Electroclinical features and phenotypic differences in adenylosuccinate lyase deficiency: Long-term follow-up of seven patients from four families and appraisal of the literature
Gianni Cutillo and Silvia Masnada contributed equally to this work.
Abstract
Objective
Adenylosuccinate lyase (ADSL) deficiency is a rare inherited metabolic disorder with a wide phenotypic presentation, classically grouped into three types (neonatal, type I, and type II). We aim to better delineate the pathological spectrum, focusing on the electroclinical characteristics and phenotypic differences of patients with ADSL deficiency.
Patients and Methods
Seven patients, from four different families, underwent serial electroencephalogram (EEG), clinical assessment, and neuroimaging. We also performed a systematic review of the cases published in the literature, summarizing the available clinical, neurophysiological, and genetic data.
Results
We report seven previously unreported ADSL deficiency patients with long-term follow-up (10–34 years). From the literature review, we collected 81 previously reported cases. Of the included patient population, 58 % (51/88) were classified as having ADSL deficiency type I, 28% (25/88) as having type II, and 14% (12/88) as having neonatal. The most frequently reported pathogenic variants are p.R426H homozygous (19 patients), p.Y114H in compound heterozygosity (13 patients), and p.D430N homozygous (6 patients). In the majority (89.2%), disease onset was within the first year of life. Epilepsy is present in 81.8% of the patients, with polymorphic and often intractable seizures. EEG features seem to display common patterns and developmental trajectories: (i) poor general background organization with theta-delta activity; (ii) hypsarrhythmia with spasms, usually adrenocorticotropic hormone-responsive; (iii) generalized epileptic discharges with frontal or frontal temporal predominance; and (iv) epileptic discharge activation in sleep with an altered sleep structure. Imaging features present consistent findings of cerebral atrophy with frontal predominance, cerebellar atrophy, and white matter abnormalities among the three types.
Significance
ADSL deficiency presents variable phenotypic expression, whose severity could be partially attributed to residual activity of the mutant protein. Although a precise phenotype-genotype correlation was not yet feasible, we delineated a common pattern of clinical, neuroradiological, and neurophysiological features.
Key Points
- Adenylosuccinate lyase deficiency is a rare inherited metabolic disorder with a wide phenotypic presentation.
- Epilepsy is a core feature of the disease, with polymorphic and often drug-resistant seizures.
- Patients display common electroencephalogram features and evolutionary trajectories.
- The severity of the phenotype seems to correlate with mutant protein residual activity.
1 INTRODUCTION
- Type I or severe: most reported cases fall into this category. The onset is generally within the first months of life, and affected patients display the whole symptomatic spectrum of the disease, that is, severe developmental delay, epilepsy with intractable seizures, marked autistic features, axial hypotonia with limb hypertonia, dystonia, and ataxia.3
- Type II or mild: the onset of symptoms is generally within the first few years of life, but they can present later in life. Patients with type II ADSL deficiency generally present with mild to moderate developmental delay, autistic features, and variable pyramidal and extrapyramidal signs. They are less often characterized by seizures compared to the other groups.4
- Neonatal (N): The most severe form that presents at birth. Affected neonates are characterized by impaired intrauterine growth, microcephaly, fetal hypokinesia, a lack of heart rate variability, severe muscular hypotonia often leading to mechanical ventilation, resistant seizures, and early death.5
Epilepsy in ADSL deficiency is present in a consistent portion of the affected individuals.1 Seizures tend to display wide semiological variability and are often intractable, severely affecting the quality of life and the outcome of these patients.6 Here we describe seven previously unreported cases from four different families. We also performed a review of ADSL cases, attempting to establish possible genotype-phenotype correlations and highlighting common clinical, neuroradiological, and neurophysiological features with specific reference to their epileptic background.
2 METHODS
We collected patients with a diagnosis of ADSL deficiency in two Italian centers (“Vittore Buzzi Children Hospital” in Milan and “ASST Spedali Civili” in Brescia) and one center in France (University Hospitals of Lyon). Clinical data were retrieved retrospectively from clinical registries and prospectively through interviews with patients, their families, and/or caregivers. Electroencephalographic (EEG) and video-EEG recordings were available for all the patients at epilepsy onset and follow-up. EEGs were obtained by a digital acquisition system, placing scalp electrodes according to the international 10–20 system. Selected patients underwent additional neurophysiologic investigations (ie, visual, auditory, and somatosensory evoked potentials and electroretinograms). All patients underwent sequential brain magnetic resonance (MRI), and the images were reviewed and discussed with a trained pediatric neuroradiologist. Five patients (Pt. 3–Pt. 7) underwent urine SAICAr and S-Ado testing. All patients and their parents underwent genetic analysis using genomic DNA extracted from peripheral blood samples. Written informed consent was obtained from the parents or legal representatives of the involved patients.
2.1 Literature review
We performed a systematic review of the literature on ADSL deficiency cases. We searched different online repositories (PubMed, EMBASE, and Google Scholar) for all the relevant articles. The search terms included “ADSL”, “ADSL deficiency” and “Adenylosuccinate lyase deficiency”. All the articles were screened by title and abstract by two reviewers (GC and SM). We then hand-searched relevant articles cited by the selected papers if they are not present in the initial search. All searches were carried out on October 10, 2022. We only included peer-reviewed case reports or series published in peer-reviewed journals in English, specifically reporting the genetic background and clinical features of the patients. The radiological data retrieved were reviewed by an expert child neuroradiologist. Patients without thorough clinical and/or genetic data were excluded from the synthesis (refer to Figure S1 for a flowchart of the screening process).
