Identification and clinical characteristics of a novel missense ADGRG1 variant in bilateral Frontoparietal Polymicrogyria: The electroclinical change from infancy to adulthood after Callosotomy in three siblings
Abstract
Objective
Bilateral frontoparietal polymicrogyria (BFPP) is a rare genetic-related migration disorder. It has been attributed to loss-of-function of the ADGRG1 gene, which encodes an adhesion G protein-coupled receptor, ADGRG1/GPR56. We report the EEG findings of BFPP in three Asian patients, and confirmed that change in protein function was caused by the novel missense variant (p.Leu290Pro).
Methods
We reviewed the medical records of three siblings with BFPP including one elder girl and two identical twin boys from birth to adulthood. The clinical symptoms, electroencephalography (EEG), brain MRI, whole-exome sequencing, treatment including medications, neuromodulation, and epilepsy surgery, and clinical outcomes were reviewed. The protein structure of a novel missense variant (p.Leu290Pro) was predicted by in silico studies, and molecular analysis was performed via typical flow cytometry and Western blotting.
Results
The elder girl (Patient 1) was 22 years old and the twin boys (Patients 2 and 3) were 20 years old at the time of publication. All of them presented with typical clinical symptoms/signs and MRI findings of BFPP. Whole-exome sequencing followed by Sanger confirmation showed that all three patients had compound heterozygous variants in the ADGRG1 gene. The missense variant (p.Leu290Pro) was confirmed to be related to a reduction in cell surface GPR56 expression. High-amplitude rhythmic activity was noted in sleep EEG during infancy, which may have been due to excessive sleep spindle, and the rhythm disappeared when they were of pre-school age. Partial callosotomy provided short-term benefits in seizure control in Patients 1 and 2, and combined vagus nerve stimulation and partial callosotomy provided longer benefits in Patient 3.
Significance
Sleep EEG findings of high-amplitude rhythmic activity in our BFPP cases were only noted during infancy and childhood. We also confirmed that the missense variant (p.Leu290Pro) led to loss of function due to a reduction in cell surface GPR56 expression.
Key Bullet Points
- The first BFPP case series in Taiwan, and the first 20 years follow-up report in BFPP.
- A special sleep EEG finding of high-amplitude rhythmic activity was found in the childhood period of BFPP patients before clinical seizure attack.
- A new loss of function missense ADGRG1 Variant (L290P) was proofed in three BFPP patients.
- Corpus callosotomy promised excellent short-term benefit in epileptic drop attacks of BFPP patients and it combined with vagus nerve stimulation promised extra benefits.
- Identical twins in BFPP patients could develop different seizure clinical course.
1 INTRODUCTION
Polymicrogyria is a heterogeneous disease with diverse etiologies and clinical manifestations, ranging from nearly normal to severe disability. The etiologies, distribution of polymicrogyria, and association with other congenital central nervous system anomalies can all influence the clinical outcomes.1 Bilateral frontoparietal polymicrogyria (BFPP) is an autosomal recessive genetic disease of cortical malformation caused by ADGRG1/GPR56 gene deletion/mutation.2 ADGRG1/GPR56 belongs to the adhesion-class G-protein-coupled receptors (aGPCRs), which are characterized by an extended extracellular region (ECR).3 The N-terminal section of the aGPCR-ECR is normally composed of diverse cell-adhesive protein modules, whereas its C-terminal section consists of a signature GPCR autoproteolysis-inducing (GAIN) domain.4 As a result, most aGPCRs are processed by self-catalytic proteolysis at the consensus GPCR proteolysis site (GPS) of the GAIN domain to form a bipartite receptor complex comprised of an extracellular fragment and a seven-span transmembrane fragment.4 Several types of ADGRG1/GPR56 genetic alterations including deletion, splicing, and missense mutations have been linked to BFPP.5 Interestingly, most BFPP-associated GPR56 missense point mutations have been identified at the ECR or extracellular loops. Moreover, all GPR56 missense variants are essentially null mutants, although they are functionally deficient due to diverse mechanisms including protein instability and stalled intracellular trafficking, inefficient GPS auto-proteolytic modification, and impaired receptor signaling.6, 7 BFPP is thought to share common clinical symptoms and brain imaging characteristics with other glycosylation disorders, such as Walker–Warburg syndrome, muscle-eye-brain disease, and Fukuyama congenital muscular dystrophy.8 The clinical symptoms include developmental delay, impaired intelligence, disconjugate gaze, oral motor dyspraxia, pyramidal, cerebellum signs, and multiple types of epilepsy.9 The brain imaging characteristics of BFPP include white matter lesions and hypo/dysgenesis of the brainstem and cerebellum, which are not seen with other types of polymicrogyria.8 While the central nervous system structure anomalies described above remain mostly constant, the electroclinical symptoms usually evolve with age. However, detailed records of the evolution of the electroclinical symptoms in BFPP patients from birth to adulthood are limited. In addition, the effect of corpus callosotomy on seizure control in BFPP patients has not been investigated systematically.
