Adrenal function during long-term ACTH therapy for patients with developmental and epileptic encephalopathy
Abstract
Some patients with developmental and epileptic encephalopathy (DEE) respond to adrenocorticotropic hormone (ACTH) therapy but relapse soon after. While long-term ACTH therapy (LT-ACTH) has been attempted for these patients, no previous studies have carefully assessed adrenal function during LT-ACTH. We evaluated the effectiveness of LT-ACTH, as well as adverse effects (AE), including their adrenal function in three DEE patients. Patients underwent a corticotropin-releasing hormone (CRH) stimulation test during LT-ACTH, and those with peak serum cortisol below 15 μg/dL were considered to be at high risk of adrenal insufficiency (AI). Two of three responded, and their life-threatening seizures with postgeneralized electroencephalogram (EEG) suppression decreased. Although no individuals had serious AE, CRH stimulation test revealed relatively weak responses, without reaching normal cortisol peak level (18 μg/dL). Hydrocortisone replacement during stress was prepared in a case with lower cortisol peak than our cutoff level. LT-ACTH could be a promising treatment option for cases of DEE that relapse soon after effective ACTH treatment. The longer duration and larger cumulative dosage in LT-ACTH than in conventional ACTH could increase the relative risk of AI. Careful evaluation with pediatric endocrinologists, including hormonal stimulation tests, might be useful for continuing this treatment safely.
1 INTRODUCTION
Epilepsy syndromes with co-occurring early-onset seizures and developmental delay and regression with frequent epileptiform activity are collectively called developmental and epileptic encephalopathy (DEE).1 West syndrome (WS) is a major syndrome of DEE, characterized by epileptic spasms (ES) occurring in clusters with hypsarrhythmia (Hyps). Adrenocorticotropic hormone (ACTH) therapy is a first-line therapy for ES.2 Nearly half of the patients who initially respond to ACTH will relapse,3 and management for relapsed cases has not been established. Okanishi et al recommended long-term weekly ACTH therapy (LT-ACTH),4 and several case reports have followed.5, 6 Considering their treatment regimes, we initiated LT-ACTH in patients who responded to ACTH but relapsed soon after.
To avoid severe adverse effects (AE), lower dose and shorter duration are recommended in both natural and synthetic ACTH regimens.7-9 Endogenous secretions of ACTH and corticotropin-releasing hormone (CRH) are suppressed due to negative feedback from hypercortisolemia, and impaired response of the hypothalamic-pituitary-adrenal (HPA) axis is thought to raise the risk of adrenal insufficiency (AI), comparable to corticosteroid therapy.10 The actual incidence of adrenal crisis has not been reported in previous studies.3, 7 Several studies, however, have investigated adrenal function following natural ACTH therapy, and while relatively few in number, some patients had a weak response to an adrenal stimulation test.11-14 Although previous case reports and the latest Japanese multicenter case series of LT-ACTH15 showed no apparent episodes of AI, detailed examinations were not performed. With the cooperation of pediatric endocrinologists, we evaluated adrenal function using a hormonal stimulation test during LT-ACTH.
2 MATERIALS AND METHODS
2.1 Patient inclusion and exclusion criteria
This study was performed with permission from the Hokkaido University Hospital Accredited Clinical Research Review Committee. We used depo, synthetic ACTH-Z-(1-24) (Cortrosyn Z, molecular formula: C136H210N40O31S・6CH3COOH). Inclusion criteria for LT-ACTH were as follows: (a) DEE patients with daily ES or tonic seizures (TS) younger than 4 years; (b) patients who responded to conventional ACTH but rapidly relapsed twice; and (c) patients who had no underlying congenital immune or heart diseases. Ten DEE patients received ACTH at the Department of Pediatrics in Hokkaido University Hospital from April 2018 to March 2021. Although eight patients relapsed, three had been in remission from the second round of ACTH and two were controlled by vigabatrin. Three individuals were enrolled for the present study. Written informed consent concerning this study was obtained from each patient's guardians. The profiles of the subjects are summarized in Table 1.