2.2 Statistical analysis
Descriptive analysis was carried out using the median and interquartile range (IQR) for the quantitative variables and percentage values for the qualitative ones. The normality distribution for quantitative variables was assessed by the Shapiro-Wilk test. Pearson's chi-square test or Fisher's exact test was used to evaluate the association between categorical variables, while the non-parametric Kruskal–Wallis test was used to evaluate the differences between continuous variables and outcomes. After the Kruskal–Wallis test, for statistically significant results, the Dunn test was calculated for the comparison between the pairs of medians for the identification of significant differences. In addition, the survival analysis was performed by applying the Kaplan–Meier estimator and log-rank test for equality of survivor functions. Statistical significance was set at the level of ≤0.05. All analyses were performed using Stata software v17.1 (StataCorp, College Station, USA).
3 RESULTS
3.1 Case series
Clinical data of the following patients are summarized in Table 1.
| Family 1 | Family 2 | Family 3 | Family 4 | ||||
|---|---|---|---|---|---|---|---|
| Pt. 1 | Pt. 2 | Pt. 3 | Pt. 4 | Pt. 5 | Pt. 6 | Pt. 7 | |
| Sex, age, ethnicity | Male, dead at 19 years, Caucasian (Italian) | Male, 34 years, Caucasian (Italian) | Male, 22 years, Caucasian (Italian) | Female, 21 years, Caucasian (Italian) | Male, 26 years, Armenian | Female, 22 years, Armenian | Male, 23 years, Caucasian (Italian) |
| Mutation | c.1277G>A, p.R426H | c.1277G>A, p.R426H | c.1288G>A, p.D430N | c.1288G>A, p.D430N | c.1288G>A, p.D430N | c.1288G>A, p.D430N | p.Y114H; R296W |
| (homozygous) | (homozygous) | (homozygous) | (homozygous) | (homozygous) | (homozygous) | (Compound heterozygosis) | |
| Age disease onset | 4 months | 6 months | 7 months | 7 months | First year of age | First year of age | 18 months |
| Presenting condition/s | Developmental delay with regression | Developmental delay with regression | Developmental delay | Hypotonia, developmental delay | Developmental delay | Developmental delay | Developmental delay |
| Developmental delay | Severe | Severe | Moderate | Moderate | Severe | Severe | Moderate |
| Cognition/language | Profound ID; no language | Profound ID; no language | Severe ID; only very few words | Moderate-severe ID; words and short sentences | Severe ID; words and few short sentences | Moderate-Severe ID; words and short sentences | Moderate-severe ID; words and short sentences |
| Autistic features | Lack of interest in the environment and in social interactions, isolation, stereotypic hand movements | Lack of interest in the environment and in social interactions, isolation, stereotypic hand movements | Echolalia, stereotypic hand movements | Echolalia, hands, stereotypic movements | Lack of interest in the environment, stereotypic hand movement | No | Lack of interest in the environment and in social interactions; isolation; stereotypic hand movements |
| Additional neurological features | Axial hypotonia, limb hypertonia, pyramidal signs, extrapyramidal signs (distonic movements of the upper limbs), distal myoclonia, strabismus, nistagmus, macrocephaly, and tetraparesis | Axial hypotonia, limb hypertonia, pyramidal signs, extrapyramidal signs (facial grimaces), distal myoclonia, strabismus, nistagmus, and tetraparesis | Pyramidal signs (hypertonia and brisk reflexes), cerebellar signs (tremor, clumsiness, dysmetria, and ataxia), macrocephaly (>97°p) | Pyramidal signs and clumsiness | Nothing significative | Nothing significative | Limbs and axial hypotonia, microcephaly (<3°percentile), strabismus |
| Seizure onset | 34 months | 13 months | 7 years | 11 years | 11 years | 9 years | 10 years |
| Seizure type | Spasms, focal seizures, FBTCS, GTCS, status epilepticus | Spasm, focal seizures, FBTCS, GTCS, status epilepticus | GTCS: myoclonic seizures of the head and upper limbs | GTS, GTCS, and FBTCS | GTCS | GTCS | GTCS, focal seizures |
| Seizure outcome | Never achieved seizure freedom | Daily GTCS and focal seizure | Seizure-free | Seizure-free | Seizure-free | Seizure-free | Seizure-free |
| ASDs | BBX, PB, VPA, CZP, CBZ, NZP | ACTH, PB, VPA, NZP, GBP, TPM | VPA, LEV | VPA | CBZ, VPA | CBZ, LTG, VPA | VPA |
| EEG | Seizure onset: hypsarrhythmia | Seizure onset: hypsarrhythmia | Seizure onset: poor general organization | Seizure onset: poor general organization | Seizure onset: paroxysmal bilateral EDs | Seizure onset: diffuse spikes and Sp-W | Seizure onset: diffuse spikes and Sp-W predominantly in the fronto-temporal regions |
| Follow-up: bilateral high-voltage spikes and spikes and slow waves predominantly in fronto-temporal regions. | Follow-up: bilateral high-voltage spikes and spikes and slow waves predominantly in fronto-temporal regions. | Follow-up: diffuse theta activity, EDs predominantly in the frontal temporal regions, generalized EDs | Follow-up: poor general organization, low amplitude theta activity, EDs in centro-temporal-parietal regions, diffuse Sp-Ws | Follow-up: diffuse Sp-Ws predominantly in the left frontal regions | Follow-up: normal after VPA | Follow-up: Poor general organization and EDs in fronto-temporal regions | |
