Use of ketogenic dietary therapy for drug-resistant epilepsy in early infancy
Abstract
Objective
There is growing evidence that ketogenic dietary therapy (KDT) can be safely and efficiently used in young children, but little evidence exists on its use in newborns. Developmental and epileptic encephalopathies starting in the neonatal period or early infancy usually present a poor prognosis. The aim of this study was to evaluate effectiveness, safety, and survival of infants younger than 3 months of age with drug-resistant epilepsy in whom KDT was used.
Methods
A retrospective study was conducted to evaluate neonates and infants younger than 3 months who started KDT for drug-resistant developmental and epileptic encephalopathies at three referral centers. Data were collected on demographic features, time of epilepsy onset, epilepsy syndrome, seizure type, seizure frequency at diet onset, etiology, details regarding diet initiation, type of ketogenic formula, breastfeeding, route of administration, blood ketones, growth, length of NICU stay, and survival.
Results
Nineteen infants younger than 12 weeks of life who received KDT with a minimum follow-up of 1 month were included; 13 had early-infantile developmental and epileptic encephalopathy, four epilepsy of infancy with migrating focal seizures, and two focal epilepsy. A >50% response was observed in 73.7% at 1 month on the diet; 37% achieved a > 75% seizure reduction, and 10.5% became seizure free. At 3 months, a >50% decrease in seizure frequency was observed in 72.2%; 15.8% had a >75% reduction; 21% became seizure free. Overall survival was 76% at 1 year on diet. Incidence of acute and late adverse effects was low and most adverse effects were asymptomatic and manageable.
Significance
Our experience suggests that KDT is safe and effective in newborns and very young infants; however, further studies on the management of the diet in this vulnerable age group are necessary.
Key points
- Few reports have been published on the use of the ketogenic diet in neonates with drug-resistant epileptic encephalopathies.
- Neonates and very young infants may respond even better to the diet than older children.
- The most common acute adverse effects were hypoglycemia and transient hypertriglyceridemia, constipation was a common late complication.
- In our study, ketogenic dietary therapy was found to be safe and effective in newborns and very young infants.
1 INTRODUCTION
Ketogenic diet therapies (KDTs) are high-fat, low-carbohydrate, and moderate-protein diets that have been used for the management of children and adults with drug-resistant epilepsy for more than a century. Currently, it has been widely accepted that KDTs can be safely and efficiently used in very young children (under 2 years old) and guidelines for their use in infants have been developed.1, 2 Nevertheless, few reports have been published on the use of KDT in neonates3-5 and although there is increasing awareness that neonates and infants produce and utilize ketone bodies as well as or better than older children,6 concerns regarding efficacy and safety remain an important issue in this vulnerable population.7
The use of KDTs in early infancy poses a special challenge but seems to be feasible, leading to improved seizure control, increased alertness, and decreased need for invasive respiratory support.3-5 In addition, it has been shown that KDTs can be initiated in infants while continuing breast milk feedings, with patients achieving and maintaining ketosis similarly to non-breastfed infants.8
Developmental and epileptic encephalopathies (DEE) starting in the neonatal period or early infancy usually present a poor prognosis regarding seizure control and are associated with severe neurodevelopmental impairment.9 The DEE of neonatal- and early–infancy onset include early-infantile developmental and epileptic encephalopathy (EIDEE), previously classified as Ohtahara syndrome and early myoclonic encephalopathy, epilepsy of infancy with migrating focal seizures (EIMFS), and early-onset infantile epileptic spasms syndrome.9
Drug resistance requires the addition of other therapeutic strategies and KDTs have become a promising alternative to reduce the neurological impact of these diseases.10, 11 Currently, KDT is considered the first-line and immediate treatment for infants with pyruvate dehydrogenase complex deficiency (PDCD) and glucose transporter type 1 deficiency syndrome (GLUT1DS).12 It is well known that these patients are sometimes identified late; however, diagnosis in the first days of life and KDT initiation in the neonatal period may lead to improved outcomes in these children.
As little is known about the use of KDTs in this age group and no data exist on the survival of the neonates and very young infants on the diet, we hypothesized that KDTs may be an effective and safe therapeutic option for the management of DEE in neonates even from the first days of life. Therefore, the aim of this study was to evaluate effectiveness, safety, and survival in a multicenter cohort of 19 patients under 3 months of age with drug-resistant epilepsy in whom KDT was used.