Herein, we report the progression of changes in electroclinical patterns from infancy to adulthood and the effect of corpus callosotomy on seizure control in three siblings with BFPP in Taiwan. We identified and characterized a novel BFPP-causing ADGRG1/GPR56 missense mutation, and showed that it was deficient in GPS proteolysis, intracellular trafficking, and cell surface expression.
2 MATERIALS AND METHODS
2.1 Clinical symptoms, signs, and medication records
We analyzed the electronic health records of three related patients with BFPP including one elder girl (Patient 1) and two identical twin boys (Patients 2 and 3) from 1 year 3 months of age and 14 days of age to 22 and 20 years of age, respectively. All three patients were followed up at our outpatient department every 3 months for prescription refills. Data on clinical symptoms/signs, electroencephalography (EEG), brain magnetic resonance imaging (MRI), whole-exome sequencing, treatment records including medications, neuromodulation and epilepsy surgery, and clinical outcomes were recorded. Structural prediction and molecular analyses of the novel missense ADGRG1/GPR56 variant (p.Leu290Pro) were performed using computational algorithms and biochemical and cellular techniques, respectively.
2.2 Brain MRI
MRI was performed at the time of initial diagnosis (1 year of age in Patient 1, and 1 month of age in Patients 2 and 3), follow-up, and post-callosotomy (19 years of age) (Figure S1). MRI was performed using 1.5 T (Magnetom, Siemens), 3 T MRI, with a slice thickness from 3 to 7 mm. The initial brain MR images during infancy were previously reported in 2000,10 with the impression of genetic-related polymicrogyria.
2.3 EEG
EEG was obtained using a Natus Nicolet monitor at 1, 5, 9, and 19 years of age in Patient 1; at 3, 9, 10, and 18 years of age in Patient 2; and at 4, 5, 6, 7, 8, 10, and 19 years of age in Patient 3 (Figure S1). The examinations were performed using silver-chloride, gold-plated electrodes placed according to the 10-20 International system, with recorded impedances of less than 5 kΩ at all electrodes. Routine and long-term video monitoring were recorded as bipolar montage and A1A2 reference montage. Initial analog signal conditioning included a 0.0-0.1 Hz high-pass filter, a 30-70 Hz low-pass filter, and a 60 Hz notch filter. The digital sampling rate was 200-500 per second. The patients were sedated with chloral hydrate oral solution during sleep EEG. Sleep and awake EEG were recorded for 30 minutes.
2.4 Genetic analysis
Whole-exome sequencing (WES)11 followed by Sanger confirmation was performed in Patient 1 at the age of 19 years, and in Patients 2 and 3 at the age of 18 years.