| Additional neurophysiological examination | Visual and auditory evoked potentials and an electroretinogram showed a progressive deterioration | Visual and auditory evoked potentials and an electroretinogram showed a progressive deterioration | Auditory, visual, and somatosensory evoked potentials are normal | Auditory, visual, and somatosensory evoked potentials are normal | NA | NA | NA |
| MRI | 8 months: normal | 14 months: cerebral atrophy | 15 months: normal | 11 years: cerebellar vermis atrophy | 17 years: diffuse cortical-subcortical atrophy, predominantly in the hippocampal regions. White matter hyperintensities with an anterior predominance | 13 years: diffuse cortical-subcortical atrophy. | 10 years: mild ventriculomegaly and periventricular white matter abnormalities |
| 10 years: progressive cerebral and cerebellar atrophy (frontal and parietal regions) and white matter hyperintensities (periventricular and posterior regions) | 10 years and 19 years: progressive cerebral and cerebellar atrophy (frontal and parietal regions) and white matter hyperintensities (periventricular and posterior regions) | 16 years: cerebellar vermis and cerebral atrophy, thin brainstem and corpus callosum, ventriculomegaly | 17 years: progression of cerebellar vermis atrophy, cerebral atrophy, ventriculomegaly | ||||
| Additional features | Scoliosis, Naso-Gastric tube feeding, hypogammaglobulinemia | Scoliosis, percutaneous gastrostomy, hypogammaglobulinemia | Scoliosis, multiple hypochromic skin lesions and “cafè au lait” macules, recurrent diarrhea, and recurrent abdominal pain | Multiple hypochromic skin lesions and “cafè au lait” macules, recurrent diarrhea, and recurrent abdominal pain | NA | NA | NA |
- Note: In the “ASDs” row the drug in bold is the one to which the patient responded better.
- Abbreviations: ACTH, adrenocorticotropic hormone; ASDs, anti-seizure drugs; BBX, barbexaclon; CZP, carbamazepine; EEG, electroencephalogram; ED, epileptic discharge; FBTCS, focal to bilateral tonic-clonic seizure; GBP, gabapentin; GTCS, generalized tonic-clonic seizure; GTS, generalized tonic seizure; ID, intellectual disability; LEV, levetirtacetam; LTG, lamotrigine; MRI, magnetic resonance imaging; mo, months; NZP, nitrazepam; PB, phenobarbital; Sp-W, spike-and-wave; TPM, topiramate; VPA; valproic acid; y, years.
3.2 Family 1 (p.R426H, homozygous)
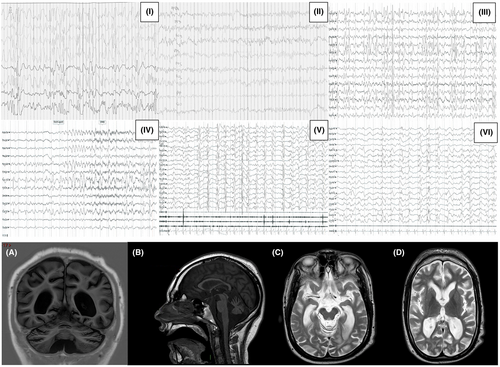
Pt. 1 and Pt. 2 were born at term to non-consanguineous Italian parents. Their prenatal and perinatal histories were unremarkable. At 4 and 6 months after birth, respectively, Pt. 1 and Pt. 2 presented with psychomotor regression of acquired motor and social skills, concomitant with the onset of epilepsy. Additionally, Pt. 1 exhibited strabismus and nystagmus. As they grew, both patients displayed profound intellectual disabilities and autistic features. Neurological examinations revealed spastic-dystonic tetraparesis with axial hypotonia and four-limb hypertonia. Extrapyramidal signs, such as upper limb dystonic movements with occasional non-epileptic myoclonus, minimal head control, and macrocephaly (only observed in Pt. 1), were also noted. At onset, electroencephalographic evaluations showed a hypsarrhythmic pattern with epileptic spasms in both patients, leading to treatment with adrenocorticotropic hormone (ACTH), which achieved a few months of seizure control. In subsequent follow-up EEGs up to adulthood, marked similarities between the two cases were observed, particularly the development of high-voltage spikes and spike-and-slow waves predominantly in bilateral frontal and temporal regions. Epileptic discharges (EDs) activation during sleep, sometimes organized in bursts, were observed, leading to a progressive loss of sleep structure. Over time, focal seizures, focal to bilateral tonic-clonic seizures, generalized tonic-clonic seizures (GTCS), and several episodes of refractory status epilepticus were reported in both. Visual and auditory evoked potentials and electroretinogram showed a progressive deterioration over the years in the two brothers. Initial MRI scans for Pt. 1 and Pt. 2 (at 8 and 10 months of age, respectively) were normal. However, follow-up MRI scans (at 10 years for Pt. 1, and 10 and 19 years for Pt. 2) revealed similar features: progressive cerebral and cerebellar atrophy, more pronounced in frontal and parietal regions, as well as ventriculomegaly, thin corpus callosum, brainstem abnormalities, and periventricular hyperintense T2 white matter signals. Genetic analysis through whole-exome sequencing identified a homozygous pathogenic variant c.1277G>A (p.R426H) in the ADSL gene, inherited from healthy parents who are heterozygous carriers. Pt.1 died at 19 years of age of complications following a hospitalization for a respiratory tract infection. Pt. 2 is currently 34 years old and bedridden with daily generalized and focal seizures (refer to Figure 1 for a summary of EEG and MRI findings).
