Experience of perampanel monotherapy beyond initial titration to achieve seizure freedom in patients with focal-onset seizures with newly diagnosed or currently untreated recurrent epilepsy: A post hoc analysis of the open-label Study 342 (FREEDOM)
Funding Information
The FREEDOM study (NCT03201900) was funded by Eisai Co., Ltd. Medical writing support for the development of this article was funded by Eisai Inc.
Abstract
Objective
This post hoc analysis evaluated whether continued treatment with perampanel monotherapy beyond initial titration may be appropriate for patients with focal-onset seizures (FOS) with currently untreated epilepsy to achieve seizure freedom with an effective dose.
Methods
Study 342 (NCT03201900; FREEDOM) is a single-arm, open-label, Phase III study of perampanel monotherapy. Patients aged ≥12 years with untreated FOS received perampanel 4 mg/d in a 32-week Treatment Phase (6-week Titration and 26-week Maintenance Periods); in case of seizure(s) during Maintenance Period, patients could enter a 30-week Treatment Phase (4-week Titration and 26-week Maintenance Periods) to be up-titrated to perampanel 8 mg/d. The primary endpoint was seizure-freedom rate during Maintenance Period in the modified Intent-to-Treat (mITT) Analysis Set (patients who had ≥1 post-dose efficacy measurement during Maintenance Period); safety was monitored. This analysis of 4-mg/d efficacy data assessed the proportion of patients achieving seizure freedom during the Maintenance Period (responders) relative to patients with an early/later response (depending on seizure status during the Titration Period).
Results
In the mITT population (n = 73), 46 patients were 4-mg/d responders; of whom, 37 (80.4%) were early responders and nine (19.6%) were later responders. The mean (standard deviation) percent reductions in FOS frequency from baseline at the end of the 4-mg/d Titration Period were 100.0% (0.0%; early responders) and 46.3% (97.3%; later responders). Among the 27 4-mg/d nonresponders, nine (33.3%) patients who had an early response experienced seizure(s) during the subsequent 4-mg/d Maintenance Period. Safety outcomes were similar, regardless of responder status, without new safety concerns.
Significance
Some patients with untreated FOS may benefit from continued treatment beyond initial titration of perampanel monotherapy to achieve seizure freedom, suggesting that it may not be appropriate to make treatment decisions to discontinue or switch from perampanel monotherapy solely based on seizure response before an effective dose has been reached.
Key Points
- The majority (37/46; 80.4%) of 4-mg/d responders achieved seizure freedom during the 4-mg/d Titration Period in Study 342
- Of 27 patients who had seizures in Titration Period, 9 (33.3%) became seizure free in the 4-mg/d Maintenance Period (later responders)
- The safety outcomes were similar, irrespective of therapeutic response, and consistent with the known safety profile of perampanel
- It is recommended to attain an effective dose before making treatment decisions to continue, switch, or withdraw perampanel therapy
1 INTRODUCTION
The main treatment goal for epilepsy is to achieve seizure freedom with a favorable safety profile.1 Antiseizure medications (ASMs) with low risk of treatment-emergent adverse events (TEAEs) could be favorable treatment options for patients with untreated epilepsy, as TEAEs have been shown to contribute to initial treatment failure in >40% of patients with epilepsy and are considered to have a negative impact on individual patients' perceptions of current health status.2 Many ASMs are titrated to an optimal dose based on clinical responses and the observed TEAEs experienced by individual patients.3 Physicians may consider withdrawing, switching, or incorporating another ASM into their patient's schedule if a patient experiences seizures.4 However, some patients may not become seizure free until an effective maintenance dose has been attained, suggesting that early withdrawal from or switching of ASMs during the Titration Period may not be an efficient strategy for the treatment of these patients.3, 5
Perampanel, a selective, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist, is a once-daily oral ASM.6, 7 In the United States and Japan, perampanel is approved for focal-onset seizures (FOS; adjunctive and monotherapy), with or without focal to bilateral tonic-clonic seizures (FBTCS), in patients aged ≥4 years, and for generalized tonic-clonic seizures (adjunctive) in patients aged ≥12 years.6, 8 Perampanel monotherapy for the treatment of FOS was approved on the basis of extrapolation of efficacy and safety data from studies of adjunctive perampanel to the monotherapy setting.9 A real-world study showed that a slow titration schedule of adjunctive perampanel was associated with improved safety outcomes, suggesting that some patients may need an extended Titration Period when initiating treatment with perampanel.10 There is a lack of information on whether patients could benefit from continued treatment with perampanel monotherapy beyond initial titration to achieve expected seizure control with manageable TEAEs, as this has not been previously assessed.
