Electroencephalography–functional magnetic resonance imaging for clinical evaluation in focal epilepsy
Abstract
Objective
We aimed to evaluate the contribution of simultaneous recording of electroencephalography–functional magnetic resonance imaging (EEG-fMRI) in the diagnosis of epilepsy syndrome, localization of the epileptogenic zone (EZ), and decision-making regarding surgical treatment.
Methods
We performed a retrospective study to evaluate patients with focal epilepsy who underwent EEG-fMRI. Two evaluators assessed epilepsy syndrome, presumed focus, and surgical candidacy and defined confidence levels. They assessed these clinical characteristics first without EEG-fMRI and then including EEG-fMRI to assess how the results of EEG-fMRI changed the evaluations. We also determined how the clinical evaluation was affected by the concordance level between the blood oxygen level-dependent (BOLD) response and the presumed focus location, and by the confidence level of the BOLD response itself based on the t-value of the primary and secondary clusters.
Results
Fifty-one scans from 48 patients were included. The BOLD map affected 66.7% of the evaluations by altering evaluation items (epilepsy syndrome, presumed focus, or surgical candidacy) or their confidence levels. EEG-fMRI results increased the confidence levels of epilepsy syndrome, presumed focus, or surgical candidacy in 47.1% of patients but reduced clinical confidence in these features in 11.8%. More specifically, the confidence levels increased for epilepsy syndrome in 28.5%, identification of presumed focus in 33.9%, and determination of surgical candidacy in 29.4%. The BOLD signal confidence level, whether high or low, did not influence these clinical factors.
Significance
Previous studies have emphasized the utility of EEG-fMRI for the localization of the epileptogenic zone. This study demonstrated the potential of EEG-fMRI to influence clinical confidence when determining epilepsy syndrome, the presumed epileptic focus, and surgical candidacy.
Key points
- Simultaneous electroencephalography–functional magnetic resonance imaging (EEG-fMRI) recording is a noninvasive method to examine patients with epilepsy.
- EEG-fMRI can provide additional information for clinical assessment in the evaluation of focal epilepsy.
- Clinical evaluation was positively affected by EEG-fMRI in 47.1% of scans.
- EEG-fMRI increased the estimated confidence of surgical candidacy in 29.4%.
1 INTRODUCTION
Pharmacoresistant epilepsy accounts for 30% of epilepsy cases,1 and intractable seizures and various comorbidities are a heavy burden to patients.2 One treatment of pharmacoresistant focal epilepsy is the surgical removal of the epileptogenic zone (EZ).3, 4 To determine a treatment plan, clinicians investigate and consider many factors including age, clinical history, seizure semiology and frequency, drug responsiveness, cognitive function, electroencephalography (EEG), and the results of various brain imaging modalities such as magnetic resonance imaging (MRI), single-photon emission computerized tomography (SPECT), and positron emission tomography (PET). However, accurate identification and localization of the EZ can be challenging in some patients. For example, scalp EEG lacks spatial resolution and the interictal epileptiform discharges (IEDs) indicate the irritative zone, which does not always reflect the EZ.5 IEDs can propagate through the cortex6 and may occur contralateral to the epileptic focus.7, 8 Intracranial EEG (iEEG) is sometimes helpful but invasive and only covers a limited space.9 Simultaneous recording of EEG and functional MRI (EEG-fMRI) is a noninvasive method that has the potential to localize the EZ, yet its clinical use remains limited.10, 11 IED-related EEG-fMRI displays hemodynamic changes in blood oxygen level-dependent (BOLD) signals related to IEDs12, 13 often in the immediate vicinity of the intracranial EEG focus.14 One study reported the accuracy of EEG-fMRI in localizing the seizure-onset zone (SOZ) with stereo-EEG (SEEG) and found that 77% of patients had at least one study with primary EEG-fMRI clusters concordant with an SEEG-defined SOZ.15 Another study claimed that the spike-related BOLD response represented intrinsic connectivity networks.16 Several studies have proposed routine clinical use of EEG-fMRI in the pre-surgical evaluation of medically refractory epilepsy patients.17-21 However, these reports did not assess the added value of EEG-fMRI in a standard clinical evaluation.