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Etiology | SCN2A pathogenic variant | post-HSV encephalitis | SCN2A pathogenic variant |
| Genetic testing | SCN2A p.D195N | N/A | SCN2A p.A853Q |
| Age at seizure onset | 1 y and 2 mo | 7 mo | 6 mo |
| Previous ASM | VPA, LTG, CLB, TPM, IVIG, MDZ, KD, B6 | LEV, VPA, LTG | VPA, VGB, B6, LTG |
| Conventional ACTH | |||
| Age at 1st | 1 y and 6 mo | 7 mo | 7 mo |
| Age at 2nd | 1 y and 9 mo | 11 mo | 9 mo |
| LT-ACTH | |||
| Age at start | 2 y and 4 mo | 1 y and 11 mo | 1 y and 2 mo |
| Induction ACTH | 0.0125 mg/kg/day/2 wk | 0.0125 mg/kg/2 wk | 0.01 mg/kg/2 wk |
| Dose of weekly | 0.0125 mg/kg | 0.01-0.0125 mg/kg | 0.01 mg/kg |
| Duration | 9 mo | 12 mo | 7 mo |
| ASM at start | VPA + LTG | VPA + LTG | VPA + LTG |
| Seizure type | TS, GTC, ES | TS, ES | TS, ES |
| EEG | Hyps or S-B, PGES | Modified Hyps | Hyps. PGES |
| Developmental status | Cannot sit | Standing | No head control |
| No social smile | Social smile | No social smile | |
| Cannot speak words | Cannot speak words | Cannot speak words | |
| Effect on seizure | Seizure-free | Relapsed | Decreased (50% reduction) |
| Effect on EEG |
Hyps, S-B disappeared PGES disappeared |
Modified Hyps remained |
Hyps disappeared PGES disappeared |
| Change of ASM |
Add PER Decrease LTG |
Add TPM, CLB, PER, VGB |
Add PER, CLB Cease LTG |
| Adverse events | Brain shrinkage |
Acne on cheeks Adrenal insufficiency |
Brain shrinkage Subdural hematoma Aspiration pneumonia |
| Effect on development | Walk | Walk | Head control, Roll over |
| Social smile | Cannot speak words | No social smile | |
| Cannot speak words | Increase gestures | Follow things with eyes | |
- Abbreviations: ACTH, adrenocorticotropic hormone; ASM, antiseizure medications; B6, vitamin B6; CLB, clobazam; ES, epileptic spasms; GTC, generalized tonic-clonic seizures; HSV, herpes simplex virus; Hyps, hypsarrhythmia; IVIG, intravenous immunoglobulin therapy; KD, ketogenic diet; LEV, levetiracetam; LTG, lamotrigine; MDZ, midazolam; N/A, data not available; PER, perampanel; PGES, postictal generalized EEG suppression; S-B, suppression-burst; TPM, topiramate; TS, tonic seizures; VGB, vigabatrin; VPA, valproate.
2.2 LT-ACTH
Weekly ACTH administration was followed by conventional ACTH as induction therapy with a single dose of 0.01-0.0125 mg/kg (equivalent to 0.4-0.5 IU/kg). ACTH was injected daily for 2 weeks, then gradually reduced over a subsequent two-week period in hospital. Weekly injections of ACTH were then administered in outpatient wards. Administration was skipped when patients had an infectious illness. The continuation of LT-ACTH was decided upon after discussion with guardians about the potential effectiveness and AE. The standard treatment period was set for 1 year.
2.3 Evaluation of the effectiveness and AE
Short-term hospitalization was scheduled every 2-3 months for evaluation. We examined several clinical parameters including overnight video electroencephalogram (EEG), brain imaging, and blood examinations. Clinical seizure outcome was assessed based on direct observation, frequency of epileptiform activity seizures during EEG, and daily records kept by guardians. AE were evaluated by clinical observation and medical interview of guardians.
2.4 Evaluation of adrenal function
Corticotropin-releasing hormone stimulation test was performed during scheduled hospitalization around 6 months after commencement of LT-ACTH. Following overnight fasting, human CRH (hCRH; Mitsubishi-Tanabe Pharma Corporation) was intravenously administered at a dose of 1.5 μg/kg at around 8:00 am Blood samples were drawn at baseline and 15, 30, 60, 90, and 120 minutes after administration of hCRH, and serum cortisol and plasma ACTH levels were measured. When peak serum cortisol levels after CRH stimulation test rise above 18 μg/dL, and the plasma ACTH level increases more than twofold, HPA axis function is usually judged to be normal.16 In light of the previous study of evaluating iatrogenic secondary AI using hCRH,17 we considered patients with serum cortisol levels under 15 μg/dL as poor responders to adrenal stimulation, and prepared hydrocortisone replacement during periods of stress.
