30.0 (20.0–40.0) | 28.0 (20.0–40.0) | 29.0 (20.0–40.0) | 0.506 |
| Duration of epilepsy, m | ||||
| Median (range) | 26.2 (12.9–49.3) | 33.1 (19.0–67.0) | 31.2 (14.9–58.6) | 0.003* |
| Type of epilepsy | ||||
| Focal | 90 (55.6) | 62 (53.0) | 152 (54.5) | 0.076 |
| Generalized | 28 (17.3) | 33 (28.2) | 61 (21.9) | |
| Combined | 26 (16.0) | 16 (13.7) | 42 (15.1) | |
| Unknown | 18 (11.1) | 6 (5.1) | 24 (8.6) | |
| Pharmacoresistant epilepsy | 30 (18.5) | 50 (42.7) | 80 (28.7) | <0.001* |
| Duration of PER treatment, m | ||||
| Median (range) | 6.3 (4.3–9.3) | 6.5 (4.2–10.5) | 6.4 (4.3–9.5) | 0.939 |
| Dose, mg | ||||
| Median (range) | 4.0 (3.0–6.0) | 4.0 (3.0–7.0) | 4.0 (3.0–6.0) | 0.445 |
| Plasma PER concentrations, ng/mL | ||||
| Median (range) | 333.0 (237.0–488.0) | 325.5 (220.5–435.0) | 332.0 (234.0–456.0) | 0.264 |
| ASMs in add-on | ||||
| 0 | 42 (25.9) | 5 (4.3) | 47 (16.8) | <0.001* |
| 1 | 69 (42.6) | 21 (17.9) | 90 (32.3) | |
| 2 | 32 (19.8) | 46 (39.3) | 78 (28.0) | |
| >2 | 19 (11.7) | 45 (38.5) | 64 (22.9) | |
| Concomitant ASMs | ||||
| VPA | 57 (35.2) | 68 (58.1) | 125 (44.8) | <0.001* |
| LEV | 40 (24.7) | 40 (34.2) | 80 (28.7) | 0.107 |
| LTG | 17 (10.5) | 45 (38.5) | 62 (22.2) | <0.001* |
| OXC | 33 (20.4) | 20 (17.1) | 53 (19.0) | 0.538 |
| CZP | 16 (9.9) | 28 (23.9) | 44 (15.8) | 0.002* |
| TPM | 13 (8.0) | 18 (15.4) | 31 (11.1) | 0.082 |
| LCM | 11 (6.8) | 19 (16.2) | 30 (10.8) | 0.018* |
| ZNS | 2 (1.2) | 10 (8.5) | 12 (4.3) | 0.005* |
| VGT | 1 (0.6) | 3 (2.6) | 4 (1.4) | 0.313 |
| CBZ | 0 | 2 (1.7) | 2 (0.7) | 0.175 |
| CLB | 1 (0.6) | 1 (0.9) | 2 (0.7) | >0.999 |
| PB | 1 (0.6) | 0 | 1 (0.4) | >0.999 |
- Abbreviations: ASMs, antiseizure medications; CBZ, carbamazepine; CLB, clobazam; CZP, clonazepam; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PB, phenobarbital; PER, perampanel; TPM, topiramate; VGT, vigabatrin; VPA, valproic acid; ZNS, zonisamide; *P <0.05.
Patients with focal onset epilepsy received better seizure control than those with generalized epilepsy (59.2% vs 45.9%), but there were no significant differences between responders and nonresponders (P = 0.093). Notably, 37.5% of patients with drug-resistant epilepsy responded to the treatment well.
In addition, patients received a median dose of 4 mg/day. Responders with a median PER trough concentration (C0) value of 333.0 ng/mL, similar to the value observed in nonresponders (325.5 ng/mL, P = 0.264). Intriguingly, 89.4% of patients who took PER MT experienced a ≥ 50% reduction in seizure frequency over 3 months. Among patients receiving more than 2 ASMs, 70.3% failed to the PER therapy (P < 0.001).
3.3 Safety outcomes
A total of 56 patients (16.5%) experienced mild or moderate adverse reactions. Irritability (n = 17), dizziness (n = 13), ataxia (n = 10), somnolence (n = 5), hypomnesia (n = 2), and nausea (n = 1) were the most common reported AEs. Moreover, eight patients reported more than one type of AEs.