2 METHODS
A retrospective study was conducted to evaluate neonates and infants younger than 3 months who started KDT for drug-resistant epilepsy at three referral centers in Argentina between 2013 and 2022. Only infants/neonates who started KDT before 12 weeks of life were included in the study. The study was approved by the ethical review board of each center. Informed consent was waived because of the retrospective nature of the study.
2.1 Ketogenic diet protocol
The classic ketogenic diet (CKD) was started in all patients in a nonfasting fashion, following the protocol of the 2016 ketogenic diet guidelines for infants.1 In three infants that were treated with CKD before 2016, energy calculation was according to the WHO 2004,13 daily fluid intake according to the Holliday-Segar formula14 based on daily allowance (RDA), and daily protein contents according to the WHO 2007.15 The diet was initiated with ketogenic formula (Ketocal 4:1®, Nutricia Metabolics, 4:1, or Ketovie 3:1®, or Ketovie Peptide 4:1®, Ajinomoto/Cambrooke) at a 1:1 ratio mixed with infant formula or, when the infant was breastfed, at a 2:1 ratio with breast milk given after formula feeding. A modular product (medium-chain triglyceride [MCT] MCT oil®, Nutricia) was also used in some patients. A ketosis induction protocol without calorie or fluid restriction was used. The ratio was increased every 2-3 days according to tolerance and β-hydroxybutyrate (BHB) levels (2-5 mmol/L). The wish of the mother regarding whether or not to continue breastfeeding during the treatment was discussed.
Body weight was checked every 48 hours and a basic metabolic panel (ionogram, acid-base status, glucose, BHB, triglycerides, amylase, lipase, urea, creatinine, albumin) was performed every 3-4 days. Plasma glucose (four times daily pre-prandially) and capillary BHB levels (twice daily) were measured during the induction phase. Table 1 shows an overview of the initiation, monitoring, and follow-up of newborns and very young infants on KDT at our centers.
| Induction phase |
|
| Monitoring |
|
| Follow-up |
|
2.2 Data collection
Data were collected on demographic features, time of epilepsy onset, epilepsy syndrome, seizure type, seizure frequency at diet onset, etiology, details regarding diet initiation, type of ketogenic formula, breastfeeding, route of administration (oral, tube-fed), glucose, and blood-ketone monitoring, growth, length of NICU stay, and survival.
Seizures16 and syndromes17 were classified according to the classifications of the International League against Epilepsy. Response to treatment was defined as a decrease in seizure frequency of more than 50% compared to baseline.
Time to ketosis was defined as the time in hours the patient needed to reach BHB >0.5 mmol/L. A ketone level between 2.0 and 5.0 mmol/L was aimed for.
Adverse events (AE) were evaluated. Hypoglycemia was defined as capillary glucose levels <40 mg%, hyperketosis as capillary BHB levels >5 mmol/L, metabolic acidosis as bicarbonate levels <18 and/or PH <7.20, hypokalemia as K < 3.6, dyslipidemia as total cholesterol levels >200 mg%, increased low-density lipoprotein (LDL) cholesterol levels >130 mg%, hypertriglyceridemia >100 mg%, hypercalciuria as a high calcium/creatinine index for age, and vitamin and micronutrient deficiencies as low levels for age.
Anthropometric measurements were recorded as weight-for-age and height-for-age Z-scores, based on the WHO Anthroplus 2009 (http://www.who.int/childgrowth/software/en/).
2.3 Outcomes
Primary outcomes were survival and effectiveness of the CKD. The secondary outcome was the occurrence of AE including hypoglycemia, vomiting, constipation, metabolic acidosis, dyslipidemia, and weight loss.
2.4 Statistical analysis
Continuous variables were expressed as median and range, while categorical variables were expressed as percentages; the Mann–Whitney test and Chi-square or Fisher exact tests were used for comparison as appropriate. The Kaplan–Meier method was used for survival analysis. For curve comparison, the log rank test was employed. To analyze associations between variables, a logistic regression model was used, adjusted for possible confounders. Relative risk (RR) was expressed with 95% confidence intervals (95% CI). A P value of <0.05 was considered statistically significant. Statistical analysis was performed with STATA 17.1 data analysis software (StataCorp LLC 4905 Lakeway Drive).