2.5 Structural prediction and molecular analysis of the novel missense ADGRG1 variant
In silico studies of DynaMut,12 DynaMut213 and mCSM-membrane14 were used to predict changes in Gibbs free energy (ΔG) and differences in vibrational entropy (ΔSvib) of several reported BFPP-associated GPR56 mutant proteins compared to those of the GPR56-WT and GPR56-L290P receptors. For the biochemical and molecular characterization of the GPR56-L290P protein, typical flow cytometry and Western blotting analyses were performed, and the results were compared with GPR56-WT and other BFPP-associated point mutants using anti-GPR56 CG2 and CG4 mAbs to verify the potential disease-causing mechanism as described previously.7
3 RESULTS
3.1 Clinical manifestation
The three siblings are offspring of non-consanguineous parents with one elder sister (Patient 1) and two identical twin younger brothers (Patients 2 and 3) (Figure 1A). There was no family history of major congenital malformations, epilepsy, developmental delay, mental retardation, or any other developmental encephalopathies. All family members of the patients were Taiwanese. The pregnancy and delivery of these patients were uneventful, and they were all born at 36 weeks of gestational age via cesarean section due to breech position and twin pregnancy, respectively. The clinical courses of the three siblings were similar, including a healthy newborn period, and first symptoms of motor and language delay during infancy. Severe mental retardation, diplegic spasticity, paroxysmal nystagmus, and seizures developed during the childhood period. Strabismus was only noted in Patient 1, and ataxia was only noted in Patient 3. All could walk independently at around 1.5 years of age and spoke their first meaningful words by the age of 2. The elder sister had one unique symptom of irritable crying every night from infancy to childhood, which has not been mentioned in previous case reports. This symptom was suspected to be a sleep-related problem, such as NREM parasomnia. Only Patient 3 had progressive hydrocephalus, with an increased head circumference, bulging anterior fontanelle, lethargy, and projectile vomiting at 3 months of age. A ventriculo-peritoneal shunt was subsequently inserted. Head drop developed at 9, 3, and 1 year of age in Patients 1, 2, and 3, respectively. Epilepsy was well controlled with monotherapy of clonazepam, phenobarbital, and valproic acid, respectively, for years. However, refractory epilepsy developed in all three siblings at 11, 12, and 8 years of age, and many types of seizures developed, including tonic, myoclonic, staring, and generalized tonic–clonic. Finally, epileptic falling seizures occurred about a hundred times per day by 18 years of age in all three patients. Several anti-seizure medications, including vigabatrin, clobazam, oxcarbazepine, zonisamide, perampanel, rufinamide, topiramate, lacosamide, levetiracetam, and lamotrigine were tried, but none provided long-term benefits. Vagus nerve stimulation (VNS) was only performed in Patient 3 at 12 years of age, with a maximum setting of 1.5 mA, after which both seizure frequency and cognitive power improved temporarily for approximately 1 year. Palliative anterior two-thirds corpus callosotomy was performed at 20, 18, and 19 years of age, respectively, after which the epileptic falling seizures disappeared totally. However, recurrent epileptic falling seizures gradually occurred at about 1 year post-operatively in Patients 1 and 2. In contrast, Patient 3 remained free from epileptic falling seizures 1.5 years post-operatively (Table S1).
3.2 Brain imaging analysis
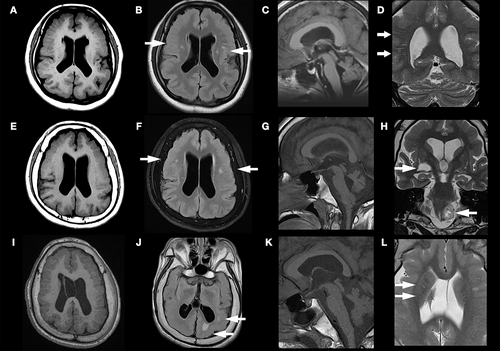
The first brain MRI was obtained at 1 year of age in Patient 1, and at 2 months of age in Patients 2 and 3 (Figure S1). Diffuse thickening of the cortical gray matter with abnormally small, crowded gyri in the bilateral frontoparietal lobes were observed in all patients, compatible with BFPP. Persistent cavum septum pellucidum and cavum vergae only presented in Patient 3. Follow-up brain MRI at 17, 18, and 19 years of age in Patient 1 (Figure 2A-D), 2 (Figure 2E-H), and 3 (Figure 3I-L) showed stationary BFPP and ventriculomegaly (Figure 2A,E,L). Multiple T2 hyperintense nodules in subcortical white matter were also noted in all three patients (Figure 2B,F,J). The nodular lesions were first seen in the brain MRI of Patients 2 and 3 at 2 and 3 years of age. The location and size of these nodular white matter lesions remained unchanged in subsequent MRI scans. There were also multiple T2 hyperintense straight lines following the orientation of penetrating arterioles at the corona radiata (Figure 2D) in Patient 1, and at the basal ganglion (Figure 2L) in Patient 3. Enlarged perivascular space was impressed. A flattened pons and small cerebellar vermis were present in all patients, and were most severe in Patient 2 (Figure 2C,G,K). One tumefactive perivascular space (1 x 1.4 cm) was found at the right inferior basal ganglion (Figure 2H), and a T2 hyperintense mass with central necrosis (2.3 x 1.5 cm) at the left peri-medullary cistern, thought to be a schwannoma, was found in Patient 2 (Figure 2H). A small cystic cavity was also noted in the right frontal periventricular white matter in Patient 1 (Figure 2A,B).