In general, PER treatment was well tolerated, and only 3.5% of patients discontinued treatment due to AEs (Figure 1).
3.4 Clinical outcomes in PER monotherapy
The clinical outcomes in PER MT and AT are shown in Figure 2.
Forty-seven children took MT. Only two patients withdrew from the treatment, and none of them were due to AEs. Forty-two patients have shown positive results, and eleven have achieved complete seizure control for at least 6 months. In the MT group, 89.4% of patients were responsive to PER, whereas patients who administered AT were only 51.7% in the responders' group (P < 0.001).
86.2% of patients have good tolerance to MT. There was no significant difference in the percentage of reported AEs between the MT and AT groups (13.8% vs 17%, P = 0.607).
3.5 Retention rate and its influencing factors
After receiving 1 month of PER treatment, a reduction in seizure frequency was observed in 340 individuals. The relapse-free survival rate of these 340 patients was 88.0% at 3 months, 69.3% at 6 months, 52.1% at 9 months, and 40.6% at 12 months (Table 2, Figure S1). The median time to relapse for responders with focal, generalized, and combined epilepsy was 11, 7, and 12 months, respectively. No statistically significant differences in time to relapse were observed with regard to epilepsy and seizure type, and duration of epilepsy. However, there was a significant difference in the numbers of ASMs (MT vs 1 or 2 ASMs vs more than 2 ASMs, P < 0.001), and types of ASMs (EIASMs vs others, P = 0.045) used in the add-on (Table 2).
| % responders (95% CI) | ||||||
|---|---|---|---|---|---|---|
| 3 m (n = 279) | 6 m (n = 153) | 9 m (n = 77) | 12 m (n = 47) | 24 m (n = 3) | P-value | |
| All patients | 88.0 (84.0–91.0) | 69.3 (63.6–74.3) | 52.1 (45.2–58.5) | 40.6 (33.2–47.8) | 14.5 (6.8–24.9) | |
| By duration of epilepsy | ||||||
| <36 m | 89.0 (83.6–92.7) | 66.9 (58.7–73.9) | 44.0 (33.6–53.9) | 33.4 (22.5–44.6) | 7.0 (0.6–24.5) | 0.066 |
| ≥36 m | 86.5 (79.9–91.2) | 71.6 (63.0–78.4) | 58.6 (49.1–67.0) | 46.2 (36.1–55.7) | 19.7 (9.2–33.0) | |
| By epilepsy types | ||||||
| Focal | 89.0 (83.5–92.8) | 75.4 (68.0–81.3) | 57.2 (48.0–65.4) | 47.8 (32.7–52.4) | 11.1 (2.6–26.5) | 0.109 |
| Generalized | 87.4 (77.2–93.3) | 58.4 (44.8–69.8) | 42.5 (28.8–55.6) | 33.4 (20.0–47.4) | 10.2 (1.1–31.5) | |
| Combined | 89.7 (76.9–95.6) | 68.7 (51.8–80.8) | 52.2 (33.1–68.3) | 44.8 (24.1–63.5) | 17.9 (3.4–41.8) | |
| By concomitant ASMs number | ||||||
| 0 | 94.8 (84.8–98.3) | 84.2 (70.7–91.8) | – | – | – | <0.001* |
| 1–2 | 88.5 (83.1–92.2) | 71.6 (64.3–77.7) | 54.0 (45.3–61.9) | 47.2 (38.1–55.8) | 22.1 (10.8–35.9) | |
| >2 | 82.1 (71.6–89.0) | 56.5 (44.3–67.1) | 38.6 (26.6–50.5) | 18.3 (9.0–30.2) | 0 | |
| By type of concomitant ASMs | ||||||
| EIASMs | 88.5 (80.6–93.3) | 79.0 (69.3–86.0) | 72.3 (60.9–80.8) | 52.1 (37.4–64.9) | 29.0 (13.1–47.0) | 0.045* |
| Others | 90.6 (86.0–93.8) | 69.9 (62.7–76.0) | 49.9 (40.9–58.2) | 41.0 (31.6–50.1) | 13.1 (4.3–26.8) | |
- Abbreviations: ASMs, antiseizure medications; EIASMs, enzyme-inducing antiseizure medications.