Study 342 is the first study of perampanel monotherapy in patients with newly diagnosed or currently untreated recurrent FOS, with or without FBTCS, in Japan and South Korea.11 Interim results from Study 342 suggest that perampanel monotherapy at 4 mg/d, or up-titrated to 8 mg/d after seizure(s), is efficacious and generally well tolerated, with no new safety signals, in patients aged 12-74 years.11 Seizure-freedom rate in the 26-week Maintenance Period, the primary endpoint of Study 342, was 63.0% (n = 46/73) among patients receiving perampanel 4 mg/d and 74.0% (n = 54/73) at the last evaluated dose of perampanel 4 or 8 mg/d.11 Herein, we report results from this post hoc analysis of Study 342 to evaluate whether continued treatment with perampanel monotherapy, irrespective of seizure(s) during titration, may be appropriate to attain an effective maintenance dose that confers seizure freedom in patients with untreated FOS, with or without FBTCS.
2 METHODS
2.1 Study design
The full methods of Study 342 (FREEDOM; ClinicalTrials.gov identifier: NCT03201900) have been published.11 Briefly, Study 342 is a multicenter, uncontrolled, single-arm, open-label, Phase III clinical trial conducted in Japan and South Korea from June 2017 to July 2020, comprising four phases: Pretreatment (≤4 weeks; clinical characteristics were assessed and documented as baseline), Treatment (4- or 6-week Titration and 26-week Maintenance Periods), Extension, and Follow-up Phases. Patients who completed the Pretreatment Phase (≤4 weeks) initiated treatment on perampanel 2 mg/d for 2 weeks then up-titrated to 4 mg/d for 4 weeks if there were no tolerability issues. Patients who tolerated perampanel 4 mg/d at the end of the Titration Period entered the 4-mg/d Maintenance Period. Patients who experienced a seizure during the 4-mg/d Maintenance Period entered a 4-week Titration Period (perampanel 6 mg/d, then perampanel 8 mg/d, each for 2 weeks), followed by a 26-week 8-mg/d Maintenance Period, per the investigator's discretion. If patients could not tolerate perampanel 8 mg/d, down-titration to 6 mg/d was allowed based on the investigator's assessment. Patients who experienced seizures or who could not tolerate perampanel 6 mg/d during the 8-mg/d Maintenance Period discontinued the Treatment Phase.
After completion of the 4- or 8-mg/d Treatment Phase, patients had the option to enter the Extension Phase to continue perampanel monotherapy at their last dose reached at the end of the Maintenance Period. Dose adjustments (within the range of perampanel 2-8 mg/d) were allowed during the Extension Phase per the investigator's discretion. Patients who finished or discontinued the study returned for the follow-up visit 4 weeks after the withdrawal of perampanel.
The primary endpoint of Study 342 was the seizure-freedom rate (defined as the number [percentage] of patients with FOS who were free from seizures) during the 26-week Maintenance Period. Secondary endpoints included seizure-freedom rate for FOS during the 52-week treatment, and the safety and tolerability of perampanel monotherapy.
2.2 Patients
Eligible patients were aged 12-74 years with newly diagnosed or recurrent FOS, with or without FBTCS, indicating that patients included in Study 342 had untreated epilepsy. Patients should have experienced ≥2 unprovoked seizures, separated by a minimum of 24 hours, within 1 year prior to the Pretreatment Phase, of which ≥1 unprovoked seizure (but below 20 seizures) occurred ≤12 weeks prior to the Pretreatment Phase. In addition, patients with recurrent seizures should have relapsed ≥2 years after the last treatment of prior ASM therapy.
2.3 Post hoc analysis by responder group
The Intent-to-Treat (ITT) Analysis Set included patients who signed the informed consent form, received ≥1 dose of perampanel, and had ≥1 post-dose primary efficacy assessment. The modified ITT (mITT) Analysis Set was a subset of the ITT Analysis Set, and included patients who entered the 4-mg/d Maintenance Period and had ≥1 post-dose primary efficacy measurement during the 26-week Maintenance Period. Efficacy endpoints, including the seizure-freedom rates during the 26-week Maintenance Period, were assessed in the mITT Analysis Set. All TEAEs and serious TEAEs were monitored and analyzed in the Safety Analysis Set, including patients who received ≥1 dose of perampanel and had ≥1 safety assessment.
