Experience with the ketogenic diet in premature neonates
Abstract
The ketogenic diet is a time-tested, potent, nonpharmacological treatment of epilepsy. However, the use of the ketogenic diet in premature neonates with epilepsy has not been previously reported. We share our experience with the use of ketogenic diet therapy in two premature neonates. Two identical twin premature neonates with SCN2A-related developmental and epileptic encephalopathy, whose seizures were refractory to multiple anti-seizure medications, were started on the classic ketogenic diet at the conceptual age of 35 weeks. Ketosis was achieved and maintained (range 2-5 mmol/L of serum beta-hydroxybutyrate level). Seizure frequency was significantly reduced (>90% reduction in both patients), and some anti-seizure medications were able to be discontinued. Initial transient weight loss and one episode of asymptomatic hypoglycemia were observed and corrected. The ketogenic diet was found to be a safe, well-tolerated, and effective treatment for seizures in two premature neonates. The side effects are tolerable and correctable. The ketogenic diet, therefore, is a treatment option for refractory seizures in this age group, when administered under expert guidance.
1 INTRODUCTION
The ketogenic diet (KD) is a high-fat, low-carbohydrate, and moderate-protein diet, which has been used as a treatment for various types of epilepsy.1 Although there are few case reports of KD use in neonates,2, 3 concerns for safety, efficacy, and tolerability in this age group remain. Moreover, to date, the application of the diet in premature neonates has not yet been reported. We successfully employed the KD in two identical twin premature neonates with SCN2A-related early-infantile developmental and epileptic encephalopathy (EIDEE).
2 CASE REPORT
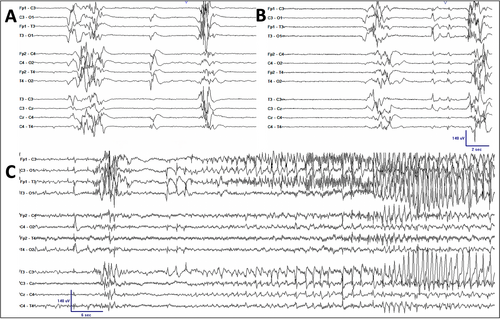
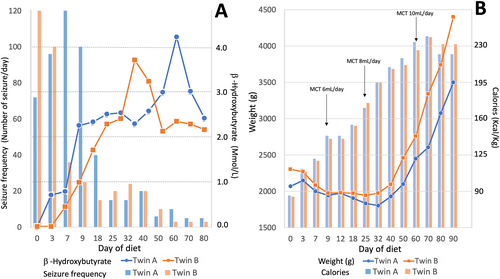
Identical twin girls were born at 32 weeks of gestational age (GA) with birth weights of 1.29 kg and 1.54 kg. At 11 and 14 h of life in twins A and B, respectively, both developed recurrent apnea with bradycardia and eventually required intubation. On day 9 of life, the Neurology service was consulted, and an electroencephalogram (EEG) was recorded to evaluate episodes of limb stiffening accompanied by apnea and bradycardia. The EEG revealed an invariant, discontinuous background activity consistent with burst suppression (Figure 1), and focal tonic and sequential seizures were seen with an occurrence of 2-5 electroclinical seizures per hour in both twins. The seizure semiology and EEG features are similar in both infants. A diagnosis of early-infantile developmental epileptic encephalopathy (EIDEE or Ohtahara syndrome) was made in both. Seizures were refractory to treatment with multiple medications, including phenobarbital (serum level 34-40 μg/mL) and levetiracetam (dose of 60 mg/kg/day), and only partially responsive to fosphenytoin load. Pyridoxine 30 mg/kg/day and folinic acid 5 mg/kg/day were administrated before the diet initiation with no clinical or electrographic improvement. Classic ketogenic diet (CKD) was initiated on the day of life 24, a conceptional age (CA) of 35 weeks, in both patients. The KD was started in a nonfasting fashion, following protocol from the international ketogenic diet study group proposed by Kossoff et al4 using KetoCal (Nutricia) formula through enteral feeding via an oral gastric tube, with an initial ratio of 1:1 (fat:nonfat) without a caloric or fluid restriction, and gradually advanced the ratio every 3-5 days, and reached the ratio of 4:1 on day 18 of the diet initiation. Body weight and basic metabolic panel were checked daily. Blood sugar was checked with