Therefore, we aimed to determine how the addition of EEG-fMRI in the pre-surgical evaluation of medically refractory epilepsy patients impacts the diagnosis of the epilepsy syndrome, the localization of the epileptic focus, and the clinician's judgment regarding surgical candidacy of the patient. We retrospectively analyzed the changes that may be realized by adding EEG-fMRI images to the standard evaluation of the epileptic syndrome, the presumed focus, and surgical candidacy.
2 METHODS
2.1 Study population
We identified consecutive patients with focal epilepsy and sufficient clinical information for assessment (i.e., seizure semiology, EEG, MRI, PET, and neuropsychological assessment) from a database of patients who underwent EEG-fMRI from January 2014 to December 2016. At our institute, we generally consider performing EEG-fMRI in cases where the EZ is difficult to identify or ambiguous by standard localization methods. We reviewed the patient's EEG to check if there were frequent IEDs to determine the suitability for EEG-fMRI. Frequent IEDs were defined as more than 5–10/h. Therefore, the cases with an EEG study with few or no IEDs before scanning did not undergo an EEG-fMRI. Each participant provided written informed consent in accordance with the Research Ethics Committee of the Montreal Neurological Institute and Hospital.
2.2 EEG-fMRI acquisition
The imaging acquisition methods were as described in previous studies.15, 20, 22, 23 EEG was recorded inside a 3 Tesla Siemens Trio MRI scanner with 25 MR-compatible electrodes placed on the scalp, according to the 10–20 (reference FCz) and 10–10 (F9, T9, P9, F10, T10, P10) electrode systems, using a Brain Amp system (Brain Products, 5 kHz sampling). fMRI functional images were collected in 6-minute runs for a total scan time of 60–90 minutes with the patient at rest, using the following T2*-weighted echo planar imaging sequences: repetition time (TR), 1.9 seconds; echo time (TE), 25 ms; 64 × 64 matrix; 33 3.7 mm slices; 3.7 × 3.7 × 3.7 mm3 voxel; flip angle 90°. The IEDs were grouped into types, based on spatial distribution and morphology, and each type constituted a single study. IED types were spike, sharp wave, spike, and wave complex or paroxysmal slow wave, and those IEDs were identified as a burst event when it lasted longer than 1 s; otherwise, it was recognized as a single event. Thus, some patients had more than one study.
fMRI data were analyzed as an event-related design,24 each type of IED was built as a regressor, and all regressors were included in the same general linear model and convolved with four hemodynamic response functions (HRFs) peaking at 3, 5, 7, and 9 seconds.25 A statistic t-map was created for each regressor using the other regressors as confounders for each event type. A combined t-map was created by taking the highest t-value at each voxel from the four t-maps created with the four HRFs (t-value = contrast value divided by the standard error). A threshold at an uncorrected t > 3.1 was applied to the single combined t-map, and the responses with a t-value higher than the significance threshold corresponding to a corrected whole-brain topological false discovery rate (FDR) of 0.05 were identified.26
2.3 Clinical evaluation protocol
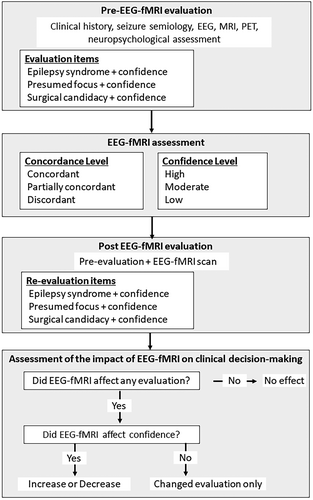