3 RESULTS
Nineteen patients younger than 12 weeks of life who received the CKD with a minimum follow-up of 1 month seen at three different tertiary centers were included. Median follow-up was 242 days (range, 80-292). 11 infants (58%) were girls and eight (42%) were boys. Median age at seizure onset was 2 days (range, 0-18 days) and mean age at CKD initiation was 7 weeks (range, 3-12 weeks). Seven of 19 patients were younger than 1 month of life at diet initiation. The etiology was unknown in seven patients (36.8%), whereas the cause was genetic in five (26.3%), structural in four (21%), and metabolic in three (15.8%).
The diet was started as first-line therapy in one patient with PDHC and in another with GLUT1DS. One patient received KDT for non-ketotic hyperglycinemia and in a patient with hemimegalencephaly the diet was used as a bridge to epilepsy surgery.
Regarding epilepsy syndromes, 13 (68%) patients had EIDEE, four (21%) patients EIMFS, and two (10.5%) had focal epilepsy.
The patients received a mean number of 3 (range, 0-5) antiseizure medications (ASM) at CKD initiation and 2.5 ASM at 6 months (r: 0-4), a difference that was not found to be statistically significant. ASM could be discontinued at 6 months in two patients.
3.1 Dietetic and metabolic management
In all but one patient (GLUT1DS), the diet was initiated on an inpatient basis in the NICU. The mean length of NICU stay was 92 days. All patients received the CKD; 13 (68.4%) at a 3:1 ratio and six (31.6%) at a 2.5:1 ratio. Route of administration was by nasogastric tube (NGT) in 14 (73.7%), oral in three (15.8%), and NGT and oral combined in two (10.5%).
Fifteen patients (79%) received formula only, and in four (21%) formula was combined with breastfeeding. Eight patients received formula with 20% MCT oil. Only one patient received hydrolyzed ketogenic formula because of non-IgE-mediated cow's milk protein allergy.
A median of 122 (range, 95-160) calories/kg were provided at CKD initiation with a median of 2.4 g (range, 1.5-3.5) protein/kg.
Mean time to nutritional ketosis was 6 days (range, 2-21), and mean time to reach the therapeutic range was 7 days (range, 1-21). Ketosis was achieved in 2-3 days in six patients (31.6%), 4 to 7 days in four (21.1%), and 8-21 days in seven (36.9%). All patients achieved ketosis within the therapeutic range.
3.2 Primary outcomes: Survival and treatment effectiveness
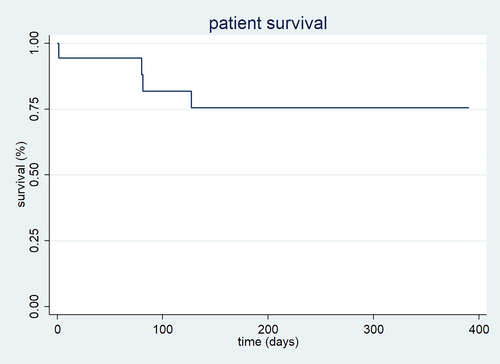
Six patients died during the course of treatment (P: 0.012) and follow-up. Three died from acute respiratory causes (influenza A, COVID-19) and three from status epilepticus (one had an ATP1A2 mutation and two with cortical dysplasia). Four deaths occurred early, in the first 4 months after diet initiation, while the remaining two occurred beyond 17 months (one of them was still on KDT). Overall survival was 95% at 1 month and 76% both at 6 months and 1 year on diet. Figure 1 shows patient survival at 1 year on KDT.
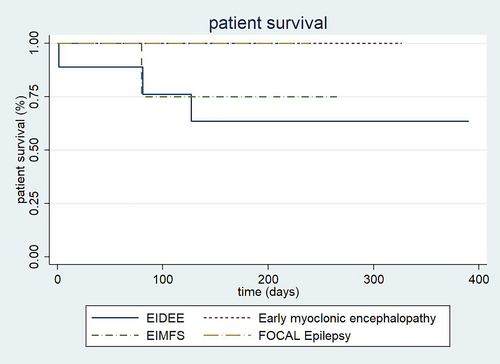
The differences between death due to the different epileptic syndromes were not found statistically significant. However, a trend toward a higher mortality was observed in infants with EIDEE (P: 0.46; Figure 2). No significant differences were observed in patient survival comparing genetic vs. non-genetic etiologies (P: 0.18). On the other hand, a significant difference was found in patient survival between those who entered ketosis before and those who entered ketosis after 7 days (66.7% vs. 85.7% at 1 year), being better for those who entered ketosis later (P: 0.03).