- *P < 0.05.
For exposure to different ages, durations of epilepsy, and types of ASMs, the univariate analysis by Cox regression did not reveal any significant hazards associated with loss of responder status. However, it was affected by the type of generalized epilepsy (hazard ratio [HR] 1.45, P = 0.045), and concomitant use of zero or more than 2 ASMs ([HR] 0.42, P = 0.019; [HR] 2.43, P < 0.001) (Table 3, Figure S2). According to the multivariate analysis by Cox regression, concomitant use of more than 2 ASMs had a significant effect on non-responder status ([HR] 1.99, P = 0.001) (Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age, y | ||||
| ≤6 | 1.37 (0.97–1.95) | 0.076 | ||
| >6 | 0.73 (0.51–1.03) | |||
| Duration of epilepsy, m | ||||
| <36 | 1.36 (0.98–1.90) | 0.068 | ||
| ≥36 | 0.74 (0.53–1.02) | |||
| Type of epilepsy | ||||
| Focal | 0.70 (0.53–1.03) | 0.070 | ||
| Generalized | 1.45 (1.01–2.09) | 0.045* | 1.30 (0.90–1.87) | 0.162 |
| Combined | 1.02 (0.64–1.63) | 0.924 | ||
| Number of concomitant ASMs | ||||
| MT | 0.42 (0.21–0.87) | 0.019* | 0.76 (0.35–1.67) | 0.492 |
| ≥2 ASMs | 2.43 (1.67–3.52) | <0.001* | 1.99 (1.31–3.04) | 0.001* |
| Type of concomitant ASMs | ||||
| EIASMs | 1.07 (0.77–1.48) | 0.684 | ||
| Others | 0.93 (0.67–1.30) | |||
- Abbreviations: ASMs, antiseizure medications; EIASMs, enzyme-inducing antiseizure medications; MT, monotherapy; Hazard ratios for non-responder status.
- *P < 0.05.
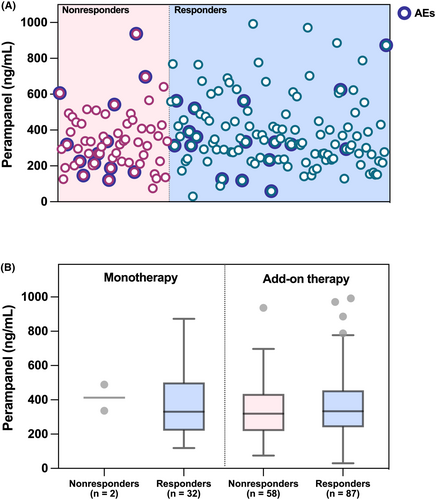
3.6 Plasma trough PER concentration
179 patients were routinely monitored for PER, and the interquartile range of the latest C0 values for these patients extends from 234.0 to 456.0 ng/mL (Table 1). The distribution of PER concentration was shown in Figure 3A. No significant difference was found in median plasma PER concentrations between patients with and without AEs (P = 0.649). That's also true between responders and nonresponders (P = 0.264). In fact, when patients were further subdivided into the MT and AT group, no differences in efficacy were observed either (Figure 3B).
4 DISCUSSION
Based on Study 311 (NCT02849626) and Study 342 (NCT03201900; FREEDOM),19, 20 PER was just available in China as an MT and an AT for partial-onset seizures (with or without secondarily generalized seizures) in patients with epilepsy 4 years of age and older in 2020. In this study, we focused on the efficacy and tolerability of PER in a cohort of 340 patients, encompassing 179 plasma PER concentrations. To the best of our knowledge, this was the first retrospective study about PER treatment for childhood epilepsy with a large sample size in China.
The responder rate to PER in observational open-label and retrospective studies was approximately 30% with an 8% seizure-free rate.2, 19 Notably, our series discovered a 58.1% responder rate, with 32.7% of patients achieving complete seizure remission. These findings were similar to the previous study conducted on a similar population of childhood epilepsy in China.21 PER was also found to be effective in different epilepsy subtypes (Table 1), as previously described in the literature.1, 22, 23 PER efficacy seemed higher in focal onset epilepsy subtypes, although no statistically significant results were found.