The evaluation pipeline is shown in Figure 1. The assessment consisted of a pre-EEG-fMRI evaluation, EEG-fMRI image evaluation, post-EEG-fMRI evaluation, and an assessment of the impact of EEG-fMRI on the clinical evaluation. First, we conducted a pre-EEG-fMRI evaluation according to the clinical history, seizure semiology, EEG, MRI, and PET. Two epilepsy experts independently evaluated the epilepsy syndrome, presumed epileptic focus, and surgical candidacy. SEEGs were included in the evaluation if they were performed within 1 year of the EEG-fMRI scan. Regarding epilepsy syndromes, patients were grouped into temporal lobe epilepsy (TLE), frontal lobe epilepsy (FLE), posterior-quadrant epilepsy (PQE), focal epilepsy, multifocal epilepsy, and undetermined. The definition of “focal epilepsy” was used in cases of suspected focal epilepsy where the region of presumed focus cannot be established, while “undetermined” was used when it cannot be determined whether there is focal or generalized epilepsy. Regarding the epileptic focus, the evaluators selected a lobe that seemed most likely to include the presumed focus from clinical information as mentioned above; patients were grouped into right (R), left (L), or bilateral (B), and grouped into frontal lobe, temporal lobe, posterior-quadrant region, multifocal, or undetermined location. Regarding surgical candidacy, patients were grouped into “yes” (a surgical candidate) or “no” (not a surgical candidate). For each item (epilepsy syndrome, presumed focus, and surgical candidacy), the confidence level was evaluated at three levels (high, moderate, and low). Definitions of estimated confidence level included: high, almost all information (clinical history, all examinations) was concordant with the diagnosis of epileptic syndrome and presumed epileptic focus; moderate, general information was concordant, some information was discordant; low, some information indicates the diagnosis, and the diagnosis is uncertain but more likely than others. Epilepsy syndrome, presumed focus, and surgical candidacy were “evaluation items” in this study.
Not all findings are consistent, and it is often unclear what type of information such as imaging, semiology, EEG, and other findings is most important. The decision on how to weigh the different, possibly contradictory factors reflects clinical judgment and experience. Some clear semiological signs may provide stronger evidence than uncertain imaging results, but the reverse can also occur. The combination of evidence is the essence of the expert's work.
The BOLD signal of EEG-fMRI was evaluated by the degree of concordance between the BOLD response and presumed epileptic focus of the pre-EEG-fMRI evaluation, using the following criteria: concordant, presumed focus and highest positive BOLD response in the same lobe; partially concordant, any BOLD response, including negative BOLD responses, intersected the presumed focus but the highest response was not in the same lobe as the presumed focus; discordant, no BOLD response intersected the presumed focus.
After the BOLD maps were visually inspected, a post-EEG-fMRI evaluation was performed, including epilepsy syndrome, presumed epileptic focus, and surgical candidacy, as for the pre-EEG-fMRI evaluation. We reported “no effect” when the level of confidence was unchanged between pre- and post-EEG-fMRI evaluations, and an “increase” when the BOLD map helped to increase evaluation confidence. “Increase” included instances when the estimated confidence level changed from low to moderate, low to high, or moderate to high in any evaluation item in epilepsy syndrome, presumed focus, or surgical candidacy. On the other hand, we reported a “decrease” if the estimated confidence level was changed upon EEG-fMRI evaluation from high to moderate, high to low, or moderate to low. We reported “changed evaluation only” when the confidence level was unchanged from pre- to post-EEG-fMRI evaluation, but at least one evaluation item was changed (for instance, focus location changed from temporal to frontal, without a change in confidence level).
2.4 Confidence level of EEG-fMRI image
2.5 Statistical analysis
Data were analyzed using SPSS (SPSS Inc.). Continuous variables were compared using the Mann–Whitney U-test, while categorical variables were compared using Pearson's chi-square test or Fisher's exact test. For all analyses, P < 0.05 was considered statistically significant.