A good response to treatment was seen in 14/19 patients (73.7%) after 1 month on the diet, of whom seven (37%) achieved a greater than 75% seizure reduction, and two were seizure free (10.5%). At 3 months of follow-up a greater than 50% decrease in seizure frequency was observed in 13/18 patients (72.2%), of whom three had a seizure reduction in more than 75% (15.8%) and four (21%) were seizure free. Of the children who were still on the diet at 6 months, 10/19 (52.6%) had a >50% decrease in seizure frequency, of whom 6/19 (31.6%) were seizure free (Table 2).
| Patient | Epileptic syndrome | Etiology | Electroclinical pattern | Age at CKD initiation (wk) | Time to ketosis /d | CKD ratio | Energy requirement at CKD initiation (kcal/kg/day) | Protein intake at CKD initiation (g/kg/day) | % seizure reduction 1 mo | % seizure reduction 3 mo | % seizure reduction 6 mo | Neurocognitive improvement | CKD duration (mo) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EIDEE | Unknown | Tonic seizures/spasms/BS | 12 | 9 | 3.1 | 120 | 2.3 | 75-99 | 51-75 | <50 | No | 12 | Death at 17 mo post diet initiation due to influenza |
| 2 | EIDEE | Unknown |
Spasms/BS myoclonic seizures |
8 | 2 | 2.5:1 | 110 | 2.4 | 75-99 | <50 | <50 | No | 6 | Death at 24 mo post diet initiation due to SUDEP |
| 3 | EIDEE | KCNQ2 | Spasms/BS | 4 | – | 2.5:1 | 125 | 2.5 | 51-75 | 51-75 | <50 | Yes | 9 | Alive |
| 4 | EIDEE | Unknown | Spasm/BS | 7 | 6 | 3.1 | 105 | 2.3 | 75-99 | 75-99 | Seizure free | Yes | 9 | Alive |
| 5 | EIDEE | ATP1A2 | Tonic seizures/spasms + apnea. Status epilepticus/focal-multifocal seizures | 9 | 6 | 3.1 | 95 | 2 | <50 | – | – | No | 1 | Death at 4 mo post diet initiation due to status epilepticus |
| 6 | EIDEE |
Glut1DS |
Myoclonic /multifocal seizures | 8 | 18 | 3.1 | 160 | 2.9 | 50-75 | 50-75 | Seizure free | No | 30 | Lost to follow-up |
| 7 | Focal epilepsy | Hemimegalencephaly | Myoclonic/focal seizures | 11 | 2 | 3.1 | 100 | 2 | >50 | - | - | Yes | 1 | Alive |
| 8 | EIDEE | Unknown | Multifocal seizures/BS | 7 | 3 | 3.1 | 101 | 2.15 | 75–99 | Seizure free | Seizure free | Yes | 11 | Alive |
| 9 | EIDEE | SCN8A | Intrauterine-onset non-epileptic myoclonic movements. Tonic seizures with apnea, status epilepticus. Focal parieto-occipital activity on EEG, focal hypomotor seizures | 11 | 2 | 3.1 | 120 | 2.5 | 50-75 | 50-75 | <50 | Yes | 9 | Alive |
| 10 | EIDEE | KCNQ2 | Tonic seizures/spasms/BS | 4 | 5 | BF+ 2.5:1 | 105 | 1.6 | Seizure free | Seizure free | Seizure free | Yes | 24 | Alive |
| 11 | EIDEE | DPDH | Spasms/BS | 3 | 10 | 3.1 | 105 | 1.6 | 75-99 | 50-75 | 50-75 | Yes | 25 | Death at 48 mo post diet initiation due to COVID-19 |
| 12 | EIDEE | Cortical dysplasia | Spasms/tonic seizures/BS | 4 | 3 | 3.1 | 105 | 1.6 | <50 | – | – | No | 2 | Death at 2 mo post diet initiation due to epilepsy |
| 13 | EIMFS | Left hippocampal subcortical heterotopias | Spasms/tonic and myoclonic seizures | 4 | 4 | 3.1 | 105 | 1.6 | <50 | – | – | No | 1 | Death at 3 mo post diet initiation due to epilepsy |
| 14 | EIMFS | SCN2A | Tonic/focal-multifocal seizures | 4 | 16 | BF+ 2.5:1 | 142 | 3.4 | 75-99 | 75-99 | 75-99 | Yes | 6 | Alive |
| 15 | EIDEE | Non-ketotic hyperglycinemia | Tonic seizures/BS | 3 | 17 | BF+ 3.1 | 139 | 3.5 | 75-99 | 75-99 | 75-99 | Yes | 5 | Alive |