3.3 Family 2 (p.D430N, homozygous)
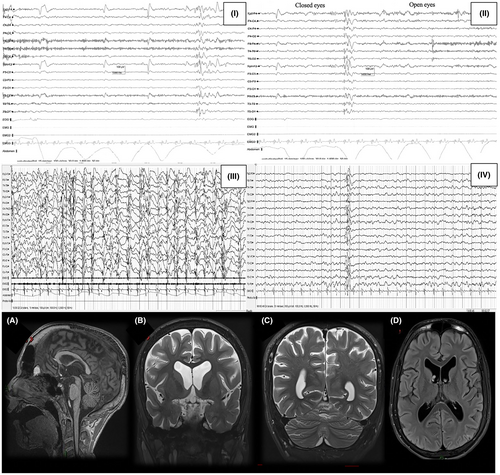
Pt. 3 and Pt. 4 are Caucasian siblings, born to non-consanguineous Italian parents. They were born at term with unremarkable prenatal and perinatal histories. However, at around 7 months of age, both patients presented with developmental delay, which progressed to severe intellectual disability accompanied by prominent autistic features. It is worth noting that Pt. 4, the female patient, displayed a relatively milder phenotype compared to her brother, retaining better social and communicative skills. Upon neurological examinations, both patients exhibited brisk reflexes and limb hypertonia. Additionally, Pt. 3 displayed cerebellar signs such as tremor, dysmetria, and ataxia, as well as macrocephaly (>97 percentile). Both patients presented with multiple hypochromic skin lesions and “cafè au lait” macules, recurrent diarrhea, and abdominal pain. Seizures manifested in Pt. 3 and Pt. 4 at the ages of 7 and 11 years, respectively. EEG evaluations showed a loss of antero-posterior gradient with spike-and-wave discharges predominantly localized over the frontal regions in both patients. In Pt. 4, diffuse epileptic discharges were also recorded. At the age of 12, Pt. 3 exhibited phases of continuous spike-and-wave activity during sleep, which progressively evolved to dedifferentiation of awake and asleep states. Treatment involved the use of valproic acid, with levetiracetam added for Pt. 3, and both achieved seizure freedom. However, no significant change in the EEG was observed. Brain MRI of the patients revealed similar features, namely, cerebral atrophy, mild periventricular leukoencephalopathy, and cerebellar atrophy (refer to Figure 2 for a summary of EEG and MRI findings). Whole-exome sequencing analysis revealed that both patients carried a homozygous variant c.1288G>A (p.D430N) in the ADSL gene, which they inherited from their healthy parents. Interestingly, only Pt. 4 displayed elevated urinary SAICAr and S-Ado levels. As of the last examinations, both patients remained seizure-free under the administration of anti-seizure medications (refer to Figure 2 for a summary of EEG and MRI findings).
3.4 Family 3 (p.D430N, homozygous)
Pt.5 and Pt.6 are French of Armenian descent. They presented with a psychomotor delay around the first year of life, more severe in Pt.5. Similar to previous cases, Pt. 6, the female patient, displayed a relatively milder phenotype compared to her brother. Pt. 6 did not exhibit overt autistic features, while Pt. 5 showed mild autistic traits. Neurological examinations were unremarkable except for the intellectual disability in both patients. Both developed epilepsy, respectively, at 11 and 9 years of age. The EEG showed diffused EDs more prominent in the frontal regions. Both patients achieved seizure freedom with valproate, associated with lamotrigine in Pt.6. Pt.6's EEG normalized after ASDs treatment. MRI showed generalized atrophy in both patients, with white matter periventricular T2 hyperintensities in Pt. 5. Only Pt. 5 showed elevated urinary SAICAr and S-Ado. Whole genome analysis revealed the mutation c.1288G>A (p.D430N) in the ADSL gene, inherited from healthy parents. Currently, both patients are still seizure-free under anti-seizure drugs.
3.5 Family 4 (p.Y114H, p.R296W; compound heterozygosity)
Pt. 7, born to non-consanguineous Italian parents, presented a relatively milder course of the disease. At around 18 months of age, the patient showed moderate psychomotor delay. Later, autistic behavior with motor stereotypies and limited social interaction became evident. Seizures developed at approximately 10 years of age, with both generalized and focal seizures observed. The EEG displayed a mild alteration of the general organization with frontal temporal spikes. The patient achieved seizure freedom with valproate monotherapy.
As of the last neurological examination, the patient displayed limb and axial hypotonia, along with strabismus. Brain MRI at the age of 10 showed mild periventricular leukoencephalopathy and ventriculomegaly, with no overt cerebral or cerebellar atrophy.
Elevated urinary levels of SAICAr and S-Ado were observed in the patient. Whole-exome analysis confirmed the diagnosis, revealing the presence of the variants p.Y114H and p.R296W of the ADSL gene. These variants were inherited from healthy parents. The patient is currently 23 years old and still seizure-free with valproate monotherapy.