3 RESULTS
3.1 Clinical profiles
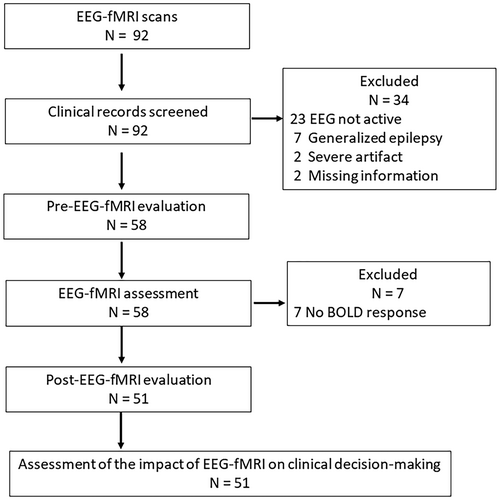
In total, 92 consecutive EEG-fMRI studies, performed on 89 patients from January 2014 to December 2016, were included in this study (Figure 2). Three patients underwent EEG-fMRI studies twice. Among the 92 studies, the following were excluded: 23 studies due to the absence of IEDs during the scan (25.0%), two due to the presence of severe artifacts, seven due to generalized epilepsy being diagnosed in the pre-evaluation, and two due to missing information. We excluded patients with presumed generalized epilepsy because this study emphasized the localization value of EEG-fMRI. Therefore, 58 studies from 55 patients were included in the EEG-fMRI evaluation. BOLD maps revealed that seven studies (12.1%) did not have a BOLD response above threshold. Finally, 51 studies from 48 patients were included in the evaluation (Table S1). The mean and standard deviation of the number of analyzed IEDs was 128.1 ± 216.6 (n = 51) in the group with a significant BOLD response, which was significantly higher than in the no significant BOLD response group with 23.7 ± 47.2 (n = 7) (P = 0.005).
The background of the patients was as follows: 15 males, 33 females; median age at the time of scanning, 28 years; median age at onset, 15 years. We performed an EEG and MRI on all patients. Thirteen patients had a pre-existing surgical resection with another intervention under consideration. Regarding the etiology of epilepsy, nine patients suffered from focal cortical dysplasia, five from periventricular nodular heterotopia, four from tumor, one from hippocampal sclerosis and 32 were unknown. SEEG was performed in 13 cases. The high proportion of unknown etiologies reflects that EEG-fMRI studies are most often requested in “difficult” patients.
3.2 Pre- and post-EEG-fMRI evaluation
The pre-EEG fMRI case evaluation was performed while considering standard elements of a phase 1 pre-surgical evaluation including EEG, PET, semiology, etc. It is noteworthy that some patients had undergone invasive studies and/or surgery prior to this assessment. The results of pre- and post-EEG-fMRI evaluation are given in Table 1A. The impact of EEG-fMRI results on the clinician's confidence level in identifying the epilepsy syndrome, the epileptic focus, and the patient's surgical candidacy are presented in Table 1B. Three cases are detailed next to demonstrate these findings.
| Pre A/B | Change post A/B | |
|---|---|---|
| Epilepsy syndrome | ||
| FLE | 10/15 | +5/+2 |
| TLE | 19/18 | −1/−2 |
| PQE | 5/4 | +2/+3 |
| Focal epilepsy | 12/7 | −7/−6 |
| Multi-focal epilepsy | 4/6 | +1/+3 |
| Undetermined | 1/1 | −1/−1 |
| Generalized epilepsy | +1/+1 | |
| Presumed focus | ||
| RF | 11/10 | −1/−2 |
| LF | 7/7 | 0/+2 |
| BF | 0/1 | 0/−1 |
| RT | 6/5 | 0/+2 |
| LT | 12/10 | −2/−4 |
| BT | 2/4 | 0/0 |
| RPQ | 3 /4 | −1/0 |
| LPQ | 2/1 | +1/+1 |
| BPQ | 1/0 | +1/+1 |
| Multi-focal | 6/6 | +1/+3 |
| Undetermined | 1/3 | 0/−2 |
| Surgical candidacy | ||
| Yes | 35/33 | −1/0 |
| No | 16/18 | +1/0 |
- Note: The numbers in the “Pre” cells are the results of the evaluation by Evaluator A/B. The numbers in the “Post” cells indicate the increase and decrease in the values compared to “Pre”.