| 16 | EIDEE | Unknown | Spasms /tonic seizures/BS | 8 | 12 | 3.1 | 125 | 3.1 | 51-75 | - | - | Yes | 4 | Alive |
| 17 | EIDEE | Unknown | Myoclonic seizures/spasms/BS | 10 | 18 | 3.1 | 131 | 2.6 | 51-75 | <50 | - | Yes | 3 | Alive |
| 18 | EIMFS | SCN2A | Tonic-focal seizures | 11 | 3 | 2.5:1 | 160 | 3.3 | Seizure free | Seizure free | Seizure free | Yes | 12 | Alive |
| 19 | Focal epilepsy | Cortical dysplasia -lobar megalencephaly | Focal seizures | 7 | 21 | BF + 2.5:1 | 160 | 3.3 | 75-99 | 75-99 | 51-75 | Yes | 9 | Alive |
- Abbreviations: BF, breastfeeding; BS, burst suppression; CKD, classical ketogenic diet; EEG, electroencephalography; EIDEE, early-infantile developmental and epileptic encephalopathy; EIMFS, epilepsy of infancy with migrating focal seizures; Glut1DS, Glut1 deficiency syndrome; PDCD, pyruvate dehydrogenase complex deficiency.
No differences were observed in diet effectiveness at 1, 3, and 6 months between patients with genetic vs. those with non-genetic etiologies (P: 0.84) or when evaluating epileptic syndromes (P: 0.91), EEG findings (burst suppression vs focal paroxysms; P: 0.25), or days of NICU stay (P: 0.14).
Regarding etiology, at the 3-month follow-up, of two patients with a SCN2A mutation one was seizure free and the other had a >75% seizure reduction. Of two patients with KCNQ2 mutations, one was seizure free and the other had a >75% seizure reduction. In the patient with a SCN8A mutation, a 50% seizure decrease was observed, and the infant could be weaned from mechanical ventilation. The patient with an ATP1A2 mutation died at 4 months due to status epilepticus. Of four patients with cortical malformations, two did not respond to the diet, one had a >50% and the other a >75% seizure reduction. The patients with GLUT1DS and DPDH became seizure free and the patient with non-ketotic hyperglycinemia had a >75% seizure reduction.
No statistical differences were found in diet effectiveness at 1, 3, and 6 months between patients who entered ketosis within 7 days and those who entered ketosis later (P: 0.621). On the other hand, patients who received breast milk were more likely to enter ketosis later than those who did not (OR: 1.28). All four patients who were breastfed during treatment responded favorably to the diet. No differences were found in effectiveness comparing patients who received MCT oil with those who did not.
3.3 Secondary outcomes: Adverse events and tolerability
Overall, 9/19 (47%) of the infants had some early AE (occurring between induction and 1 month on the diet), of which asymptomatic hypoglycemia (3/19; 15.8%) and transient hypertriglyceridemia (3/19; 15.8%) were the most common.
Weight loss and vomiting were observed in two patients (10.5%) each. Hyperketosis was seen in one (5%). The occurrence of AE decreased after 1 month on the diet. The most common late AE was constipation (3/19; 15.8%). Metabolic acidosis and transient hypertriglyceridemia were seen in two patients at 3 months after CKD initiation.
There were no statistically significant differences in the frequency or time of appearance (early or late) of AE between patients who received formula only, formula plus MCT oil, or formula plus breastfeeding. Nor were there any statistically significant differences in the occurrence of constipation between patients receiving formula with or without MCT oil.
