Progressive myoclonus epilepsies due to SEMA6B mutations. New variants and appraisal of published phenotypes
Barbara Castellotti and Laura Canafoglia equally contributed to the study.
Abstract
Variants of SEMA6B have been identified in an increasing number of patients, often presenting with progressive myoclonus epilepsy (PME), and to lesser extent developmental encephalopathy, with or without epilepsy. The exon 17 is mainly involved, with truncating mutations causing the production of aberrant proteins with toxic gain of function. Herein, we describe three adjunctive patients carrying de novo truncating SEMA6B variants in this exon (c.1976delC and c.2086C > T novel; c.1978delC previously reported). These subjects presented with PME preceded by developmental delay, motor and cognitive impairment, worsening myoclonus, and epilepsy with polymorphic features, including focal to bilateral seizures in two, and non-convulsive status epilepticus in one. The evidence of developmental delay in these cases suggests their inclusion in the “PME plus developmental delay” nosological group. This work further expands our knowledge of SEMA6B variants causing PMEs. However, the data to date available confirms that phenotypic features do not correlate with the type or location of variants, aspects that need to be further clarified by future studies.
1 INTRODUCTION
Progressive myoclonus epilepsies (PMEs) are a group of rare, clinically and genetically heterogeneous diseases, occurring mainly in childhood or adolescence, presenting with cortical myoclonus, generalized tonic-clonic seizures, and progressive neurological decline with variable severity.1
In addition to PMEs described in case series with different genetic causes,2, 3 the recent advances in next-generation sequencing (NGS) allowed to discover further pathogenic variants in genes not previously associated with the PME phenotype.4 Among these, truncating mutations in the SEMA6B gene (NM_032108.4) were first described in five unrelated patients with PME.5 SEMA6B maps to chromosome 19p13.3; it encodes for a transmembrane protein member of the semaphorin family, playing a role in the early development of the central nervous system, affecting axonal guidance by interacting with neuropile receptors.6 Twenty-nine patients have been reported so far, showing non-homogeneous phenotypes, ranging from developmental encephalopathy (DE) without epilepsy to developmental and epileptic encephalopathy (DEE) or PME.7-10
With the aim of sharpening the distinctive features of the PME phenotype and to compare our observations with the available literature; here, we present the genetic and clinical characteristics of three adjunctive patients with PME, carrying different truncating SEMA6B pathogenetic variants, two of which are novel.
2 METHODS
2.1 Genetic analysis
Experimental protocols were approved by the Institutional Review Boards of the Fondazione IRCCS Istituto Neurologico Carlo Besta of Milan, Italy. Written informed consent was obtained from patients and their parents. Genomic DNA was prepared from peripheral blood lymphocytes using standard procedures. NGS panel (Agilent Sure Design, Santa Clara CA USA) for the analysis of 300 genes (list of genes available as Appendix S1) was applied to 155 patients with undetermined DEE and PME. The average coverage at 20× for this panel was 99%. The resulting sequences were aligned to the reference genome GRCh37/hg19 (MiSeq software). Data analysis was obtained using MiSeq Reporter vs 2.4.60, Variant Studio vs 2.2 (Illumina) and CLC Genomics Workbench vs 7.0 (Qiagen). Variants with MAF > 1% were considered benign. Other variants were classified according to ACMG criteria.11 The parental segregation was performed by direct sequencing using ABI 1330 XL Applied Biosystems automatic sequencer (Life technologies).
2.2 Data collection and reviewof the literature
We collected the main clinical features of the patients here reported and compared with those previously described. To perform the literature review, we screened the PubMed database (https://pubmed.ncbi.nlm.nih.gov) for SEMA6B variants and associated phenotypes. We extracted the following information: type of mutation/exon, earliest sign(s), phenotype features, seizure’ type, response to treatment, presence of cognitive regression, ataxia, spasticity/pyramidal signs, intention tremor, myoclonus, electroencephalographic (EEG) pattern, somatosensory evoked potentials (SEPs), brain magnetic resonance image (MRI) findings. We added a classification distinguishing the following syndromic groups: PME, DEE, DE without epilepsy, or epilepsy as a unique symptom.