- Abbreviations: BF, bi-frontal region; BPQ, bi-posterior-quadrant region; BT, bi-temporal region; EEG-fMRI, electroencephalography–functional magnetic resonance imaging; FLE, frontal lobe epilepsy; LF, left frontal region; LPQ, left posterior quadrant region; LT, left temporal region; PQE, posterior-quadrant epilepsy; RF, right frontal region; RPQ, right posterior-quadrant region; RT, right temporal region; TLE, temporal lobe epilepsy.
| Pre-EEG-fMRI evaluation | |||
|---|---|---|---|
| High | Moderate | Low | |
| Epilepsy syndrome | |||
| Post-EEG-fMRI evaluation | |||
| High | 8/9 | 8/3 | 0/0 |
| Moderate | 3/1 | 19/20 | 4/10 |
| Low | 2/1 | 3/3 | 4/4 |
| Presumed focus | |||
| Post-EEG-fMRI evaluation | |||
| High | 7/7 | 7/3 | 1/0 |
| Moderate | 1/0 | 13/12 | 8/16 |
| Low | 0/1 | 3/4 | 11/8 |
| Surgical candidacy | |||
| Post-EEG-fMRI evaluation | |||
| High | 14/11 | 3/5 | 0/1 |
| Moderate | 3/0 | 11/14 | 12/4 |
| Low | 0/0 | 0/4 | 8/10 |
- Note: The numbers in each cell are the results of the evaluation by Evaluator A/B.
- Abbreviation: EEG-fMRI, electroencephalography–functional magnetic resonance imaging.
Case 1 (Figure 3A) is an example in which EEG-fMRI increased the confidence level of the evaluators when assessing the various elements of the pre-surgical investigation. The patient was a 24-year-old woman with nocturnal seizures manifesting with hyperventilation and fear. Interictal EEG showed occasional spikes in the right frontal region (F4, Fz). Her MRI was non-lesional and ictal EEG was not recorded. Therefore, the evaluators assumed the presumed focus was right frontal with low-to-moderate confidence because her seizure semiology did not indicate any laterality, and there was little information indicating the localization of the EZ other than the location of IEDs. The BOLD map showed the maximum positive BOLD response in the right frontal region (peak t-value, 7.9), which was concordant with the clinical information. In the post-EEG-fMRI evaluation, both evaluators increased their confidence in the localization of the presumed focus and in surgical candidacy categories.
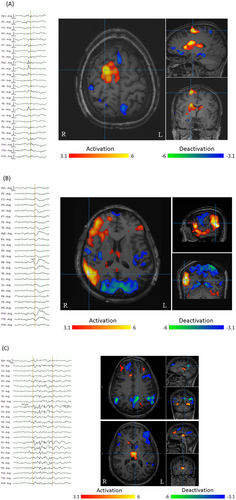
Case 2 (Figure 3B) is an example in which EEG-fMRI decreased the evaluators' confidence. The patient was a 35-year-old man with seizures manifesting with right oculo-cephalic deviation and vocalization. Interictal EEG showed spikes and fast activity mostly in the right frontal regions and rarely right temporal regions independently. His MRI was non-lesional and ictal EEG was not recorded. Therefore, the evaluators considered the presumed focus in the frontal region as the most likely possibility with a low confidence in laterality, but the maximum positive BOLD response was in the right posterior-quadrant and BOLD responses were widely scattered in other regions in the hemisphere. Therefore, the BOLD map raised doubts about the presumed focus and suggested the possibility of an EZ including a wider right hemispheric area, and thus lowered the confidence level of the presumed focus and surgical candidacy.