3.3 EEG analysis
Sleep EEGs were obtained at 1 and 5 years of age in Patient 1; at 3, 9, and 10 years of age in Patient 2; and at 4, 5, 6, and 8 years of age in Patient 3. Awake EEGs were obtained at 9 and 19 years of age in Patient 1; at 18 years of age in Patient 2; and at 7, 10, and 19 years of age in Patient 3. Video EEGs were obtained before callosotomy in Patient 1 and 2 (Table 1).
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| EEG Timing (years old) | |||
| Sleep EEG | 1, 5 | 3, 9, 10 | 4, 5, 6, 8 |
| Awake/Sleep EEG | 9, 19 | 18 | 7, 10, 19 |
| Video EEG | 19 | 18 | |
| EEG abnormalities | |||
| HARA | |||
| Age (years old) | 1, 5 | Nil | 4, 5 |
| Frequency (Hz) | 8-12 | 8 ~ 12 | |
| Location | Frontal, Temporal, Parietal | Frontal, Temporal, Parietal | |
| Amplitude (uV) | 300–400 | 150 ~ 400 | |
| Intermittent Theta/Alpha Epileptiform Activity | |||
| Onset (years old) | 9 | 3 | 6 |
| Disappearance | Post-callosotomy | Post-callosotomy | Post-callosotomy |
| Duration (seconds) | 1-4 s | 0.5 ~ 1 s | 0.5-2 s |
| Amplitude (uV) | 100-150uV | 100 ~ 200uV | 90-150uV |
| Location | Diffuse | Bilateral Frontal, Central, Temporal | Diffuse |
| Typical Epileptic Discharges | |||
|
Spike or sharp wave Onset (years old) |
19 | 3, 9 | 6, 8, 10, 11 |
| Location | Bilateral F, C, P | Bilateral F, C | Bilateral F, C, T, O |
| GSW | |||
| Onset (years old) | Nil | 10, 18 | Nil |
| GPFA | |||
| Onset (years old) | Nil | 18 | Nil |
- Abbreviations: C, Central; F, Frontal; GPFA, generalized paroxysmal fast activity; GSW, generalized spike and wave; HARA, high-amplitude rhythmic activity; O, Occipital; P, Parietal; T, Temporal.
- *Post-Callosotomy.
3.3.1 High-amplitude rhythmic activity
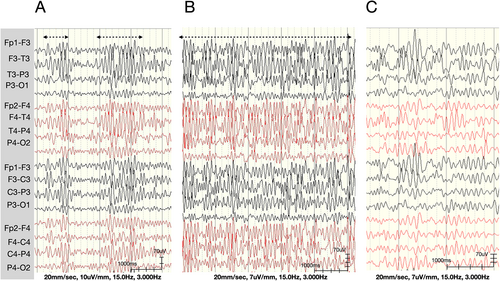
Intermittent diffuse high-amplitude (>300 uV) rhythmic alpha range (8 ~ 12 Hz) activity with frontal, central, and temporal predominance (Figure 3A,B) was noted in the sleep EEG at 1 and 5 years of age in Patient 1, and at 4 and 5 years of age in Patient 3 (Table 1). The background was normal at 10 ~ 12 Hz and 8 ~ 10 uV during these examinations, and there were no interictal epileptic discharges. This activity could have been a sleep spindle, as no normal sleep spindle was seen in the examinations. However, it had a higher amplitude and longer duration (up to 13 seconds) compared to a normal sleep spindle. In Patient 1, this high-amplitude rhythmic activity became continuous in the whole sleep EEG examination at 5 years of age (Figure 3B). In addition, this activity disappeared and was replaced by multifocal sharp waves at 9 and 6 years of age in Patients 1 and 3 (Table 1).
3.3.2 Interictal epileptic discharge
Interictal epileptic discharges were first noted at 9, 3, and 6 years of age in Patients 1, 2, and 3, respectively (Table 1). They presented as multifocal sharp waves and resembled brief (1 ~ 2 s) alpha activity (Figure 4A). During follow-up, more spiky multifocal interictal epileptic discharges developed in all three patients. Generalized spikes and waved were noted in Patient 2 at 10 and 18 years of age. The background was within theta activity during these periods.