To investigate whether continued treatment, beyond initial titration, with perampanel monotherapy may be appropriate for patients with untreated epilepsy to attain an effective dose and achieve seizure freedom, a post hoc analysis of the relationship between early response (no seizures reported) during the Titration Period and seizure freedom during the Maintenance Period was performed using efficacy (seizure frequency) data from Study 342. Baseline patient characteristics and efficacy and safety data were collected and analyzed in the mITT population; results were stratified by responder status, which was defined based on the presence or absence of seizures during the 4-mg/d Treatment Phase. Patients who were seizure free during the 4-mg/d Maintenance Period were deemed 4-mg/d responders; otherwise, patients were deemed 4-mg/d nonresponders. Responders were further subcategorized into early responders and later responders, depending on seizure response during the Titration Period. Definitions for 4-mg/d responders with early or later response and 4-mg/d nonresponders are presented in Figure 1. A Mann-Whitney-Wilcoxon test was conducted to compare baseline seizure frequency per 4 weeks between early responders and later responders within the 4-mg/d responder group, and between patients with early response and no early response within the 4-mg/d nonresponder group.

3 RESULTS
3.1 Patients by responder status
A total of 91 patients were enrolled in the study; of these, 89 patients received ≥1 dose of perampanel and were included in both the Safety and ITT Analysis Sets.11 The mITT Analysis Set included 73 patients; of these, 46 patients completed the 4-mg/d Treatment Phase and 21 patients entered the 8-mg/d Treatment Phase per the investigator's discretion after experiencing seizures during the 4-mg/d Maintenance Period. Six patients discontinued during the 4-mg/d Maintenance Period.
Overall, 46 patients (63.0% [n = 46/73]) achieved seizure freedom at perampanel monotherapy 4 mg/d and 54 (74.0% [n = 54/73]) were seizure free at the last evaluated dose of 4 or 8 mg/d. The 46 patients who achieved seizure freedom during the 4-mg/d Maintenance Period were deemed 4-mg/d responders; of these, 37 (80.4%) patients were early responders and nine (19.6%) patients were later responders (Figure 2). Among the 4-mg/d nonresponders (n = 27), nine (33.3%) patients had an early response but went on to experience seizures during the 4-mg/d Maintenance Period, and 18 (66.7%) patients experienced seizures during both the 4-mg/d Titration and Maintenance Periods.

Baseline clinical characteristics and demographics of patients from the mITT Analysis Set, stratified by responder status, are presented in Table 1. The majority of 4-mg/d responder (97.8% [n = 45/46]) and nonresponder (92.6% [n = 25/27]) patients were newly diagnosed with epilepsy. The median (range) baseline seizure frequency per 4 weeks was 0.7 (0.3-7.1) for the overall mITT population. As observed in Table 1, baseline seizure frequency per 4 weeks was significantly lower for early responders compared with later responders in the 4-mg/d responder group (P < .001). In the 4-mg/d nonresponder group, patients with early response had a significantly lower baseline seizure frequency per 4 weeks relative to those with no early response (P = .001).
| 4-mg/d responders (N = 46) | 4-mg/d nonrespondersa (N = 27) | |||||
|---|---|---|---|---|---|---|
| Early responders (n = 37) | Later responders (n = 9) | Total (N = 46) | With early response (n = 9) | No early response (n = 18) | Total (N = 27) | |
| Mean (SD) age,b years | 42.3 (19.3) | 39.8 (16.8) | 41.8 (18.7) | 45.0 (19.6) | 39.9 (18.4) | 41.6 (18.6) |
| Female, n (%) | 19 (51.4) | 3 (33.3) | 22 (47.8) | 4 (44.4) | 8 (44.4) | 12 (44.4) |
| Median (range) time since last diagnosis of epilepsy, monthsc | 0.1 (0-3) | 0.3 (0-2) | 0.1 (0-3) | 0.0 (0-120) | 0.3 (0-13) | 0.2 (0-120) |
| Median (range) seizure frequency per 4 wk | 0.7 (0.3-2.0) | 2.2 (0.7-5.2) | 0.7 (0.3-5.2) | 0.7 (0.3-1.0) | 1.7 (0.3-7.1) | 1.3 (0.3-7.1) |
| P-value | <.001d | .001d | ||||
| Seizure history, n (%) | ||||||
| Newly diagnosed epilepsy | 36 (97.3) | 9 (100.0) | 45 (97.8) | 9 (100.0) | 16 (88.9) | 25 (92.6) |
| Recurrent epilepsy | 1 (2.7) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 2 (11.1) | 2 (7.4) |
| Seizure type,e n (%) | ||||||
| Focal aware without motor signs | 0 (0.0) | 2 (22.2) | 2 (4.3) | 0 (0.0) | 2 (11.1) | 2 (7.4) |
| Focal aware with motor signs | 3 (8.1) | 3 (33.3) | 6 (13.0) | 2 (22.2) | 1 (5.6) | 3 (11.1) |
| Focal impaired awareness | 16 (43.2) | 8 (88.9) | 24 (52.2) | 1 (11.1) | 16 (88.9) | 17 (63.0) |
| FOS with FBTCS | 29 (78.4) | 2 (22.2) | 31 (67.4) | 8 (88.9) | 9 (50.0) | 17 (63.0) |
- Abbreviations: FBTCS, focal to bilateral tonic-clonic seizure; FOS, focal-onset seizure; mITT, modified Intent-to-Treat; SD, standard deviation.