3.4 Growth
Overall, 14/19 patients were born at term, with adequate weight and length for gestational age. Five patients were late preterm infants; two were born at 36 weeks and three at 37 weeks, also with adequate weight and length for gestational age. None of the infants had microcephaly at birth. Mean head circumference (HC) at birth was 35 cm (range, 30-37.5) with a Z-score of 0.8 (range, −1.7 to 3.5), while at 6 months mean HC was 42.5 cm (range, 36-47) with a Z-score of −0.86 (range, −8.2 to 1.8).
An HC deficit (HC < −2 SDS) was observed in four patients (one with an SCN8A mutation, one with EIDEE, one with EIMFS, and one with homozygous GLUT1DS) at 6 months after diet initiation. Both the SCN8A patient and the GLUT1DS patient also developed a severe height deficit with chronic growth delay during the treatment.
From birth until the start of the diet, a delay in weight and height was observed with a statistically significant difference in weight Z score (P: 0.0040) and height Z score (P: 0.0175). But this difference was not seen at 1, 3, and 6 months after diet initiation (Table 3).
| Birth | KD initiation | 1 mo | 3 mo | 6 mo | P value | |
|---|---|---|---|---|---|---|
| Weight (g) | 3190 (2400–4790) | 4150 (2900–6220) | 5040 (3500–7090) | 6200 (5140–10 000) | 7160 (4830–11 000) | 0.0001 |
| Weight Z score (SDS) | 0 (−2-3.8) | −1.1 (−3.8-0.84) | −1 (−3.9-0.8) | −0.6 (−2.7-2.2) | −0.6 (−3.9-2) | 0.5039 |
| Height (cm) | 49.5 (45-53) | 52.7 (48-62.5) | 56.7 (51-66.5) | 61 (53.5–71.5) | 67 (57-76.5) | 0.0001 |
|
Height Z score (SDS) |
0.4 (−1.2-2.7) | −0.9 (−4.1-1.1) | −0.8 (−4.4-2.1) | −1.3 (−5.5-2.2) | −0.7 (−8.1-2.1) | 0.7216 |
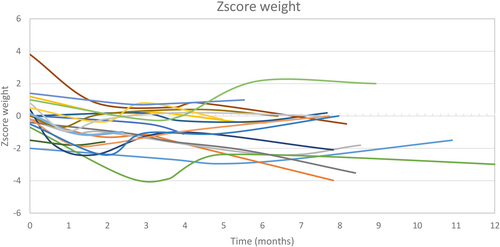
A growth deficit (height < −2 SDS) was observed in three patients (one with an SCN8A mutation, one with DPDH, and one with homozygous GLUT1DS) at diet initiation. All of them developed chronic growth delay during the treatment. The individual growth trajectories in weight and height of each patient are shown in Figures 3 and 4.
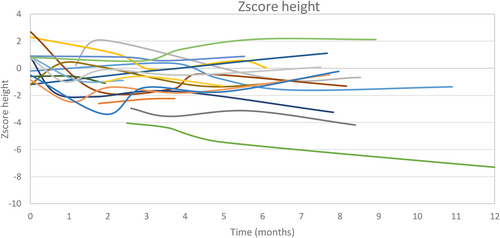
The diet was discontinued in 11 patients. Reasons for diet discontinuation were ineffectiveness in two, loss to follow-up in one, non-compliance in two, and surgical treatment of hemimegalencephaly in one. Five patients died due to causes unrelated to the diet.
4 DISCUSSION
In this multicenter study, we report 19 infants under 3 months of age with drug-resistant epilepsy who were treated with KDT. Survival was found to be 95% at 1 month after treatment initiation, 76% at 1 year of follow-up, and 38% at 24 months of follow-up. We found a trend toward higher mortality in patients with EIDEE. In a systematic review18 analyzing mortality in patients with EIDEE with burst suppression (BS), median time of death was at 12.9 ± 14.1 months, and 58.8% of the infants survived less than 1 year. KDT has been described to have an epigenetic and neuroprotective effect, which may be useful in these children.19
Although KDT is a common treatment in infants with drug-resistant epilepsy, the diet is still underutilized in neonates or young infants. Indeed, the first study reporting this treatment in the NICU was published only in 2017 describing four infants younger than 3 months who received the diet at a single center3 and to our knowledge there are no reports on the survival of the neonates receiving KDT.