Case 3 (Figure 3C) is an example where the diagnosis of epilepsy syndrome was changed to generalized epilepsy due to the EEG-fMRI findings. The patient was a 27-year-old woman with seizures manifesting with right oculocephalic deviation as well as elevation and abduction of the right arm, occasionally evolving to focal to bilateral tonic–clonic seizures. Interictal EEG showed diffuse spikes or poly-spike and wave complexes predominantly seen in the bifrontal head regions. Her MRI was non-lesional. Ictal EEG was controversial because the only change prior to the seizure becoming bilateral tonic–clonic was a low-amplitude fast activity over bilateral frontal head regions. Therefore, the evaluators considered that the seizure semiology indicated focal epilepsy, particularly FLE; however, EEG findings indicated bi-hemispheric involvement. The pre-evaluation was focal epilepsy, but the negative BOLD response was in the default mode network (DMN) and the positive BOLD response was in the thalamus bilaterally. There were positive BOLD responses in the right mesial frontal cortex and bilateral dorsolateral frontal activations. Such features of subcortical regions are observed in generalized epilepsy.27 Considering the fact that the seizure semiology of generalized epilepsy can show focal manifestations, the BOLD map raised the possibility of changing the diagnosis to generalized epilepsy and decreased the confidence level of surgical candidacy.
3.3 EEG-fMRI effect on the evaluation
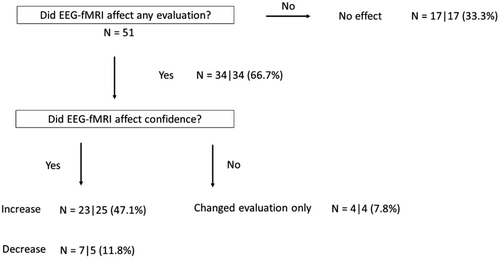
The main results are shown in Figure 4. Of the 51 studies completed, EEG-fMRI results influenced the clinician's confidence level in 66.7% of cases. In 47.1% of studies, the confidence level was increased, and in 11.8% of studies, the confidence level was decreased. There was a change in evaluation items without a change in confidence level in 7.8% of cases. The confidence level was increased with regards to the classification of epilepsy syndrome in 28.5%, identification of presumed focus in 33.9%, and determination of surgical candidacy in 29.4%. (These figures include duplicates, i.e., cases with the increase of confidence level on two or more evaluation items). The results of surgical candidacy in pre- and post-EEG-fMRI are shown in Table 1A,B. The BOLD map increased the confidence of surgical candidacy in 29.4% of cases (percentage of increased confidence cases in the “yes” group), and 17.7% were confirmed as poor surgical candidates (percentage of increased confidence cases in the “no” group), due to the uncertain epileptic focus localization or the BOLD map demonstrating the presence of multiple foci.
3.4 BOLD signal concordance
The distribution of BOLD signal concordance with the presumed focus was as follows: 27.5% were concordant; 43.2% were partially concordant; 28.5% were discordant. Among the concordant group (concordant and partially concordant), 59.5% showed increasing confidence in any of the evaluation items; among the discordant group, 18.2% showed decreasing confidence. The mean and standard deviation of the number of IEDs in all studies was 128.1 ± 216.6. The mean and standard deviation of the number of IEDs in the concordant group were 126.1 ± 229.5 and 134.4 ± 178.6 in the discordant group. There was no significant group difference in the number of IEDs (P = 0.90).