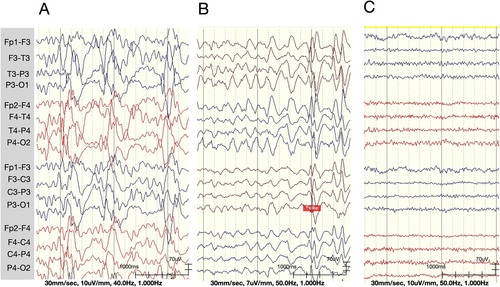
3.3.3 Post-callosotomy EEG changes
The multifocal sharp waves disappeared, and the theta background changed to a low-amplitude beta rhythm after callosotomy in all patients (Figure 4C). A few bilateral focally independent spikes were still noted in Patient 1, with intermittent generalized spikes and waves, and generalized paroxysmal fast activity, especially during sleep, in Patient 2. No focal spikes, sharp waves, generalized spikes and waves, or generalized paroxysmal fast activity were noted in Patient 3.
3.4 Genetic analysis
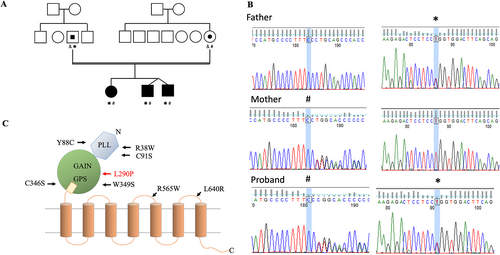
Whole-exome sequencing followed by Sanger confirmation showed that all three patients shared the same compound heterozygous ADGRG1 alleles (reference transcript: NM_001145773). One allele contained a single-nucleotide deletion at exon 4 leading to a frameshift variant with a premature stop codon which was inherited from the mother (Chr16:57685260C > -, exon 4: c.213delC: p.Pro72Leu fs*40). The other harbored a single-nucleotide missense variant at exon 7 which was inherited from the father (Chr16:57689411, exon 7: c.T869C: p.Leu290Pro) (Figure 1B). Neither allele was found in control population databases (such as ExAc or gnomAD) or a Taiwanese population database (Taiwan Biobank). The frameshift variant was reported to be pathogenic in the ClinVar database, while the missense variant was predicted to be deleterious by multiple in silico algorithms including PolyPhen2, PROVEAN, MutationTaster2, and CADD (data not shown). The L290P missense variant was novel and classified as being likely pathogenic based on the ACMG guidelines under a recessive in trans model, which is consistent with the known inheritance pattern of BFPP-associated ADGRG1 gene variants (Figure 1B).
3.5 Structural prediction and molecular analysis of the GPR56-L290P variant
Previous studies have shown that almost all BFPP-causing GPR56 mutant receptors are structurally unstable with very weak or no surface expression.7 To investigate the possible effect of the L290P mutation on protein stability, we first compared the amino acid sequences of the ECR of the GPR56 receptor from different animal species. Interestingly, multiple sequence alignment showed that all previously reported BFPP-associated mutation residues in the GPR56-ECR, namely R38, Y88, C91, C346, and W349, are highly conserved in all animal species. In contrast, the leucine (L) residue at amino acid 290 of human GPR56 is located within the GAIN domain and conserved in all primates, but is changed to valine (V) in rodents (Figure 5A). Regardless of the change, leucine (L) is still very similar to valine (V) biochemically, but a change in leucine (L) to structurally different proline (P) is likely to cause significant alterations in intramolecular interactions among residues. To explore the impact of the L290P mutation on protein structural stability, we used DynaMut,12 DynaMut2,13 and mCSM-membrane14 to predict changes in Gibbs free energy (ΔG) and differences in vibrational entropy (ΔSvib) of several mutant proteins compared to those of the WT receptor to predicted via either the AlphaFold215, 16, RoseTTAFold17or TrRosetta.18 The results showed that the GPR56-L290P isoform, as with the GPR56-C91S and -C346S variants, also displayed a decrease in stability (negative predicted ΔΔG) and increase in vibrational entropy (positive ΔΔSvib) (Figure 5B). These features suggested that the GPR56-L290P isoform would be pathogenic with structural instability and increased molecular flexibility.