3.6 Review of the literature
Clinical characteristics and demographics are summarized in Tables 2 and 3, with 88 individuals in the patient population, 81 retrieved from 29 articles in the literature, and 7 newly described patients (Figure S1). Among the study population, 47.7% (42/88) were female. Globally, 58% (51/88) had ADSL deficiency type I, 28.4% (25/88) had type II, and 13.6% (12/88) had neonatal (type N) presentation. The age of disease onset varied: from birth in neonatal cases to 4 years: the majority (89.2%) within the first year of life, 29.5% within the first week, 15.9% between day 7 and day 30, 44.3% after the first month but within a year, and only 10.2% after the first year. Significant differences in median onset age were found between the groups (P < 0.0001). Specifically, type I versus type II (P < 0.0001), type I versus type N (P = 0.002), and type II versus type N (P < 0.0001). Epilepsy onset was earlier in type I and N patients compared to type II (P < 0.0001). The most common presenting symptom across disease types was developmental delay, observed in 47.7% (42/88) of patients, with varying severity across the disease spectrum, followed by seizures in 28.4% (25/88). Cardio-respiratory deficits were seen in 10 patients, mostly in neonatal forms. Autistic features were reported in 56.9% (51/88) of patients, mainly in type I and type II, but sporadically in type N due to disease severity and early death. Hypotonia was present in 53 patients, more frequently in neonatal and type I groups (83% of neonatal form and 70.6% of type I ADSL deficiency, compared to 28% of milder variants). Pyramidal signs (spasticity and hyperreflexia) were in 33% (29/88) and extrapyramidal signs (dystonia and ataxia) in 12.5% (11/88), without specific group prevalence. Strabismus and ocular problems were in 26.1% (23/88) and microcephaly in 22.7% (20/88) of patients. Imaging data showed cerebral atrophy in 47.7% (42/88) of patients, most in type I (64%), and cerebellar atrophy, white matter abnormalities, and ventriculomegaly in significant proportions without specific group prevalence. Cerebral hemorrhages and gyrification deficits were mostly in the neonatal group. Data on EEG patterns and/or therapeutic strategies were collected and summarized in Table S1. From 16 articles specifically reporting on EEG features or therapeutic strategies, there were 26 patients. EEG features and localizations of the EDs varied considerably among different patients. EEG background was altered in all patients; hypsarrhythmia or a burst suppression pattern were reported in, respectively, four and six patients. Localization of the EDs varied and was reported as diffuse in five patients and focal in seven patients, mostly in the fronto-temporal and more rarely in the occipital-parietal regions. There were GTCS (17/26), followed by focal seizures (11/26), myoclonic seizures (11/26), spasms (5/26), and atypical absences (3/26). Valproic acid and levetiracetam were the most commonly reported ASDs used in these patients. Complete seizure control was reported in 5/26 patients.
| Gender, n (%) | |
| Female | 42 (47.7%) |
| Male | 46 (52.3%) |
| Type, n (%) | |
| I | 51 (58.0%) |
| II | 25 (28.4%) |
| N | 12 (13.6%) |
| Age at presentation, n (%) | |
| <7 days | 26 (29.5%) |
| 8–30 days | 14 (15.9%) |
| 31–359 | 39 (44.3%) |
| ≥360 | 9 (10.2%) |
| Presenting symptom, n (%) | |
| Seizure | 25 (28.4%) |
| Developmental delay | 42 (47.7%) |
| Hypotonia | 14 (15.9%) |
| Cardiac and/or ventilatory disfunction | 10 (11.4%) |
| Epilepsy, n (%) | 72 (81.8%) |
| Seizure type, n (%) | |
| Generalized | 33 (37.5%) |
| Focal | 32 (36.4%) |
| Spasm | 9 (10.2%) |
| Status | 7 (8.0%) |