a finger stick test every 4 h with a confirmatory plasma glucose level if the measurement is less than 40 mg/dL until reaching the goal diet ratio. Electrolytes, bicarbonate, total protein, calcium, magnesium, phosphates, and kidney profile (blood urea nitrogen and creatinine) were monitored every 3-4 days. Complete blood count with platelets, liver, and lipid profile were monitored every 2 weeks and as needed. Serum β-hydroxybutyrate (BHB) level was checked every 2-3 days. Ketosis was achieved in both patients with a serum BHB level of 2.5 mmol/L in twin A and 2.2 mmol/L in twin B on day 18 of the diet. Weight loss was initially noted in both twins with a peak of 12% reduction from the prediet weight on day 29 in twin A, and 15% on day 25 in twin B. Total calorie intake was adjusted with the addition of the medium-chain triglyceride (MCT) oil (Nestlé), up to 18% of total calories with a maintained diet ratio, which resulted in both patients gradually and consistently gaining weight (Figure 2). Total calories per kilogram per day were started at 82 and 81 kcal and gradually increased up to 241 kcal and 242 kcal in twin A and twin B, respectively. MCT oil was increased in proportion to total calories (Figure 2), with approximately 18 (8-18) % of the total calories, to maintain the diet ratio. Besides the initial weight loss and one episode of asymptomatic hypoglycemia (plasma glucose of 32 mg/dL) seen in twin A during the diet initiation, which was corrected with a bolus of dextrose 10% in water, no other significant complications, such as severe acidosis (HCO3 less than 16 mEq/dL), hyperlipidemia, constipation, or diarrhea, were observed in either patient. Both twins were able to be extubated on day 18 after the diet initiation.
MRI brain and cerebral spinal fluid studies were obtained and were unrevealing. Genetic testing with next-generation gene sequencing with an epilepsy gene panel was performed and showed a de novo pathogenic missense variant in SCN2A gene (c.4462A > G [p.Ile1488val], heterozygous). Considering the clinical phenotype, EEG findings, and genotype, a diagnosis of SCN2A developmental and epileptic encephalopathy (SCN2A-DEE) was confirmed.
Seizure frequency was gradually reduced since the diet was started with approximately 10-20 brief focal tonic seizures per day at the discharge (CA 42 weeks). In addition to the KD, carbamazepine was also added to the treatment regimen after the genetic test result, at CA of 40 weeks or day 25 after the diet initiation. Of note, phenobarbital, levetiracetam, and midazolam were discontinued before the discharge without any worsening of seizures.
At the last follow-up visit, patients aged 6 months old (corrected age of 4 months), both patients were feeding by mouth. The body weight was 5.7 kg (9th percentile) and length of 58 cm (3rd percentile) in twin A, and 6.0 kg (18th percentile) and 57.2 cm (3rd percentile) in twin B. Seizures were well controlled in both twins with approximately 1-2 focal tonic seizures per day on the KD and sodium channel blocker drugs (carbamazepine and lacosamide). A repeat EEG showed a continuous but poorly-organized background admixed with multifocal pleomorphic epileptiform discharges while awake and a discontinuous background during sleep with an epoch of amplitude attenuation lasting 1-2 s. The EEG findings are similar in both twins. Nevertheless, parents reported a significant improvement in alertness and energy. Developmentally, both twins were able to fix and track faces with some head control, but not able to reach, smile, or roll over yet. Laboratory tests show serum BHB levels of 5.36 and 4.50 mmol/L in twins A and B, respectively, with a normal lipid panel and liver functions.