- a Patients were considered nonresponders even if they went on to achieve seizure freedom during the 8-mg/d Treatment Phase.
- b Age is calculated at the date of informed consent.
- c Defined as (screening date - date of diagnosis + 1)/30.5, rounded up to one decimal place.
- d P-values were derived from comparisons of baseline seizure frequency in the responder/nonresponder groups as a whole (not medians, Mann-Whitney-Wilcoxon test).
- e Multiple seizure types may be recorded.
3.2 Efficacy during the 4-mg/d Treatment Phase by responder status
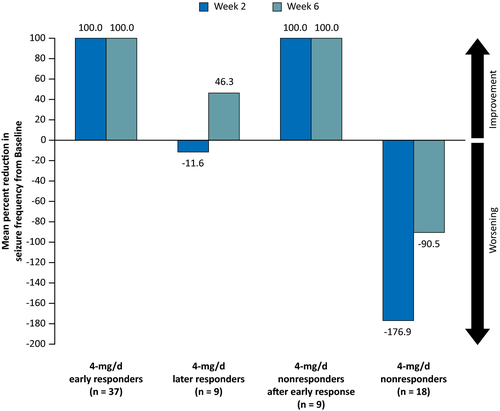
The mean percent reduction in FOS frequency from Baseline, stratified by responder status, at Weeks 2 and 6 of the 4-mg/d Titration Period is presented in Figure 3. Positive values of percent reduction indicate improvements in seizure control from Baseline, whereas negative values of percent reduction indicate worsening of seizures relative to Baseline. Based on the seizure-freedom criteria of early response, by definition, all patients with early response (4-mg/d responder, n = 37; 4-mg/d nonresponder, n = 9) had a 100.0% reduction in FOS frequency from Baseline during the 4-mg/d Titration Period. In contrast, later responders (n = 9) initially experienced a mean (standard deviation [SD]) increase from Baseline in FOS frequency of 11.6% (129.1%) at Week 2 and then reported a mean decrease from Baseline in FOS frequency of 46.3% (97.3%) at Week 6, suggesting clinical responses to perampanel monotherapy 4 mg/d improved during the course of titration. For the 4-mg/d nonresponders (n = 18), there was a mean (SD) increase from Baseline in FOS frequency of 176.9% (468.9%) at Week 2 and of 90.5% (357.3%) at Week 6.
3.3 Safety outcomes
To assess safety outcomes in patients with or without early therapeutic response, an overview of TEAEs during the 4-mg/d Treatment Phase, stratified by responder status, is presented in Table 2. Overall, TEAEs, regardless of causality, occurred in 31 (67.4%) patients who were deemed 4-mg/d responders and in 25 (92.6%) patients who were deemed 4-mg/d nonresponders. The incidences of serious TEAEs among 4-mg/d responders were generally comparable with those among 4-mg/d nonresponders. No patients in the 4-mg/d responder group discontinued from the study due to TEAEs; two (7.4%) of the 4-mg/d nonresponder patients discontinued due to TEAEs. Overall, safety outcomes were consistent with the known safety profile of perampanel, regardless of therapeutic response.