| Developmental delay, n (%) | 82 (100%) |
| Degree, n (%) | |
| Mild | 17 (25.0%) |
| Moderate | 12 (17.6%) |
| Severe | 39 (57.4%) |
| Autistic features, n (%) | 51 (57.9%) |
| Hypotonia, n (%) | 53 (60.2%) |
| Pyramidal signs, n (%) | 29 (33.0%) |
| Extrapyramidal signs, n (%) | 11 (12.5%) |
| Microcephaly, n (%) | 20 (22.7%) |
| Respiratory system involvement, n (%) | 17 (19.3%) |
| Cardiac disfunction, n (%) | 6 (6.8%) |
| Strabismus/eye problems, n (%) | 23 (26.1%) |
| Dysmorphism, n (%) | 8 (9.1%) |
| Imaging features, n (%) | |
| Cerebral atrophy | 42 (47.7%) |
| Cerebellar atrophy | 15 (17.0%) |
| White matter abnormalities | 36 (40.9%) |
| Ventriculomegaly/enlarged sulci | 21 (23.9%) |
| Cerebral hemorrhage | 5 (5.7%) |
| Gyrification deficits | 4 (4.5%) |
| Type (I, II, N) | ||||
|---|---|---|---|---|
| I (n = 51) | II (n = 25) | N (n = 12) | P-value | |
| Age at presentation (days), median (IQR) | 30.0 (7.0–150.0) | 150.0 (120.0–360.0) | 0.0 (0.0–2.5) | <0.0001 |
| Epilepsy onset (days), median (IQR) | 37.5 (13.5–375.0) | 1800.0 (1440.0–3240.0) | 0.0 (0.0–7.0) | <0.0001 |
| Epilepsy, n (%) | ||||
| Not reported | 7 (13.7%) | 9 (36.0%) | 0 (0.0%) | 0.014 |
| Present | 44 (86.3%) | 16 (64.0%) | 12 (100.0%) | |
| Autistic features, n (%) | ||||
| Not reported | 17 (33.3%) | 9 (36.0%) | 11 (91.7%) | 0.001 |
| Present | 34 (66.7%) | 16 (64.0%) | 1 (8.3%) | |
| Aggressive behavior, n (%) | ||||
| Not reported | 41 (80.4%) | 21 (84.0%) | 12 (100.0%) | 0.292 |
| Present | 10 (19.6%) | 4 (16.0%) | 0 (0.0%) | |
| Hypotonia, n (%) | ||||
| Not reported | 15 (29.4%) | 18 (72.0%) | 2 (16.7%) | <0.0001 |
| Present | 36 (70.6%) | 7 (28.0%) | 10 (83.3%) | |
| Pyramidal signs, n (%) | ||||
| Not reported | 30 (58.8%) | 18 (72.0%) | 11 (91.7%) | 0.082 |
| Present | 21 (41.2%) | 7 (28.0%) | 1 (8.3%) | |
| Extrapyramidal signs, n (%) | ||||
| Not reported | 45 (88.2%) | 20 (80.0%) | 12 (100.0%) | 0.279 |
| Present | 6 (11.8%) | 5 (20.0%) | 0 (0.0%) | |
| Microcephaly, n (%) | ||||
| Not reported | 37 (72.5%) | 21 (84.0%) | 10 (83.3%) | 0.551 |
| Present | 14 (27.5%) | 4 (16.0%) | 2 (16.7%) | |
| Respiratory symptoms, n (%) | ||||
| Not reported | 44 (86.3%) | 25 (100.0%) | 2 (16.7%) | <0.0001 |
| Present | 7 (13.7%) | 0 (0.0%) | 10 (83.3%) | |
| Cardiac symptoms, n (%) | ||||
| Not reported | 51 (100.0%) | 25 (100.0%) | 6 (50.0%) | <0.0001 |
| Present | 0 (0.0%) | 0 (0.0%) | 6 (50.0%) | |
| Strabismus/eye problems, n (%) | ||||
| Not reported | 34 (66.7%) | 19 (76.0%) | 12 (100.0%) | 0.045 |
| Present | 17 (33.3%) | 6 (24.0%) | 0 (0.0%) | |
| Dysmorphism, n (%) | ||||
| Not reported | 47 (92.2%) | 23 (92.0%) | 10 (83.3%) | 0.509 |
| Present | 4 (7.8%) | 2 (8.0%) | 2 (16.7%) | |
| Cerebral atrophy, n (%) | ||||
| Not reported | 18 (35.3%) | 17 (68.0%) | 11 (91.7%) | <0.0001 |
| Present | 33 (64.7%) | 8 (32.0%) | 1 (8.3%) | |
| Cerebellar atrophy, n (%) | ||||
| Not reported | 39 (76.5%) | 22 (88.0%) | 12 (100.0%) | 0.125 |
| Present | 12 (23.5%) | 3 (12.0%) | 0 (0.0%) | |
| White matter abnormalities, n (%) | ||||
| Not reported | 26 (51.0%) | 17 (68.0%) | 9 (75.0%) | 0.209 |
| Present | 25 (49.0%) | 8 (32.0%) | 3 (25.0%) | |
| Ventriculomegaly/enlarged sulci, n (%) | ||||
| Not reported | 38 (74.5%) | 18 (72.0%) | 11 (91.7%) | 0.434 |
| Present | 13 (25.5%) | 7 (28.0%) | 1 (8.3%) | |
| Cerebral hemorrhage, n (%) | ||||
| Not reported | 50 (98.0%) | 25 (100.0%) | 8 (66.7%) | 0.001 |
| Present | 1 (2.0%) | 0 (0.0%) | 4 (33.3%) | |
| Gyrification deficits, n (%) | ||||
| Not reported | 50 (98.0%) | 25 (100.0%) | 9 (75.0%) | 0.007 |
| Present | 1 (2.0%) | 0 (0.0%) | 3 (25.0%) | |
- Note: The values in bold are the statistically significant one.
