Claustrum hyperintensities: A potential clue to autoimmune epilepsy
Summary
In a cohort of 34 patients with autoimmune limbic encephalitis and/or epilepsy, we identified 4 patients exhibiting claustrum fluid-attenuated inversion recovery (FLAIR) hyperintensities. All 4 patients presented with explosive onset of seizures and developed medically intractable epilepsy, and 2 exhibited a marked response to immunotherapy. Associated features included cognitive and behavioral disturbances (4/4), cerebrospinal fluid (CSF) lymphocytic pleocytosis (3/4), and a neural autoantibody (2/4). Electroencephalogram (EEG) features consisted of slow wave activity and epileptiform discharges in frontal and parasagittal regions, where ictal patterns were captured in 1 patient. In 1 patient, magnetoencephalographic source imaging of interictal spikes revealed dipole sources in anterior insular or subinsular localizations, mirroring claustrum FLAIR hyperintensities, which developed after a short lag from presentation and resolved in all but 1 patient. These MRI abnormalities were isolated (2/4) or associated with mesial temporal hyperintensities (2/4). Claustrum FLAIR hyperintensities may be a useful MRI marker of autoimmune epilepsy.
Accumulating evidence that intractable epilepsy may follow limbic encephalitis,1, 2 and that isolated refractory epilepsy may be associated with neural autoantibodies,3 has led to increased recognition of autoimmune epilepsy. Reported radiologic features of autoimmune epilepsy include either unilateral or bilateral mesial temporal changes, neocortical hyperintensities involving any lobe,4 and basal ganglia hyperintensities,5 specifically in leucine-rich glioma-inactivated 1 (LGI1) epilepsy.
In a cohort of 34 patients with autoimmune limbic encephalitis and/or epilepsy, we noted striking bilateral claustrum T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in 4 patients. We describe the clinical, neurophysiologic, and radiologic features of the patients harboring this abnormality.
Methods
Thirty-four patients with autoimmune limbic encephalitis and/or autoimmune epilepsy were retrospectively identified within the academic hospitals affiliated with the University of Toronto, through an autoimmune clinic at the University Health Network where patients are typically referred for follow-up care after the initial diagnosis of autoimmune encephalitis is made. The cases satisfy the previously published criteria for autoimmune epilepsy6 and/or for autoimmune limbic encephalitis.7
Among this cohort of patients, 4 patients were noted to have claustrum FLAIR hyperintensities and were selected for further chart review. The clinical course and electroencephalogram (EEG) findings were obtained and serial MRI results were reviewed in detail by a board-certified neuroradiologist (T.K.). Infectious causes of encephalitis were excluded in all cases and are not outlined in detail in the following case descriptions. Antineuronal autoantibody panels included antibodies to amphiphysin, Ri, Yo, Hu, PNMA2 (Ma2/Ta), CV2/CRMP-5, Recoverin, SOX1, Titin, NMDA, VGKC (LGI1, CASPR2), GAD65, and AMPA-R. GABAA receptor antibodies could not be assessed resulting from lack of commercial availability. Age-appropriate malignancy screens were negative in all patients.
Magnetoencephalography (MEG) was performed in 1 patient as part of a presurgical investigation for intractable epilepsy. MEG acquisition and MEG source imaging (MSI) methods are described in Appendix S1.
The study was approved by the institutional Research Ethics Board, and consent was waived because of the retrospective nature of the study.
Results
Patient 1
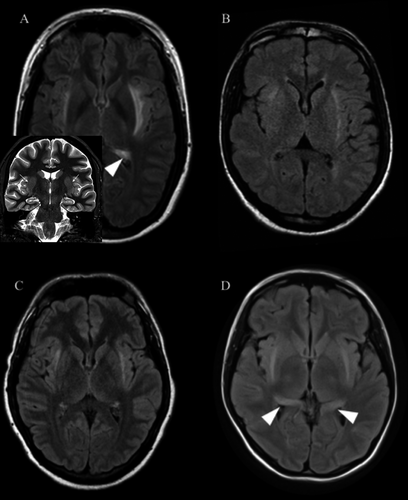
A 23-year-old woman had developed spells of unexplained falls and staring that were unresponsive to multiple antiepileptic drugs (AEDs) 5 years prior to presentation. She then presented to our institution 4 months after the onset of generalized tonic-clonic (GTC) seizures, episodes of unresponsiveness, and rapidly progressive memory impairment. MRI showed bilateral mesial temporal and claustrum hyperintensities (Fig. 1A). Cerebrospinal fluid (CSF) analysis revealed high titers of glutamic acid decarboxylase (GAD) antibody (also present in serum), lymphocytic pleocytosis (10 WBC), oligoclonal banding, and an elevated immunoglobulin G (IgG) index. EEG revealed left and right parasagittal sharp waves. She did not show any significant clinical improvement after receiving an initial immunotherapy trial in the form of pulse steroids and intravenous immunoglobulin (IVIg). Thereafter, she started experiencing frequent seizures consisting of somatosensory auras, followed by unresponsiveness, along with severe memory impairment and mood disturbance. Continuous EEG recordings in the Epilepsy Monitoring Unit (EMU) revealed right centroparietal, frontocentral, and left anterior temporal interictal epileptiform discharges and captured several seizures with right frontotemporal ictal onsets that quickly propagated to the parasagittal region. She remained intractable to multiple AEDs and further immunotherapy trials (steroids, mycophenolate mofetil). Repeat MRI scans continued to demonstrate mesial temporal and claustrum hyperintensities.