| 4-mg/d responders (N = 46) | 4-mg/d nonrespondersa (N = 27) | |||||
|---|---|---|---|---|---|---|
| Early responders (n = 37) | Later responders (n = 9) | Total (N = 46) | With early response (n = 9) | No early response (n = 18) | Total (N = 27) | |
| All TEAEs, n (%) | 23 (62.2) | 8 (88.9) | 31 (67.4) | 9 (100.0) | 16 (88.9) | 25 (92.6) |
| Serious TEAEs, n (%) | 3 (8.1) | 1 (11.1) | 4 (8.7) | 1 (11.1) | 2 (11.1) | 3 (11.1) |
| Treatment-related TEAEs, n (%) | 10 (27.0) | 7 (77.8) | 17 (37.0) | 8 (88.9) | 10 (55.6) | 18 (66.7) |
| Most common TEAEs (occurring in ≥7 patients in total), n (%) | ||||||
| Dizziness | 8 (21.6) | 2 (22.2) | 10 (21.7) | 5 (55.6) | 8 (44.4) | 13 (48.1) |
| Nasopharyngitis | 5 (13.5) | 4 (44.4) | 9 (19.6) | 2 (22.2) | 1 (5.6) | 3 (11.1) |
| Headache | 3 (8.1) | 2 (22.2) | 5 (10.9) | 2 (22.2) | 2 (11.1) | 4 (14.8) |
| Somnolence | 2 (5.4) | 3 (33.3) | 5 (10.9) | 1 (11.1) | 2 (11.1) | 3 (11.1) |
| Most common treatment-related TEAEs (occurring in ≥7 patients in total), n (%) | ||||||
| Dizziness | 7 (18.9) | 2 (22.2) | 9 (19.6) | 5 (55.6) | 7 (38.9) | 12 (44.4) |
| Somnolence | 1 (2.7) | 3 (33.3) | 4 (8.7) | 1 (11.1) | 2 (11.1) | 3 (11.1) |
- Abbreviations: mITT, modified Intent-to-Treat; TEAE, treatment-emergent adverse event.
- a Patients who were considered nonresponders even if they went on to achieve seizure freedom during the 8-mg/d Treatment Phase.
TEAEs that were considered to be related to the treatment by the investigator occurred in 17 (37.0%) 4-mg/d responders and 18 (66.7%) 4-mg/d nonresponders. The most common treatment-related TEAEs, irrespective of responder status, were dizziness and somnolence. The proportion of patients who experienced treatment-related dizziness was greater in the 4-mg/d nonresponders group compared with the 4-mg/d responders group (44.4% vs 19.6%, respectively), although the patient population of nonresponders was relatively small (n = 27). The incidences of treatment-related somnolence were comparable between the 4-mg/d responder and nonresponder groups (8.7% vs 11.1%, respectively).
4 DISCUSSION
Study 342 was designed to investigate the efficacy and safety of perampanel as a monotherapy, initiated at 2 mg/d and then up-titrated to 4 or 8 mg/d, in patients with newly diagnosed or currently untreated recurrent FOS, with or without FBTCS. For patients with newly diagnosed epilepsy, ASM monotherapy is commonly prescribed for the management of seizures and associated with increasing likelihood of achieving seizure freedom.3, 5 If patients do not tolerate the initial monotherapy or experience seizures during titration, physicians typically either switch to an alternative ASM monotherapy or initiate combination therapy with two or more ASMs. Appropriate titration of ASMs is critical to attain an effective dose as well as to improve tolerability. As such, the titration schedule is often individualized based on several factors, including the pharmacokinetic profile of each ASM, and the clinical characteristics and therapeutic responses of individual patients.3 As the half-life of perampanel is approximately 105 hours, it takes a relatively long time (e.g., 2-3 weeks) before steady-state plasma perampanel concentrations are reached. Therefore, an adequate Titration Period is warranted to allow steady state to be reached before therapeutic response is assessed, to inform treatment decisions such as continuing, switching, or discontinuing perampanel therapy. This post hoc analysis of Study 342 efficacy data aimed to examine the association between early response during the 6-week 4-mg/d Titration Period and seizure freedom during the 26-week 4-mg/d Maintenance Period, and evaluate whether continued treatment with perampanel monotherapy may be appropriate for patients to achieve seizure freedom with an effective dose.