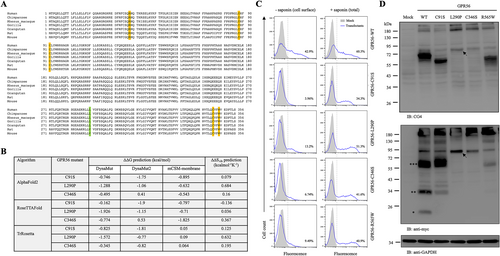
To verify the disease-causing mechanism of the GPR56-L290P isoform, we next determined the cell surface and total expression levels of the receptor using flow cytometry and Western blotting analyses and compared the results with GPR56-WT and other BFPP-associated point mutants. Highly similar to those of the GPR56-C91S, -C346S, and -R565W isoforms, a markedly reduced surface level of GPR56-L290P was detected in transiently transfected HEK-293 T cells (Figure 5C). In contrast, the total cellular expression of GPR56-L290P was comparable with those of GPR56-WT and BFPP-associated mutants (Figure 5C). These results indicated that the L290P mutation caused a reduction in cell surface GPR56 expression as a result of impaired subcellular trafficking, likely due to receptor instability. This conclusion was confirmed using Western blotting analysis, which showed much weaker protein signals in the lysates of cells expressing GPR56-L290P than in those expressing the WT receptor (Figure 5D). Furthermore, the Western blotting results revealed that the L290P isoform, as with the C346S isoform, was not processed by GPS proteolysis, as protein bands of the same size were detected by two different Abs used to detect the extracellular fragment and transmembrane fragment, respectively (Figure 5D). Thus, we concluded that the GPR56-L290P variant is a GPS proteolysis-deficient unstable receptor which is retained mostly in the intracellular compartment.
4 DISCUSSION
The three members of the same family reported in this study are the first genetically confirmed BFPP cases in Taiwan, in whom we detected one frameshift mutation and one novel missense variant (Leu290Pro) of ADGRG1/GPR56 (Figure 1). There are two novel findings in this study, the epileptogenic course in BFPP and EEG abnormalities after callosotomy, and the pathogenic mechanism of the Leu290Pro variant.
Polymicrogyria is associated with high epileptogenicity, and more extensive distribution of polymicrogyria is associated with early seizure onset.19 All three patients underwent a similar epileptogenesis course, including pre-epilepsy, pharmacoresponse, and pharmacoresistant periods. The onset of seizures began after developmental delay, and ranged from 1 to 9 years of age. No focal epileptic discharges were noted before seizure onset, however bilateral high-amplitude rhythmic activity over frontal, parietal, and temporal regions developed during sleep. This could have been an extreme sleep spindle, which has been reported in patients with lissencephaly syndrome,20 but this is the first reported case in patients with GPR56-related polymicrogyria. According to Gibbs and Gibbs in 1973,21 an extreme spindle is abnormal and correlated with the organic type of mental retardation. These spindles can be classified into six types, of which type 3 involves more spikes and is associated with the highest incidence of epilepsy. Sleep spindles are generated from the thalamic reticular nucleus, regulated in thalamocortical loops, and are associated with memory consolidation and cognitive abilities.22 An extreme spindle may indicate cortical excitation and inhibition imbalance, as in our cases.
Head drop was the first type of seizure in our patients, followed by several other kinds of seizures including tonic, generalized tonic clonic, myoclonic, staring, and epileptic falling. Various types of seizures have been reported in patients with BFPP, including generalized tonic clonic,5, 23, 24 atypical absence,5, 23 epileptic spasm,5, 25 tonic,25, 26 atonic,23 and recurrent nonconvulsive status epilepticus.5 Our three cases presented with multiple types of seizures, epileptic falling, mental retardation, slow background, and multiple focal spikes in EEG. However, slow generalized spikes and waves (1.5 Hz) and generalized paroxysmal fast activity were only noted in Patient 2 (Table 1).
Other than typical brain imaging findings of BFPP in our patients, the straight linear T2 hyperintense white matter lesions vertical to the cortex in Patients 1 and 3 were distinct, and may reflect an enlarged perivascular space. These lesions have not been discussed in patients with BFPP before, however, they have been reported in patients with polymicrogyria.27 These lesions may be due to focal white matter atrophy.