3.7 Focus on genotype-phenotype correlation
The most frequently reported variants in our sample are: (i) the homozygous p.R426H variant found in 19 patients; (ii) the p.Y114H variant reported in compound heterozygosity in 13 patients (4 of them presented the variant in conjunction with the p.R426H); and (iii) the variants p.D430N reported in 6 patients (4 homozygous and 2 heterozygous with the p.R426H variant). Most variants in the literature were inherited from healthy carrier parents. Kmoch et al. categorized seven ADSL variants based on their residual activities: null variants without detectable activity (p.Y114H and p.D268N), variants with substantially compromised activity (p.R194C and p.R426H), and variants with activities similar to the wild-type enzyme (p.A3V, p.R190Q, and p.D430N).7 Patients with combined p.Y114H and p.R426H variants showed the most severe phenotype. Mouchegh et al.5 reported four cases with p.Y114H and p.R426H variants in compound heterozygosis, presenting severe cardio-respiratory complications and refractory seizures immediately after birth. Other patients with at least one p.Y114H allele also showed early presentation with a similar clinical picture, though some had milder phenotypes when p.Y114H was present in compound heterozygosity, for example, Pts. 4 and 5 in Kmoch et al.7 whose other allele was p.R190Q, had a relatively milder phenotype without cardio-respiratory complications or epilepsy. Three patients with p.Y114H and p.G418A were reported (Pts. 6–8 in Mastrogiorgio et al.8), two with type II and one with type I, all having mild developmental delay, and two with epilepsy. Homozygous p.R426H carriers reported in the literature, like our Pts. 1 and 2, presented early with developmental delay, sometimes associated with regression of acquired skills, drug-resistant epilepsy, and often with spastic-dystonic tetraparesis. An exception is seen in five cases harboring the same homozygous variant (Pt. 1 and Pt. 2 in Marie et al.9; Pt. 3 in Edery et al.10; Pt. 5 in Donti et al.3; Pt. 5 in Mastrogiorgio et al.8) where no epilepsy was reported, suggesting the involvement of unaccounted gene-gene interactions or non-genetic factors in the phenotype. The p.D430N variant encodes a mutant protein with modest residual function and was reported in two patients (Pt. 1 in Jurecka et al.4 and Pt.16 in Mastrogiorgio et al.8) in heterozygosity with p.R426H, resulting in a mild phenotype without epilepsy. Our patients with homozygous p.D430N (Pts. 2–6) had a milder phenotype with developmental delay and autistic features within the first year and epilepsy onset in late childhood, achieving seizure freedom with appropriate treatment. The newly reported p.P24L variant's protein activity is not yet characterized. The four patients with p.P24L variants are compound heterozygous, and their phenotype, while consistent in terms of developmental delay and epilepsy presence, displays different degrees of severity even between siblings.11 Only a few patients were reported with additional variants, severely limiting the description of peculiar traits related to specific protein changes.
3.8 Survival analysis
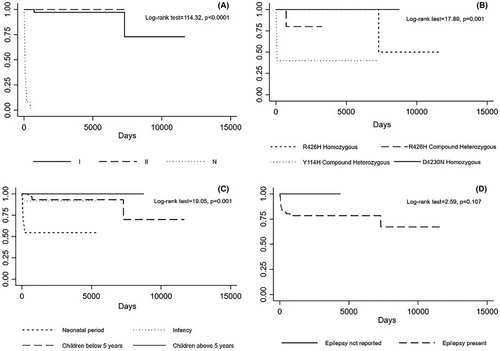
As expected, neonatal forms are associated with lower survival compared to the other two groups (Figure 3A). We computed the survival of the carriers of the four most frequent variants, that is, homozygous p.R426H, compound heterozygous p.Y114H, compound heterozygous p.R426H (excluding the p.Y114H and p.R426H carriers computed in the previous group), and homozygous p.D430N. The analysis revealed poor survival in the compound heterozygous p.Y114H and p.R426H groups and a better prognosis of the homozygous p.R426H and p.D430N carriers (Panel B, log-rank test = 17.89, P = 0.001). The neonatal onset, not accounting for mutation type, was associated with a poorer prognosis (Panel C). The presence of epilepsy is not associated with a statistically significant difference in terms of survival (Panel D). However, an earlier onset of epilepsy correlated with a worse prognosis (log-rank test = 8.92, P = 0.030). Seizures as the presenting condition and white matter abnormalities did not show significant differences in terms of survival (log-rank test = 0.37, P = 0.540; log-rank test = 1.07, P = 0.300, respectively).
4 DISCUSSION
Although ADSL deficiency is classified into three broad phenotypes, the condition remains highly heterogeneous. The mechanisms by which ADSL deficiency can give rise to its symptomatology are not fully elucidated and may include deficiency of purine nucleotides, impairment of cellular bioenergetics, and toxic accumulation of SAICAr and S-ADO.6 Despite a diverse clinical presentation, epilepsy is present in a substantial portion of the affected patients (ie, 81.8%), with polymorphic and often intractable seizures, severely affecting the quality of life of the patients and their caregivers. Earlier epilepsy onset and drug-resistant seizures appear to be more frequent in patients with type I and neonatal phenotypes and in patients harboring pathogenic variants encoding proteins with reduced residual activity. Different EEG patterns have been observed in ADSL patients: (i) a poor background organization with diffuse theta-delta activity was commonly reported; (ii) a burst suppression pattern or hypsarrhythmic pattern with epileptic spasms, especially at onset, has been described. As reported in our patients and by other authors,12, 13 the hypsarrhythmic pattern is generally responsive to ACTH or steroidal therapy, although the response is only limited in time. Also, burst suppression patterns have been reported. (iii) These patterns may evolve during childhood or adolescence into focal spike-and-waves with a predominance over the frontal temporal regions of EDs, as observed in our two first siblings and in previous data from the literature.14-16 Despite the wide clinical spectrum, the frontal or fronto-temporal predominance of EDs observed in all our cases and reported in previous literature,14-16 could be considered a possible specific localization of EDs in this condition and seems to coincide with the areas that show the most significant atrophy on MRI.17 Sporadically, occipital predominance or diffused spike-and-waves have also been observed,15, 18 (iv) sleep patterns were rarely reported,19 and like our patients, the sleep structure was disorganized with no recognizable physiological sleep elements. No overt activation of spike-and-wave activity in sleep was previously reported; however, sleep recordings of our Pt. 3 and, to a lesser extent, Pts.1 and 2, showed EDs activation, also in bursts, evolving to a progressive impoverishment of the EEG background with loss of physiological sleep elements. EDs sleep activation in our Pt. 3, despite not configuring a typical case of developmental and/or epileptic encephalopathy with spike-and-wave activation in sleep (DEE-SWAS) due to a different clinical evolution and genetic background, may account for his more severe cognitive phenotype in comparison with the sibling. Concerning the anti-seizure treatment for ADSL deficiency, to date, no standard of care can be recommended. However, in our milder patients (Pts. 3–7), valproate, levetiracetam, and lamotrigine were found to be effective. Ketogenic diet, D-ribose, and S-adenosyl-L- methionine1, 11, 12, 17, 20 were implemented as possible therapeutic strategies in these patients with limited success (refer to Table S2). Clinical features, for example, psychomotor delay, intellectual disability, epilepsy, frequently associated with autistic traits, pyramidal and extrapyramidal signs, albeit common to many childhood encephalopathies, can be suggestive of this condition and configure a spectrum of disease presentation with differences in severity among the types of the disease.