3 DISCUSSION
The KD has been widely used as a treatment of epilepsy, particularly drug-resistant epilepsy since its introduction in 1921.5, 6 The application of the diet was, however, initially limited mainly to children and some adults until an initial report regarding the efficacy and tolerability of the KD in infants was published in the early 2000s.7 Since, the use of the diet has expanded to include wider age groups. Nevertheless, the application of the dietary therapy for epilepsy in neonates remains controversial and has been rarely employed. In fact, to date, there are only few case reports in the literature on the KD in neonates with the youngest patient was a term neonate at 30 days of life.3 In this study, we are reporting the first case of the KD use in premature neonates.
Seizures are one of the most common neurological disorders seen in neonates.8 Epilepsy comprises approximately 10%-15% of all seizures with the neonatal onset.9 Despite the advancement and increased number of available anti-seizure medications, many neonates with epilepsy continue to have seizures, which can potentially have a negative impact on their neurodevelopmental outcome.10 The KD is a potent, nonpharmacological treatment of epilepsy but has rarely been used in this age group.2, 3 Although there have been reports considering the potential adverse effects of KD, most of them are relatively mild, preventable, and correctable.11 Among those, the common concerns in its use in neonates are inadequate nutrition for growth, weight loss, hypoglycemia, and inability to achieve or maintain ketosis.2 Initial weight loss was reported from the study in full-term neonates by Thompson et al,2 which was also observed in our study (Figure 2). This may be at least partially caused by reduced efficiency in fat absorption in neonates, which is partially attributed to a decreased capability of lipase and bile secretion.12 The addition of MCT oil has helped with weight loss in our patients with a similar experience reported from other studies in term neonates.2, 13, 14 This may be explained by the different mechanisms of absorption and metabolism of MCT compared with long-chain triglyceride commonly used in the CKD since there is direct absorption of MCT oil via portal circulation without bile emulsification and MCT bypasses the carnitine shuttle in its beta-oxidation.15 Of note, carnitine levels in both infants were low at the beginning of the diet (11 μmol/L, 16 μmol/L in twins A and B, respectively), requiring carnitine supplements, which might also have contributed to initial weight loss and favorable response to MCT oil. Both patients, however, later gradually and consistently gained weight and reached the prediet weight at day 36 and day 34 in both twin A and twin B, respectively, after the introduction of MCT oil and calory adjustment (Figure 2).
In addition to weight loss, hypoglycemia and inability to achieve or maintain ketosis in this age group were common concerns. In our study, both patients tolerated the diet well with only 1 episode of asymptomatic hypoglycemia observed in twin A during initiation, with a plasma glucose level of 32 mg/dL, which was corrected with an administration of 10% dextrose water. Ketosis was achieved and well maintained in both patients with the level of serum BHB ranging between 2 and 3 mmol/L during the hospital stay (Figure 2). The ketone level appears to be slightly lower than what other authors reported in a full-term neonate and may be explained by the limitation of ketogenesis in preterm compared with full-term neonates.12 Nevertheless, newborns and infants can generally utilize ketone bodies as an energy fuel very efficiently, especially in the neonatal period when the rate of brain ketone uptake and utilization is faster and more efficient than in other age groups.16
The efficacy of KD therapy for seizures in SCN2A-DEE has been prior reported with seizure reduction observed over the course of the treatment.17, 18 In our study, at the most recent visit, both patients showed a remarkable improvement in seizure control with more than 90% seizure reduction in frequency (Figure 2). Moreover, the patients were able to successfully discontinue 3 anti-seizure medications since the start of the KD, which may potentially help reducing the side effects of polypharmacy.
Our experience with the ketogenic diet in our two premature patients shows that it can be safe, well-tolerated, and effective treatment for seizures. The side effects were relatively low and correctable. The KD, therefore, is a treatment option for seizures even in this age group. Careful attention, close monitoring, and a team approach are imperative for a successful implementation of the dietary therapy. A further study with a larger number of patients should be considered to confirm the findings.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
PRIVACY PROTECTION
Parental consent was obtained regarding the publication of cases.