Traditional anterior 2/3 corpus callosotomy has been shown to have an excellent effect on seizure control, especially epileptic drop attacks.28 However, there was a honeymoon effect with regard to seizure control after surgery in our cases. To achieve a more persistent therapeutic effect, more extensive disconnection should be considered, including total corpus callosotomy and/or transection of the anterior and posterior commissure, although both surgeries are associated with increased risks of paraoperative and postoperative complications. Of note, Patient 3 received concurrent VNS treatment, and his epilepsy remained well controlled with no epileptic falling seizures noted 1.5 years later. In palliative surgery, corpus callosotomy is thought to potentially be more effective than VNS in reducing seizure frequency.29 On the other hand, Katagiri et al. reported that combining callosotomy and VNS resulted in satisfactory treatment outcomes in patients with Lennox–Gastaut syndrome,30 while Hong et al. also supported that callosotomy can help reduce seizures in patients with medically refractory epilepsy following VNS.31 The better outcome in Patient 3 suggests that these two surgical interventions are complementary and can work synergistically in particularly refractory patients. In other words, surgery would be more beneficial when combined with VNS than either one alone.
According to the ClinVar database, there are 32 ADGRG1 pathogenic and likely pathogenic mutations, of which 21 are protein-truncating variants and 11 are missense variants located in both the extracellular and transmembrane domains. In our cases, the novel frameshift variant (p.Pro72Leufs*40) was located at the pentraxin/laminin/neurexin/sex-hormone-binding-globulin-like (PLL) domain of GPR56-ECR, while the missense variant (p.Leu290Pro) was within the GAIN domain. As the frameshift mutation was predicted to produce a non-functional truncated GPR56 protein, we focused on the molecular analysis of the GPR56-L290P isoform. Structural prediction based on multiple computational algorithms suggested that this variant was pathogenic and likely an unstable receptor molecule (Figure 5B). Indeed, our expression and biochemical analyses confirmed that the GPR56-L290P isoform was weakly expressed on the cell surface due to structural instability and impaired intracellular trafficking (Figure 5C). In addition, it was not modified properly by GPS auto-proteolysis (Figure 5D). This is probably because the L290P mutation is located within the GAIN domain, which is known to be essential for the GPS proteolytic modification of most aGPCRs. This conclusion was supported by the fact that the GPR56-L290P isoform shared very similar expression and biochemical characteristics with the GPR56-C346S isoform, in which the mutated residue was located within the GPS motif that disrupted the GPS auto-proteolysis (Figure 1C).
Our data were obtained from three cases of one family, and the timing of data collection was inconsistent during long-term follow-up. This limits the strength of the findings. However, the findings of EEG changes from childhood to adulthood, and post-callosotomy in our cases with BFFF may lead to further well-designed studies to elucidate the relationship between polymicrogyria and brain function development.
In conclusion, we found sleep EEG findings of high-amplitude rhythmic activity in our cases with BFPP only during infancy and childhood, and confirmed that the missense variant (p.Leu290Pro) led to loss of function due to impaired receptor auto-proteolysis, intracellular trafficking, and surface expression.
AUTHOR CONTRIBUTIONS
The electroclinical data were analyzed by Cheng-Yen Kuo, MD, Kuang-Lin Lin, MD, Jainn-Jim Lin, MD, and Po-Cheng Hung, MD. The genetic analysis was performed by Meng-Han Tsai, MD, PhD, and the Kaohsiung Epilepsy and Neurogenetics Research Program of Chang Gung Memorial Hospital, and the Genomic & Proteomic Core Laboratory at the Department of Medical Research, Kaohsiung Chang Gung Memorial Hospital. The biological analysis of GPR56-L290P variant structural and function analysis was performed by Hsi-Hsien Lin, PhD and Abhishek Kumar Singh of the Department of Microbiology and Immunology, College of Medicine, Chang Gung University, Taoyuan, Taiwan. Surgery was performed Yu-Chi Wang MD, PhD. The brain image analysis was performed by Chang-Chih Chen, MD.
ACKNOWLEDGMENTS
The authors thank Min-Lan Tsai MD for support with the electroclinical analysis.
FUNDING INFORMATION
This study was supported in part by a grant from Chang Gung Memorial Hospital (CMRPG3H0761–3).
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL APPROVAL
This study was approved by the Chang Gung Memorial Hospital Institutional Review Board (202101571B0).