3.5 Confidence level of BOLD response
Confidence levels of the BOLD maps were analyzed (reflecting the strength of the BOLD response); 32 BOLD maps had high BOLD signal confidence, and 19 BOLD maps had low signal confidence. Among the 32 maps in the high confidence group, 20 were positive and 12 were negative BOLD response. Among the 19 maps in the low confidence group, 12 were positive and seven were negative. There was no association between these positive and negative BOLD responses and etiology. The increases in the confidence level in the clinical assessment were found in 54.7% of the high BOLD signal confidence group and in 34.2% of the low BOLD signal confidence group. There was no significant difference between these two proportions. We evaluated the relationship of the number of IEDs to the BOLD signal confidence: high confidence group, mean 150.6 ± 268.6; low confidence group; mean 91.3 ± 110.3. There was no significant group difference in the number of IEDs (P = 0.28).
4 DISCUSSION
In this retrospective simulation study, we determined how EEG-fMRI may affect clinical evaluation and decision-making regarding the diagnosis of epilepsy syndrome, localization of presumed epileptic focus, and surgical candidacy in focal epilepsy. The results are likely influenced by the perceived clinical value that the evaluators attribute to EEG-fMRI results. A similar situation arises whenever one considers the contribution of a new diagnostic method to standard practice. The gold standard for such an evaluation would be a randomized control trial; however, this is rarely done when introducing new localization methods for epilepsy due to high cost and complexity. The evaluators' confidence in the method was based on the literature on EEG-fMRI, which showed the frequent but far from perfect concordance of BOLD responses with epileptic foci28 as well as personal experience in their clinical practice. A similar methodology has been used in evaluating EEG-fMRI for surgery,29 the contribution of EEG source analysis,30-32 resting-state functional connectivity,33 and interictal PET findings.34
The current study demonstrates that EEG-fMRI provides additional value when combined with traditional pre-surgical investigations of medically refractory epilepsy by increasing confidence in the identification of epilepsy syndrome, localization, and surgical candidacy. In the 51 studies where BOLD maps were used for evaluation, clinical confidence was increased in 47.1% of cases, which is significant. The clinical confidence was reduced in a minority of cases (11.8%) (Figure 4). A PET study reported comparable results: PET contributed to the presurgical evaluation and decision-making process in 47% of cases with drug-resistant focal epilepsy.34 Additionally, the fact that the two evaluators in this study had very similar results reinforces the potential clinical applicability of EEG-fMRI in the context of the evaluation of focal epilepsy and surgical candidacy.
4.1 EEG-fMRI effect on epileptic syndrome and localization
Approximately 50% of EEG-fMRIs added confidence regarding epileptic syndrome, presumed focus, and/or surgical candidacy in the present study. A previous report shows that EEG-fMRI contributed to the localization of the epileptic focus in half of the patients in comparison to EEG alone.11 Another study reported that EEG-fMRI was concordant with the spike location of 8/9 patients and it may provide different types of information supporting topography, concordance with PET and SPECT, and structural peculiarities for patients with non-lesional FLE.18 We used several modalities in addition to EEG in the pre-evaluation, and we confirmed that EEG-fMRI could add information to a relatively standard clinical evaluation.
4.2 EEG-fMRI effect on surgical candidacy
EEG-fMRI increased the estimated confidence of surgical candidacy in 29.4% of patients. A study by Kowalczy et al29 reported that EEG-fMRI results critically influenced the decision to offer surgery in 17% (10/59) of the patients in their cohort. This led to resection in one patient and opened new prospects of surgery in three others. Their results align with our study. On the other hand, there is some evidence10, 35 that EEG-fMRI supports the classification of patients as non-surgical candidates in cases when the BOLD response has multiple activation regions. For example, a case in which EEG-fMRI indicated the possibility of multiple EZs was not considered a surgical candidate and would not be reflected in previous studies of surgical outcome. Our study shows that 17.7% of cases were no longer considered surgical candidates, due to uncertainty of the focus or the presence of multiple foci after this was clarified by EEG-fMRI. Therefore, EEG-fMRI may be useful for the localization of the epileptic focus prior to surgical treatment but also in the generation of supportive information for exclusion of a surgical candidate.