In our series, treatment effectiveness was high, as 14/19 patients (73.7%) showed a good response in terms of seizure control at 1 month after CKD initiation, of whom seven (37%) achieved a > 75% seizure reduction and two (10.5%) became seizure free. This good response was maintained at 3 months (72.2%), with three patients achieving a >75% seizure reduction and four becoming seizure free. Surprisingly, at 6 months 10/19 (52.6%) had a >50% decrease in seizure frequency, of whom 6/19 (31.6%) were seizure free. In a previous study published by our group in infants younger than 24 months,2 effectiveness was 62.4% at 3 months and 60.7% at 6 months on the diet.
In six of our patients, a genetic cause could be established, consisting of a KCNQ2 in two, a SCN2A mutation, and a ATP1A2 and SCN8A mutation in one patient each. In the patients with SCN2A and KCNQ2 mutations response to the diet was excellent. KDT was previously found to be effective in children20 as well as neonates and very young infants3, 4, 7 with these mutations. Our patient with severe SCN8A-DEE associated with gain of function of the channel a seizure decrease in 50% was observed and the patient could be weaned from mechanical ventilation. Although there is currently no experience in neonates, reports in infants and children with this mutation show variable results.20-22 Finally, our patient with severe ATP1A2-EIDEE23 did not respond to the diet and died 4 months after KDT initiation due to status epilepticus. To our knowledge, no patients with ATP1A2-EIDEE treated with KDT were previously reported.
Four of our patients had cortical malformations associated with heterogeneous epileptic syndromes. Response to the diet was variable, with a <50% response in two, a >50% response in one, and a 75%-99% response in the remaining patient. The efficacy of KDT for cortical dysplasia has been previously reported.24
Inui et al.25 described two neonates who were clinically diagnosed with PDHC deficiency and were started on parenteral KDT on the first day of life followed by the enteral CKD at a 3.1 ratio 1 week later. Both infants became seizure free. In our patient with PDHC deficiency, KDT was initiated at Week 3 of life with excellent results.
In patients with Glut1DS, KDT initiation at a younger age has been shown to result in better outcomes.18, 26, 27 Our patient started the CKD at 8 weeks and was therefore the only patient in whom treatment was not started in the NICU; nevertheless, response to the diet was excellent.
One of our patients had non-ketotic hyperglycinemia. KDT was previously used for this condition28, 29 in infants between 3 and 6 months of age with a 50% reduction in seizures, symptom improvement, and better quality of life. Our patient, who received the diet in the neonatal period with a very good response, showed that early KDT initiation combined with NMDA blockers may be an option to improve the outcome of this disease.
One of the main reasons for the limited use of KDT in this age range is the concern about the possibility of AE in these vulnerable infants.3 In our series, AE were observed in almost 50% of the patients during the induction phase and the first month on the CKD. Asymptomatic hypoglycemia and transient hypertriglyceridemia were the most common (both found in 15% of patients), similar to reports by other authors.3, 5, 7, 30 Nevertheless, we found that AE were easily resolved at treatment initiation. Constipation was the most frequent late AE, observed in 15% of the infants. In the study by Thompson et al.,3 50% of the patients had constipation and they were successfully treated with the addition of MCT oil to the formula. In our series, 8/19 patients received MCT from diet initiation, which may explain the lower frequency of constipation in our series.
Although in our previous study in infants younger than 2 years we found a trend toward a higher rate of AE in infants younger than 1 year,2 in our current study a rate of less than 50% was found. We may speculate that the use of a less strict protocol (lower ketogenic ratio and slower ketosis induction) may have led to the lower AE rate.
Little is known about the nutritional status of neonates during KDT. In our series, we found that weight and height significantly decreased from birth to onset of the diet; however, once the diet was started this difference disappeared. Indeed, between diet initiation up to 6 months of the diet the weight Z score improved 0.5 while height remained stable. Therefore, KDT may have stopped the growth delay, allowing children to continue growing in the same percentile. Nevertheless, to evaluate growth in height, a longer follow-up is necessary.
In our cohort, mean caloric intake prescribed was 122 cal/kg/day, above the RDA requirements for infants on KDT recommended in the infant guidelines (100-95)1 and higher than the WHO 2007.15 We may speculate that before KDT, these patients have a high energy demand due to the uncontrolled epilepsy and high caloric intake is needed. On the other hand, improved growth might also be the result of the close monitoring of the expert team and dietitian during KDT.