3 RESULTS
3.1 Evaluation of effectiveness and AE
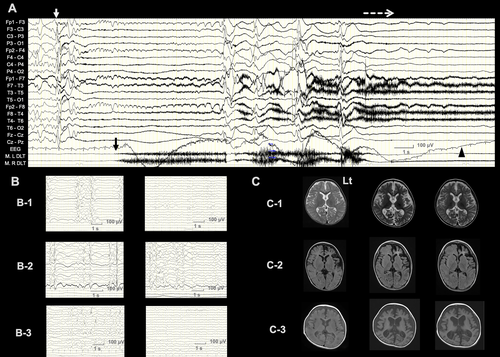
Two of three individuals responded well to LT-ACTH, seizure-free (case 1) and 50% seizure reduction (case 3), and one patient relapsed ES (case 2). Cases 1 and 3 had the same genetic etiology, SCN2A-related epileptic encephalopathy. Both had ES followed by TS with postictal generalized EEG suppression (PGES), a high-risk factor for sudden unexpected death in epilepsy (SUDEP; Figure 1).18 Along with seizure control, PGES disappeared in both cases. Case 2 had large structural cerebral lesions from herpes simplex virus (HSV) encephalitis. LT-ACTH was effective at first, but the patient then relapsed.
No AE associated with discontinuance of LT-ACTH, such as severe infectious disease, hypertension, and cardiac symptoms, were observed in any individual. Recurrent aspiration pneumonia occurred in case 3, although she responded to antibiotics and recovered smoothly. Mild Cushing syndrome-like symptoms, moon face, hirsutism, or acne was appeared in two patients. Electrolyte or glucose abnormalities did not occur and extreme hyperphagia and irritability were not apparent. In case 3, a nonsymptomatic subdural hematoma occurred after the first round of ACTH, but eventually resolved after commencing LT-ACTH and did not recur. In two cases, brain shrinkage was present and persisted, but did not progress during LT-ACTH.
3.2 Evaluation of adrenal function
No patients had apparent episodes of AI during the follow-up period. The results of CRH stimulation tests are summarized in Table 2. The morning baseline serum cortisol levels ranged from 5.8 to 10.3 μg/dL (average, 6.7 μg/dL). The peak serum cortisol levels ranged from 14.3 to 16.6 μg/dL (average, 15.3 μg/dL). Peak serum cortisol levels were lower than normal peak level (18 μg/dL), showing weaker responses compared with a normal population.19 The morning and peak plasma ACTH were 18.0-22.4 pg/mL (average, 19.5 pg/mL) and 37.1-51.5 pg/mL (average, 44.9 pg/mL), respectively. In case 3, CRH stimulation tests were performed two times, before and during the weekly injection period. The cortisol peak level decreased from 20.8 to 16.6. In one individual (case 2), the peak cortisol level was below our cutoff level (<15 μg/dL). We prepared hydrocortisone replacement for him during severe physical stress, fevers, or bouts of infectious disease. He did not show clinical symptoms of AI for 3 months after withdrawal of LT-ACTH.
| Case | 1 | 2 | 3 | Normal standarda | |
|---|---|---|---|---|---|
| BW (kg) | 16.8 | 15.5 | 12.0 | 13.5 | |
| HT (cm) | 97.0 | 91.1 | 85.0 | 93.0 | |
| BSA (m2) | 0.7 | 0.6 | 0.5 | 0.6 | |
| Age CRH stimulation test performed | 3 y 0 mo | 2 y and 8 mo | 1 y and 3 mo | 1 y and 8 mo | |
| Duration of weekly ACTH | 6 mo | 7 mo | 0 mo (before) | 6 mo | |
| Cumulative dose of ACTH (mg) | 11.0 | 11.1 | 6.2 | 11.0 | |
| Morning serum cortisol (μg/dL) | 5.8 | 6.7 | 11.3 | 10.3 | 3.9-21.3 |
| Peak serum cortisol (μg/dL) | 15.0 | 14.3 | 20.8 | 16.6 | 13.1-35.6 |
| Morning plasma ACTH (pg/mL) | 18.0 | 18.2 | 22.2 | 22.4 | 5.3-51.1 |
| Peak plasma ACTH (pg/mL) | 51.5 | 37.1 | 44.9 | 46.2 | 17.2-135.3 |
- Abbreviations: ACTH, adrenocorticotropic hormone; BSA, body surface area; BW, body weight; CRH, corticotropin-releasing hormone; HT, height.