4.3 Confidence level of BOLD response
We assessed the confidence of the BOLD response according to our previous report.23 A formula was used to classify the images into high and low BOLD signal confidence. In our previous study, if the region of the peak response of the high confidence level was not resected, no patient had a good outcome. For medium and low confidence levels, only 18% and 28% had a good outcome, respectively. The authors concluded that the negative predictive value remained high for all confidence levels, and that resection of the primary cluster is necessary but not sufficient to obtain a good outcome because the positive predictive value was low.23 In the present study, the concordance and confidence level of the BOLD response did not affect the results. The reason may be that some cases of concordant BOLD responses only confirmed the evaluation but did not change it, whereas some discordant cases were considered to have provided important information resulting in a changed evaluation. This suggests that EEG-fMRI may be useful whether or not the BOLD response is concordant with the presumed focus. Another reason may be the limitation of the evaluation of the level of confidence, which is calculated by the difference of t-values between primary and secondary clusters, since even secondary clusters were consistent with EZ in previous reports.22, 36
4.4 Limitations
The key limitation of our study is that it was a retrospective single-center study. A prospective randomized study is desirable, and postoperative outcomes may be a good indicator of whether the EEG-fMRI results lead to better EZ localization and better surgical decisions. A single-center study may contain bias with respect to the patients' background. There may be a selection bias affecting our results since EEG-fMRI is typically requested in complex patients. However, this bias, which exists in all surgical candidacy studies, is seemingly unavoidable. Our successful EEG-fMRI rate (55.4%; 51/92) was similar to that of another study (50%).29 In our investigation, 25% of studies reported inactive IEDs during recording and 7.6% were excluded due to the absence of a significant BOLD cluster. A previous report excluded 46% of scans due to a lack of epileptic activity in the scalp EEG during the recording.29 A lower rate of exclusions in our study may be due to patient selection. Performing a scalp EEG is important to determine whether a patient has active or inactive IEDs, and long-term EEG recording assists in this conclusion.29 At our institute, inactive IED patients were not EEG-fMRI candidates, and this different selection of patients may have affected our results. Conventional IED-related EEG-fMRI is only analyzed when IEDs are recorded during scanning; thus, one of the limitations of EEG-fMRI is that it is only adapted to patients with active IEDs.37 The number of BOLD responses appears to correlate to the number of IEDs analyzed with fewer BOLD responses recorded in patients with few IEDS and vice versa, a phenomenon previously reported in the literature.37 Our results were similar, with significantly fewer IEDs in the no BOLD group than in the group with BOLD responses.
Although not critical for positive identification of the EZ, a test with high negative predictive value could improve patient selection of surgical candidacy, decreasing cost and exposure to unnecessary risk of surgery by this non-invasive technique. Thus, the strength of this study is that it included cases that were not considered for surgery, and it assessed both the increase and decrease of the confidence level by adding EEG-fMRI to a standard clinical evaluation for focal epilepsy.
5 CLINICAL RELEVANCE/FUTURE DIRECTIONS
Previous studies have advocated for routine clinical use of EEG-fMRI when investigating medically refractory epilepsy, but the impact of this noninvasive modality on clinical decision making had not been previously explored. The current study revealed that EEG-fMRI can increase the confidence level of the identification of the epilepsy syndrome, localization, and/or influence surgical candidacy in nearly half the cases, whereas it decreases the clinician's confidence in assessing these factors in only 11.8% of cases. Additionally, EEG-fMRI increased the estimated confidence of surgical candidacy in 29.4% of studies while proposing to exclude surgery in 17.7% of cases. This study demonstrated the potential of EEG-fMRI results to influence clinical assessment and surgical candidacy. Further studies are needed to confirm these findings, which are nevertheless encouraging.
ACKNOWLEDGMENTS
SI was supported in part by the Uehara Memory Foundation. This work was supported by Grant FDN 143208 of the Canadian Institutes of Health Research.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICS STATEMENT
Each participant provided written informed consent in accordance with the Research Ethics Committee of Montreal Neurological Institute and Hospital.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.