3 RESULTS
3.1 Case reports
3.1.1 Patient 1
25-year-old male with congenital cryptorchidism. This patient showed mild early psychomotor delay with autonomous walking at 2 years, with first words pronounced at 14 months. He presented the first seizure at the age of 29 months, characterized by clonic movements of the right arm evolving to bilateral; 3 months later reported non-convulsive status epilepticus (NCSE). Treatment with valproate (VPA) led to complete seizure freedom for 6 years; thereafter, he presented sporadic non-convulsive seizures with loss of awareness, occasionally followed by head version.
During the second decade of life, the patient developed cognitive and motor regression, becoming wheelchair-bound from the age of 14. Action myoclonus was recognized when he was 11 years old and progressively worsened, despite multiple therapeutic attempts. At last neurological examination (25 years old), he showed severe cerebellar ataxia, marked spasticity, and dysarthria. He was able to interact only with a few stereotyped sentences.
Serial EEGs performed since 3 years of age showed diffuse slowing of background activity, with frequent multifocal epileptic discharges. Over the years, EEGs showed mild increase of epileptic abnormalities and occurrence of photo paroxysmal response (PPR). EEG polygraphy revealed myoclonic jerks occurring spontaneously in association with diffuse spike and wave discharges or movement-activated, without obvious EEG changes. At 25 years, SEPs showed delayed and attenuated cortical responses, with enhanced C-reflex. Repeated MRIs (from the age of 3 to 25 years) were normal.
NGS panel identified a heterozygous deletion of one nucleotide (c.1976delC) in exon 17, resulting in the slippage of the reading frame, leading to the formation of a premature stop codon 26 amino acids downstream the deletion (p.Ala659GlyfsTer26) (Figure 1). This variant is not reported in databases of patients and control subjects (https://gnomad.broadinstitute.org/); prediction tools indicated its damaging effect. Segregation analyses excluded the variant in the healthy father, while it was not possible to analyze the DNA of the mother who died of cancer, but was reported healthy until the age of 49.

3.1.2 Patient 2
16-years-old female. First observed at 11 years, she presented speech delay and learning disability (TIQ = 72), with normal motor development. First tonic-clonic seizure presented at 11 years old, followed by a second episode 2 months later. EEGs showed slow background activity, bilateral posterior spikes, diffuse spike-wave discharges, occasionally associated with absence seizures (Figure 2A), with PPR at 10-17 Hz intermittent light stimulation (Figure 2B). Repeated brain MRIs (12 and 15 years old) were normal. Treatment with VPA allowed complete seizure freedom. Mild jerky movements were present from the first year of life. However, at the age of 14 years, they became disabling, interfering with motor activity and walking. EEG polygraphic recording identified repeated myoclonic jerks during active movements, mixed with brief lapses of tone (Figure 2C); jerk-locked back averaging indicated their cortical origin (Figure 2D). SEPs showed an increased amplitude of the cortical responses. Clonazepam added to VPA resulted in mild improvement of myoclonic jerks.
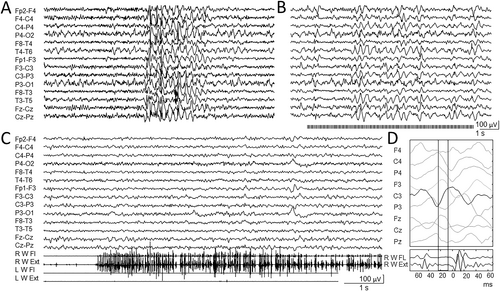
NGS genetic screening identified the novel de novo heterozygous nucleotide substitution c.2086C > T in exon 17, resulting in the formation of a premature stop codon (p.Gln696Ter). This variant is not present in the Gnomad database; segregation analysis ruled out the presence of the variant in both parents (Figure 1).
3.1.3 Patient 3
30-year-old female with mild motor delay, with some “babble” pronounced at 25 months. Subsequently, she had limited verbal production, but maintained partial language comprehension. At 11 months, the patient presented a first febrile convulsion, followed by rare, generalized tonic-clonic episodes, and daily atonic and absences seizures. She also had focal seizures with head version and loss of awareness. Despite several changes of antiseizure medications (including phenobarbital and VPA), she never reached seizure control. The patient developed progressive ataxia and worsening myoclonus, leading to a wheelchair since the age of 21. At our last observation (30 years old), she showed severe intellectual disability, ataxia, and severe action myoclonus affecting limbs, axial and face muscles, and negative myoclonic component.
Repeated EEGs showed slow background activity, diffuse slow activity, and diffuse epileptic discharges. Brain MRI (22 years) was normal, SEPs (23 years old) showed increased latency but normal amplitude of the cortical responses.