One situation that needs our attention was that about 30% of patients will be refractory to currently available drugs.24 If PER can play a role in the treatment of pharmacoresistant epilepsy, it will greatly improve the condition and quality of life of patients with pharmacoresistant epilepsy. In our study, indeed, it is noteworthy that 37.5% of patients with drug-resistant epilepsy responded to the treatment, similar to previous studies.13, 25 Thus, patients resistant to certain ASMs may benefit from the PER therapy.
Behavioral and psychiatric side effects were frequently reported with PER treatment.26 In fact, a total of 56 patients (16.5%) experienced mild or moderate AEs, particularly for irritability (17/56, 30.3%). Overall, PER could be well-tolerated in our cases, only with 3.5% of patients discontinuing the treatment due to AEs.
One of the core contents of this study was to evaluate the clinical outcomes of the PER therapy. In order to avoid the impact of underlying factors on the evaluation results, we further analyzed the efficacy and safety of PER MT. Impressively, our findings revealed great efficacy and safety of PER MT in children, which was in line with a previous report.27 Of note, 89.4% of patients have good responses to the MT, and 11 cases achieved seizure-free by the time the treatment reached at least 6 months. Furthermore, compared to the AT subgroup, patients with the MT counterpart have better seizure control (P < 0.001). Intriguingly, there was no significant difference in the percentage of reported AEs between them.
Next, we further evaluated the long-term efficacy of PER and its potential factors. The retention rates by 3 and 12 months were 88.0% and 40.6%, respectively, which was plain to see a downward trend. Indeed, PER improved seizures in most patients regardless of the type and duration of epilepsy. However, different numbers of administrated ASMs did influence the long-term efficacy. Cases that took more than 2 ASMs seemed to fail to completely control seizures.
This finding was worthy of further discussion. As mentioned above, patients with PER MT obtained better seizure control than those with AT, especially better than cases who were administered more than 2 ASMs. To be honest, this could not be simply interpreted. The progression of the disease itself or complex drug–drug interactions might be involved. Not surprisingly, the late AT or polytherapy was often performed in patients with refractory epilepsy in the current study. 62.5% of patients were refractory to previous ASMs and still in a state of non-responsive after receiving the PER AT therapy. We suspected that the seizure remission might be limited by the pharmaco-resistance in those patients. Obviously, this conundrum needs to be well explored in the future.
One more question needs to be concerned. In other words, does PER treatment require routine TDM in clinical settings? As we previously reported,15 180.0–610.0 ng/mL might be an alternative and more suitable target range for Chinese children with epilepsy. Herein, we expanded the sample size from the previous 80 patients to 179 cases in this study, but we did not obtain a different conclusion. However, like previous studies,28, 29 no association was found between the plasma PER concentration and efficacy or safety. Therefore, it is suggested that TDM should be implemented flexibly where conditions permit, so as to better provide personalized therapy for children with epilepsy.
This study also has several limitations, including the small sample size of the study population (particularly the MT group), the retrospective study design nature, and the lack of control and blinding groups. Secondly, the indication of PER's MT therapy was newly approved in China and the follow-up period for patients receiving MT was much shorter than for AT. Longer follow-up would provide stronger evidence of the long-term efficacy of MT. Nevertheless, the real-world clinical findings of this study in terms of efficacy and safety, especially monitoring plasma PER concentration in children, may prove highly beneficial to pediatric clinicians when selecting first or later add-on ASMs.
5 CONCLUSIONS
This study revealed the effectiveness and safety of PER in Chinese pediatric patients with epilepsy, whether in MT or AT. While the responder rate was significantly higher in the MT group than in the AT or late add-on group, the tolerability of PER in these groups did not differ significantly throughout the follow-up. In addition, no association was found between the plasma PER concentration and efficacy or safety.
AUTHOR CONTRIBUTIONS
Miss Li had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Chen and Lu. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Miss Li and Dr. Chen. Critical revision of the manuscript for: Dr. Chen. Administrative, technical, or material support: Chen and Lu. Supervision: Chen and Lu.
ACKNOWLEDGMENTS
This research was supported by the Specially Appointed Medical Expert Project of the Jiangsu Commission of Health (2019), Jiangsu Research Hospital Association for Precision Medication (JY202108, JY202238), and the CAAE Epilepsy Research Fund of China Association Against Epilepsy (CU-2022-024).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.