Among the 46 4-mg/d responders, the median seizure frequency at baseline was <1 seizure per 4 weeks. Hence, the absence of seizures during the 4-mg/d Titration Period may not be enough to indicate therapeutic response given the short observation time (6 weeks). However, the majority of 4-mg/d responders (80.4% [n = 37/46]) showed an early response with sustained seizure freedom for up to 32 weeks (starting from the initiation of perampanel monotherapy until the end of the 4-mg/d Maintenance Period). Patients who recorded a higher seizure frequency every 4 weeks prior to the initiation of perampanel treatment were more likely to experience seizures during the Titration Period; however, some of these patients (33.3% [n = 9/27]) were able to achieve seizure freedom during the 26-week Maintenance Period, therefore becoming later responders, having experienced ≥1 seizure during the Titration Period. Furthermore, eight of the nine later responders had maintained seizure freedom for up to 52 weeks during the Extension Phase of Study 342 (data on file, Eisai Co., Ltd., Tokyo, Japan). Indeed, the efficacy results of this post hoc analysis are in line with results from a previous post hoc analysis of Study 342,12 which showed that baseline seizure frequency was the best predictor of 26 weeks of seizure freedom. Together, these data suggest that baseline seizure frequency could be a relevant clinical factor to guide physicians when evaluating a patient's therapeutic response to perampanel monotherapy early on.
TEAEs experienced during the initial Treatment Period of an ASM could limit a patient's ability to tolerate an effective ASM dose, and it is inappropriate to evaluate clinical responses when an effective dose is not reached. Physicians may then adopt a “start slow, go slow” approach in routine practice to minimize the risk of TEAEs.13 A previous study showed that a slow titration schedule of adjunctive perampanel (increments of 2 mg/d no more frequently than at biweekly intervals) was associated with a lower overall incidence of TEAEs.10 Therefore, starting treatment with perampanel at 2 mg/d and then up-titrating to an effective dose of ≥4 mg/d over the course of several weeks, irrespective of the occurrence of seizure(s) during the initial titration, could be an appropriate approach to improve tolerability and to ensure adequate opportunity to reach a therapeutically effective dose for achieving treatment goals (seizure reduction and/or seizure freedom). Findings from this post hoc analysis of Study 342 suggest that continued treatment beyond initial titration may be appropriate to attain an effective dose of perampanel monotherapy in patients who had higher baseline seizure frequency before switching or discontinuing from perampanel monotherapy per clinical response during titration.
5 CONCLUSION
These results indicate that perampanel monotherapy could be an efficacious and well-tolerated treatment option in patients aged ≥12 years with untreated FOS, with or without FBTCS. It is recommended that treatment decisions should not be solely based on the presence or absence of seizures and/or TEAEs during perampanel titration, as efficacy may not be apparent until perampanel dose reaches ≥4 mg/d, which is the minimum effective dose recommended for perampanel monotherapy.7
ACKNOWLEDGMENTS
The authors would like to thank the study participants. The FREEDOM study (NCT03201900) was funded by Eisai Co., Ltd. Medical writing support, under the direction of the authors, was provided by Can Huang, PhD, of CMC AFFINITY, McCann Health Medical Communications, funded by Eisai Inc., in accordance with Good Publication Practice (GPP3) guidelines. The data reported in this paper have been previously presented at the Virtual American Academy of Neurology Annual Meeting, April 17-22, 2021; the 13th Virtual Asian and Oceanian Epilepsy Congress, June 10-13, 2021; the 34th International Epilepsy Congress, August 28-September 1, 2021; and the 54th Annual Congress of the Japan Epilepsy Society, Nagoya, Japan, September 23-25, 2021.
CONFLICTS OF INTEREST
Ryan Edbert Husni, Hirofumi Senokuchi, Hidetaka Hiramatsu, and Kazuaki Watanabe are employees of Eisai Co., Ltd. Leock Y Ngo is an employee of Eisai Inc. Anna Patten is an employee of Eisai Europe Ltd. Takamichi Yamamoto has received speaker's honoraria from Daiichi-Sankyo, Eisai, LivaNova, Otsuka Pharmaceutical, and UCB Japan; participated in advisory boards for Eisai; and served as Sponsor's Responsible Medical Officer for the FREEDOM Trial (Study 342).
AUTHOR CONTRIBUTIONS
Ryan Edbert Husni, Leock Y Ngo, Hirofumi Senokuchi, Hidetaka Hiramatsu, and Kazuaki Watanabe contributed to the conception, design, and data analysis and interpretation of this post hoc analysis. Anna Patten conducted the statistical analysis of the data. Takamichi Yamamoto served as Sponsor's Responsible Medical Officer of this study. He contributed to the study design and protocol development. All authors had access to the study data, were involved in the decision to submit this article for publication, contributed to data interpretation, reviewed the manuscript, and approved the final version.
ETHICAL APPROVAL
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.