Also, imaging can aid in suspecting of ADSL deficiency, with similar features but with different grade of severity among the different phenotypes: cerebral, cerebellar atrophy, and white matter abnormalities tend to be more pronounced in Pts.1 and 2 (type I) compared to the patients we have described in families 2 and 3 (type II). These data were confirmed by the literature, where cerebral and cerebellar atrophy were reported in a significant proportion of patients, as well as white matter abnormalities (ie, periventricular or semioval center T2-hyperintensities). A previous review17 specifically focusing on the MRI features of ADSL patients reported cerebral atrophy with frontal predominance as a common finding in older patients, in addition to cerebellar, specifically vermian, atrophy and white matter periventricular abnormalities. Such findings, in our cases and in literature,17 tend to be more prominent in the most severe types of ADLS deficiency (type I) compared to the milder (type II) and more evident in older patients. They are not often reported in neonatal forms, in which cortical development abnormalities or cerebral hemorrhages are most commonly encountered, contributing to their poor prognosis and early death (preventing the establishment of overt cerebral atrophy).
Considering the features described in our new patients reported, in the literature revision, and the previous functional analysis conducted, the phenotype of the patients affected by ADSL deficiency seems to suggest a possible correlation with the residual activity of the ADSL protein.7 Patients harboring the p.Y114H variant, with minimal residual function, presented with neonatal forms of the disease, while patients with variants encoding for proteins with an almost normal residual function (eg, p.R190Q and p.D430N) presented with a relatively milder course. However, rarely, compound heterozygous patients can show a milder phenotype, for example, our Pt. 7 (p.Y114H and p.R296W), suggesting the influence of the residual activity of the mutant protein encoded in the second allele in the establishment of the phenotype. Patients with the homozygous p.R426H variants, like our first family described, generally presented with a type I phenotype even if considerable variability could be observed between affected individuals, while patients with variants encoding for proteins with an almost normal residual function (eg, p.R190Q and p.D430N, like our reported patients) presented with a relatively milder course. Functional studies on new variants and the identification of new patients would be required to delineate a stronger phenotype-genotype correlation.
4.1 Limitations
In our review, we included only a part of the literature published on ADSL patients because we focused only on peer-reviewed English-language journals and we included only studies or clinical cases reporting both genetic and clinical data. To date, many variants are present only in small groups of patients and are not fully clinically characterized. For a database of the variants identified up to June 2013, please refer to http://www1.lf1.cuni.cz/udmp/ADSL/. The low number of people with the disease and the high variability between the reported variants did not allow for a cluster analysis of the symptoms based on specific protein alterations. This analysis will be the goal of future work expanding the sample.
5 CLINICAL RELEVANCE AND FUTURE DIRECTIONS
We present a series of previously unreported patients with ADSL deficiency with long-term follow-up documenting the electroclinical features of the syndrome and comparing our patients with previously published cases. Despite the fact that a precise genotype-phenotype association is not feasible due to the limited number of patients reported, clinical phenotype severity seems to correlate with residual protein activity. Also, clinical, neuroradiological, and neurophysiological data seem to display common features and developmental trajectories in ADSL patients: the development of cerebral and cerebellar atrophy and white matter periventricular abnormalities associated with an EEG pattern of EDs with frontal temporal predominance. In patients with psychomotor delay, epilepsy, prominent autistic features, and pyramidal-extrapyramidal signs, ADSL deficiency might be considered in the differential diagnosis of epileptic encephalopathies.
ACKNOWLEDGMENTS
The present study did not receive any funding. We greatly appreciate the patient's parents for their support of the research and for kindly participating in the present study.
CONFLICT OF INTEREST STATEMENT
The authors have no disclosures related to the present work.
ETHICS STATEMENT
Written informed consent was obtained from the parents or legal representatives of the involved patients. The study adheres to the principles of the Code of Ethics of the World Medical Association-Helsinki Declaration and concerns data gathered during routine diagnostic activity. The study also complies with institutional regulations for anonymized retrospective studies. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data to support the findings of this study are included in the article and supplementary materials. Additional data may be available from the corresponding author, PV, upon reasonable request.