NGS genetic assessment identified the heterozygous de novo deletion c.1978delC on exon 17, already reported.10 This results in the reading frame slippage, leading to the formation of a premature stop codon 25 amino acids downstream the deletion (p.Gln660ArgfsTer25) (Figure 1).
3.2 Review of the literature
As far as we know, until now 29 patients with 22 different SEMA6B variants have been described, 18 of which within exon 17.4, 5, 7-10, 12, 13 SEMA6B variants include 5 missense, 5 nonsense, and 12 frameshift mutations (Table S1).
Seizures were present in 25 of 29 patients, including two with non-convulsive status epilepticus (NCSE), with variable age of onset between 11 months to 10 years. Sixteen patients had a progressive impairment of motor ability; 10 cases reported ataxia and spasticity. In 10 subjects severe cognitive regression with loss of speech ability was reported.
Sixteen patients had myoclonic jerks. Of these, only 10 had a clear-cut PME phenotype with prominent action-activated myoclonus. The remaining six cases presented myoclonic jerks in the context of polymorphic seizures, suggestive of DEE (Lennox-Gastaut or epilepsy with myoclonic atonic seizures). Three patients showed a milder phenotype, in one case characterized only by epilepsy.8, 10 The graphic in Figure 1 shows the genotype-phenotype correlation in all the patients with SEMA6B variants (including those here reported). Frameshift/nonsense amino acidic alterations were more frequently associated with PME phenotype; however, few missense variants were reported so far. Those occurring in more than one subjects showed a variable association with distinct syndromic groups, according to the classification reported in Table S2.
4 DISCUSSION
SEMA6B variants are associated with a wide phenotypic spectrum, ranging from DE without epilepsy to DEE. However, a relatively frequent presentation is a pure PME phenotype, previously defined by the Marseille Consensus Group,1 characterizing the three patients here reported and the other 10 present in the literature. PME phenotype followed developmental delay, was associated with a progressive moderate-to-severe intellectual disability and motor regression due to both myoclonus worsening and additional neurological deficits. In the patients here reported, neurological and cognitive impairment was variable: severe in patients 1 and 3, and mild in case 2, showing a neurological picture milder than previously observed.
In two of the subjects here described, similarly to other cases previously reported, seizures were polymorphic, including both generalized and focal episodes. Accordingly, epileptic abnormalities at the EEG were both diffuse and multifocal; PPR was present in two cases. SEPs presented variable amplitude (either increased or attenuated), probably in relation to the disease stage, since elder patients had attenuated responses.
Based on the discovery of several new genes associated with PME, a novel classification was proposed.4, 14 Specifically, the presence of cognitive delay preceding the PME phenotype identified the nosological group of “PME plus developmental delay,” which could include PMEs associated with SEMA6B mutations.
Similarly to the majority of the previously reported cases, the three subjects here reported have pathogenetic variants located in the last exon of SEMA6B, leading to truncated proteins. Hamanaka et al.5 hypothesized that this type of variants could cause the escape from nonsense-mediated mRNA decay. Thus, the translation of truncated proteins may have a toxic effect or alter semaphorin signaling. The different severity of the PME phenotype observed in the patients here reported did not correlate with the type nor with the localization of the mutations. Moreover, by the revision of literature, severity does not clearly correlate with genotype, and patients with the same genetic alteration could present different phenotypes.
In conclusion, our observation confirms the causative role of SEMA6B mutations in PME plus developmental delay. The presence of different clinical presentations indicates a variable relationship between the genetic alterations and the resulting phenotype that need further studies to be clarified.
AUTHOR CONTRIBUTIONS
B.C, L.C., E.F, and S.F. were involved in the conception and design of the study, in the acquisition and analysis of data, drafted a significant portion of the manuscript and figures and critically reviewed the manuscript. M.T., G.M., S.M, J.C.D., M.F., C.D.B, A.M., T.G., C.G., and R.M. were involved in the acquisition and analysis of data, and critically reviewed and revised the manuscript.
ACKNOWLEDGMENTS
The present work was supported by the Italian Ministry of Health Project Ricerca Finalizzata Giovani Ricercatori GR-2016-02363337 to JCD and SM; RF-2019-J45F21000050001 to BC and supported by the Italian Ministry of Health (RRC). Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. All the authors have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.