Patient 2
A 36-year-old man presented with severe encephalopathy after a febrile illness, followed by convulsive status epilepticus, the latter continuing despite first-line treatment and requiring anesthesia and up-titration of three AEDs. CSF lymphotic pleocytosis (20 WBC) was noted, and antineuronal antibody panel was negative. Although initial MRI was unrevealing, a repeat study 12 days after presentation showed bilateral claustrum hyperintensities (Fig. 1B). He received empiric immunotherapy trials with pulse steroids and plasmapheresis. Seizures abated, and upon weaning of anesthesia and extubation, the patient exhibited severe cognitive impairment. Further immunotherapy was administered with IVIg and high-dose oral steroids. Two months after discharge from the hospital, he began experiencing seizures characterized by an auditory and psychic aura, followed by loss of awareness and, on occasion, GTC seizures. Prednisone taper was associated with worsening of seizures, which then responded to reinitiation of immunotherapy rather than conventional AED up-titration. Repeat MRI showed resolution of claustrum hyperintensities. EEGs revealed right frontal and frontotemporal intermittent slow waves and sharp waves, along with left frontal intermittent slow wave activity.
Patient 3
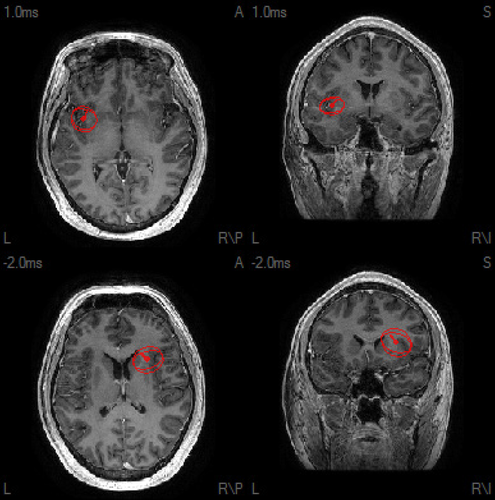
A 25-year-old man was transferred to our institution with a refractory convulsive then nonconvulsive status epilepticus that was preceded by a febrile illness. CSF lymphocytic pleocytosis (31 WBC) was noted, and antineuronal antibody panels were negative. After up-titration of several AEDs, the status epilepticus resolved. After anesthesia was weaned and he was extubated, he developed frequent brief hypermotor or akinetic seizures involving either arm, preceded by painful somatosensory auras, that were refractory to further AED titration. EEGs and EMU recordings showed independent bifrontal and frontocentral parasagittal interictal epileptiform discharges and ictal patterns. Pulse steroids led to significant reduction in frequency of seizures, which then rebounded, leading to further immunotherapy (high-dose oral steroids and IVIg). Over the next months, he exhibited fluctuation of seizure burden, mirroring immunotherapy pulses, then stopped showing immunotherapy response. Additional seizure semiology included evolution to axial hypermotor behavior. MEG of bilateral independent frontal interictal epileptiform discharges revealed bilateral anterior insular and subinsular dipole source solutions (Fig. 2 and Appendix S1), mirroring the claustrum hyperintensities initially noted upon presentation (Fig. 1C), which had resolved by the time MSI was performed.