In 42% of patients in our cohort, 20% of MCT oil was used. In half of these children the formula used already contained MCT. In others, we decided to add MCT because of its positive effects. It has been found that human milk is a natural source of medium-chain fatty acids (MCFAs), comprising approximately 10%-35% of the total fatty acids. MCFAs are important for infants with an immature digestive system and, therefore, the addition of MCT to infant formula may facilitate better absorption of lipids.31 This may be especially important for this group when they have risk of weight loss and growth failure. For infants, a total amount of 10%-25% MCT has been observed to be well tolerated.1
In previous studies weight loss was observed in most neonates and very young infants on KDT and calorie intake was increased with the addition of MCT oil.3, 7, 32 Only two of our patients lost weight, which may have been due to the use of MCT oil in almost half the infants from diet initiation. MCT oil is better absorbed in the immature intestine and may be used from diet initiation to prevent constipation and weight loss.33, 34 Nevertheless, it should be noted that MCT does not contain essential fatty acids that are crucial for the neurodevelopment of neonates and infants. Careful energy prescription is advised in these very young infants.
Patients who received ketogenic formula together with breast milk did not have a higher AE rate than those who received formula alone, similar to a previous study on the use of KDT combined with breast milk for infants with severe epilepsy.8 In our series, all four patients who were breastfed during treatment responded favorably to the diet; however, this number is too small to draw definitive conclusions.
The mean time to enter ketosis was 6-7 days in our series; 37% entered ketosis between 8 and 21 days. This faster ketosis in our cohort may be explained by the use of MCT oil in almost half the patients. It is well known that MCFAs are ketogenic and the ideal ketone precursors and a higher level of plasma BHB was observed in the preterm infants fed with the MCT formula compared with a control formula without MCT.35
On the contrary, the newborn twins reported by Phitsanuwong et al.7 entered ketosis on day 18. Our patients who received ketogenic formula together with breast milk also achieved late ketosis, but the outcome was good. Therefore, late ketosis does not seem to affect seizure control.
Although most studies use the CKD in a 4:1 ratio,3, 5, 29, 30 in our series and in the case reported by Turkdogan et al.4 a 3:1 ratio was used with good seizure control. Therefore, we believe that increasing the ketogenic ratio higher than 3:1 is not necessary. A lower ratio was also effective in a randomized trial of 38 infants demonstrating that a 2.5:1 ratio diet was as effective as a 4:1 ratio with less side effects.36
Our study has several limitations. First, its retrospective and multicenter nature may have led to differences in data collection and there may have been slight differences in the treatment protocol. Nevertheless, although the sample size is small, this is currently the largest study evaluating the use of KDT in very young infants with a follow-up of 6 months and assessing survival and response to the diet according to epileptic syndrome and etiology. As this group of very young infants has particular features regarding their epilepsy syndrome and seizure types9 as well as nutritional requirements, further studies including larger series of patients are necessary to evaluate the management of the diet in early infancy.
5 CONCLUSIONS
Our experience suggests that KDT is safe and effective in newborns and very young infants; however, additional studies on the management of the diet in this vulnerable age group are necessary.
In our 19 infants under 3 months of age with drug-resistant epilepsy on KDT, we found that 95% survived 1 month after initiating treatment and 76% at 1 year of follow-up with a trend toward higher mortality in patients with EIDEE.
It is important to consider the possibility of maintaining breastfeeding under close supervision and monitoring of AE that are mostly asymptomatic and manageable.
This group of very young infants has specific nutritional concerns which urge the need to define specific recommendations concerning caloric and protein needs for KDT treatment.
AUTHOR CONTRIBUTIONS
MA: Study design; data acquisition; data interpretation; writing of the original draft. SC: Data acquisition; writing of the original draft. MV, JA, DG, AC, RV, FV: Data acquisition. RC: Conception and design of the study; drafting and critically revising the manuscript. All authors read and approved the final submitted paper.
ACKNOWLEDGMENTS
We would like to thank Julia Minetto for her support in the statistical analysis and Janneke Deurloo for her assistance in writing and technical editing.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose relevant to this article. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL APPROVAL
The study was approved by the IRBs of the participating centers.