- a Data of CRH stimulation test of nonendocrine short stature children in Japan reported by Tanaka et al (Ref. [19]).
- Bold indicates the values without reaching normal serum cortisol peak level (18μg/dL) after CRH stimulation test.
4 DISCUSSION
In the latest multicenter case series of LT-ACTH for WS, the nonrelapse rate was 60.6%.15 These findings generally match those found in the present study. Continued case accrual and large prospective cohort studies are needed to confirm whether LT-ACTH has a role in preventing relapse. Two DEE individuals had a life-threatening seizure type, TS with PGES. When DEE individuals have life-threatening seizures and only responded to ACTH, LT-ACTH is one of the valuable therapy choices.
There were no serious AE; however, care should be taken due to the possibility of serious incidents because it is challenging to administer in infants for such a considerable period of time. Studies of posttreatment assessment of adrenal function in patients with ES were limited, and the dosage, duration, and tapering periods were different from those in the present study.11-13 Since the ACTH dose in Japan is lower than in the other countries—roughly equivalent to 1/6-1/8 that used in the United States, and equivalent to half in Finland or the United Kingdom—the risk of AI might be considered to be lower. Routine hormonal assessment using stimulation testing is not performed in Japan. Sakaguchi et al assessed HPA axis function in WS patients who were treated by conventional ACTH with standard Japanese doses.20 Of thirty-five patients who underwent CRH testing, four patients (11%) had insufficient response. Mytinger and Bowden assessed the adrenal function in the modern hormonal treatment regimens of high-dose natural full-length ACTH or prednisolone.14 They found two of 12 patients had insufficient adrenal response by adrenal stimulation testing. They called attention that adrenal suppression could occur after modern hormone therapy regimens. They also reported another one patient treated with both ACTH and prednisolone in tandem for 2 months, who did not have a posthormone laboratory assessment, who developed signs of AI and required hydrocortisone replacement. In our study, one patient who was assessed before and during LT-ACTH exhibited a decreased cortisol response. We posit that a longer duration can increase the risk of secondary AI. As such, we suggest that not all patients need a hormonal stimulation test after ACTH, but while in LT-ACTH, we recommend a more detailed adrenal assessment, ideally during and post a hormonal stimulation test, as well as monitoring for signs of AI for at least 3 months after withdrawal. It would also be practical to prepare prophylactic coverage with stress dose hydrocortisone for times of stress, such as febrile illness and operative treatment. It is essential to educate caregivers on the signs of AI.
Although there are some kinds of stimulation tests to assess adrenal function, the ACTH stimulation test is standard, is safe, and can be performed at low cost. The CRH stimulation test is more expensive, but can be done safely. Administered ACTH may cause adrenal dysfunction through negative feedback to the hypothalamus. In addition, excessively secreted cortisol may suppress the HPA axis. We consider the CRH stimulation test has the advantage of evaluating ACTH and cortisol responses simultaneously.
Our study has the limitations of small sample size, and in lacking a comparison with other hormonal assessment studies in ACTH therapy. DEE is a rare disease, and the candidates were limited to a single center. Research collaborations with other facilities will be required to further investigate the benefits and risks of this treatment. Efforts should be made to avoid iatrogenic harm from longer hormonal exposure. While we focused on AI in this study, other influences (such as immunosuppression and brain shrinkage) are assumed to have an effect. In the future study, we would like to validate our findings using a multidirectional approach by cooperating with various experts.
5 CONCLUSIONS
Long-term ACTH therapy is a promising option for DEE patients when immediate response cannot be obtained with treatments other than ACTH. Our results on hormonal stimulation assessment suggest that the impact on the HPA system is not small. Paying close attention to AI is important for continuing LT-ACTH safely.
ACKNOWLEDGMENTS
We are grateful to the patients and their families, who agreed to share the patients' information in the current study. We thank Professor Atsushi Manabe of the Department of Pediatrics, Hokkaido University Graduate School of Medicine, for his valuable editorial opinion.
CONFLICT OF INTEREST
Neither of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.