Patient 4
A 19-year-old woman presented with cognitive and behavioral disturbance and seizures with unresponsiveness and oroalimentary automatisms that evolved to convulsive status epilepticus requiring anesthesia and up-titration of five AEDs. Upon extubation, disinhibition and confusion in conjunction with mesial temporal and claustrum hyperintensities (Fig. 1D) led to a presumptive diagnosis of autoimmune limbic encephalitis and empiric treatment with pulse steroids and IVIg. An initial response was noted, but her seizures and behavioral disturbance reemerged upon steroid taper. Later, in our institution, high titers of anti-Ma2 antibodies were demonstrated in CSF and serum. She continued to experience frequent seizures characterized by psychic auras and out-of-body experiences, followed by unresponsiveness. EEGs showed right frontotemporal and parasagittal sharp waves. Despite immunotherapy, the patient continues to have intractable epilepsy and severe cognitive impairment.
Imaging features
Initial MRI was normal in 2/4 cases and showed unilateral or bilateral mesial temporal hyperintensities in the other 2/4 cases. There was a lag between time of clinical symptom onset and time of claustrum changes on MRI from 10 days to 4 months (on average 41 days). Claustrum hyperintensities resolved an average of 102 days after first appearance but did not resolve in 1 patient (Patient 1). In 2 patients, claustrum hyperintensities were the only radiologic finding.
Discussion
Four patients with autoimmune epilepsy—diagnosed on the basis of the clinical presentation, CSF profile, presence of antineuronal autoantibody, and/or MRI features—exhibited striking claustrum FLAIR hyperintensities that resolved upon follow-up imaging despite ongoing medically intractable epilepsy.
Bilateral claustrum hyperintensities have been described in patients with refractory status epilepticus following febrile illness,8 none of whom harbored neural autoantibodies, in addition to 14 previous isolated reports of this radiologic finding in association with new-onset refractory seizures.8 Bilateral claustrum lesions had also previously been reported in a patient with voltage-gated potassium channel antibodies and limbic encephalitis in whom no information was available regarding the presence of seizures.9 Subinsular changes have been described in GABAA encephalitis,10 but also acute hyperammonemic encephalopathy,11 albeit accompanying other widespread cortical and subcortical changes. Meanwhile, insular cortex itself has been shown to be abnormal in N-methyl-D-aspartate receptor (NMDAR) encephalitis.12 However, in the cohort described here, there was no evidence of insular cortex changes in association with the described claustrum hyperintensities. To our knowledge, this is the first reported case series of patients with autoimmune epilepsy exhibiting claustrum hyperintensities, although one may postulate a similar immune-mediated mechanism in previously reported cases of new-onset refractory status epilepticus. Despite the absence of known neuronal autoantibodies in 2/4 patients, the clinical presentation and ancillary testing enabled a diagnosis of autoimmune encephalitis and epilepsy.7
Common electroclinical correlates among these 4 patients include a variety of auras (somatosensory, psychic, auditory, “out of body”) with progression to loss of awareness or, in one case, hypermotor or akinetic seizures, and corresponding frontal or parasagittal ictal and interictal EEG patterns. In 1 patient, MSI of interictal spikes revealed dipole sources in bilateral anterior insular and subinsular localizations. Although formulating a unifying electroclinical syndrome is challenging, epilepsy characteristics expand far beyond mesial temporal epilepsy, typically thought of in association with limbic encephalitis and subsequent mesial temporal sclerosis,1 and invoke additional areas such as perisylvian opercular and insular and frontal regions. Indeed, mirroring the multiple aura types experienced by the 4 patients, electrical stimulation studies13 have evoked multimodal responses from the insula. Processing and integration of such responses may occur in conjunction with various subcortical structures, leading to homeostasis and emotional regulation.14 Therefore, the electroclinical syndrome described here may involve the insula and its connected regions. Previously, formulation of distinct electroclinical syndromes associated with autoimmune mechanisms of epilepsy has included the description of LGI1 faciobrachial dystonic seizures,15 tonic-dystonic seizures,16 tonic spasms,17 and subclinical temporal lobe electrographic seizure patterns.18 These findings help to widen the electroclinical spectrum and enable suspicion and diagnostic testing for these treatable entities.
Similar to the basal ganglia hyperintensities noted in LGI1 epilepsy,5 we hypothesize that activation of an epileptic network in these autoimmune epilepsies leads to subcortical hyperintensities in areas connected to epileptogenic cortex. Involvement of the claustrum may reflect its well-recognized bidirectional connections to multiple neocortical and allocortical areas, including the limbic system.19 Therefore, claustrum hyperintensities seen in the initial stage of the illness may represent a concentration of dysfunction of connected areas undergoing immune-mediated epileptogenesis. Further studies are needed to explore these findings in a larger group of patients with autoimmune epilepsy.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.





