Vascular syndrome predicts the development and course of epilepsy after perinatal stroke
The data provided in this manuscript has not been presented elsewhere.
This study is not a clinical trial and therefore is not registered as one.
Abstract
Objective
Epilepsy develops in one third of the patients after perinatal stroke. It is still unclear which vascular syndrome of ischemic stroke carries higher risk of epilepsy. The aim of the current study was to evaluate the risk of epilepsy according to the vascular syndrome of perinatal stroke.
Methods
The study included 39 children with perinatal arterial ischemic stroke (13 with anterior or posterior trunk of the distal middle cerebral artery occlusion, 23 with proximal or distal M1 middle cerebral artery occlusion and three with lenticulostriate arteria infarction), and 44 children with presumed perinatal venous infarction. Magnetic resonance imaging obtained at the chronic stage was used to evaluate the vascular syndrome of stroke.
Results
The median follow-up time was 15.1 years (95% CI: 12.4–16.5 years), epilepsy developed in 19/83 (22.9%) patients. The cumulative probability to be without epilepsy at 15 years was 75.4% (95% CI: 65.8–86.4). The probability of having epilepsy was higher in the group of proximal or distal M1 artery occlusion compared to patients with periventricular venous infarction (HR 7.2, 95% CI: 2.5–26, p = .0007). Patients with periventricular venous infarction had significantly more often status epilepticus or spike–wave activation in sleep ≥85% of it compared to patients with anterior or posterior trunk of the distal middle cerebral artery occlusion (OR = 81; 95% CI: 1.3–5046, p = .029).
Significance
The emphasis of this study is placed on classifying the vascular syndrome of perinatal stroke and on the targeted follow-up of patients for epilepsy until young adulthood. The risk for having epilepsy after perinatal stroke is the highest in children with proximal or distal M1 middle cerebral artery occlusion. Patients with periventricular venous infarction have a more severe course of epilepsy.
Abbreviations
-
- AIS
-
- arterial ischemic stroke
-
- AT
-
- anterior trunk of the distal middle cerebral artery infarction
-
- DMI
-
- distal M1 middle cerebral artery infarction
-
- IED
-
- interictal epileptiform discharges
-
- LLS
-
- lateral lenticulostriate arteries
-
- MCA
-
- middle cerebral artery
-
- PMI
-
- proximal M1 middle cerebral artery infarction
-
- PT
-
- posterior trunk of the distal middle cerebral artery infarction
-
- PVI
-
- periventricular venous infarction
Key points
- The probability of developing epilepsy is different in distinct vascular syndromes of perinatal stroke.
- The highest probability of developing epilepsy occurs in children with proximal or distal M1 middle cerebral artery infarction and the risk persists until young adulthood.
- In children with periventricular venous infarction, epilepsy develops before school age, but they are at the highest risk for having status epilepticus or spike–wave activation in sleep ≥85% of it.
1 INTRODUCTION
Perinatal stroke is focal vascular brain injury which occurs between 20 weeks of fetal life through the 28th postnatal day.1 Based on the time of diagnosis, ischemic perinatal stroke is classified into fetal ischemic stroke, neonatal ischemic stroke and presumed perinatal ischemic stroke. The most frequent vascular types of ischemic perinatal stroke are arterial ischemic stroke (AIS) and periventricular venous infarction (PVI).1-3 According to anatomical and vascular classifications, the following vascular syndromes are recognized: (1) proximal M1 middle cerebral artery (MCA) infarction (PMI), (2) distal M1 MCA infarction (DMI), (3) anterior trunk (AT) of the distal MCA infarction, (4) posterior trunk (PT) of the distal MCA infarction, (5) lateral lenticulostriate arteries (LLS) infarction, and (6) PVI.2 According to population-based data, estimated birth prevalence is 1:3000 for neonatal AIS, 1:7900 for presumed perinatal AIS and 1:6000 for PVI.4
Epilepsy is one of the serious consequences after perinatal stroke.5, 6 According to one of the latest population-based studies, the risk of epilepsy is the highest during the first 6 months, but remains elevated 20 years after ischemic stroke.7 The incidence of epilepsy after perinatal stroke varies in different studies depending on follow-up time and the vascular type. At 2 years it is 11% for neonatal AIS and 19% for presumed perinatal AIS, but at 10.4 years it is 27.2% for perinatal AIS.8, 9 Previously we have found a rate of 71% of poststroke epilepsy for neonatal AIS and 6% for PVI.10 At 3 years the probability of remaining seizure free after perinatal AIS was 73%.11
Other studies have linked the development of epilepsy to extensive cortical damage, multiple strokes, and simultaneous cortical and basal ganglia impairment.8, 10, 12 A recent volumetric study revealed association of smaller thalamus and basal ganglia with development of epilepsy after perinatal ischemic stroke.13
It is still unclear which vascular syndrome of ischemic stroke carries higher risk of epilepsy.
The aim of the current study was to evaluate which vascular syndrome has higher probability for developing poststroke epilepsy and affecting the course of epilepsy after perinatal stroke. We hypothesized that epilepsy develops more often in children with PMI or DMI, compared to children with AT or PT infarction, or compared to children with PVI.
2 MATERIALS AND METHODS
This is an observational regional population-based cohort study of patients with ischemic perinatal stroke. The study is part of a larger research on outcome in children with perinatal stroke.3, 10, 13-17
2.1 Participants
Patients were identified from the Estonian Pediatric Stroke Database and all patients with perinatal ischemic stroke were invited to participate in the outcome study. All children with pediatric stroke admitted to the Children's Clinic of Tartu University Hospital are included in the Pediatric Stroke database. The Children's Clinic of Tartu University Hospital is one of the two third level centers for neonatal intensive care and child neurology in Estonia and serves the southern and eastern parts of the country. Data in the Estonian Pediatric Stroke Database was collected retrospectively within an epidemiological study from 1994 to 2003 and prospectively from 2004.10, 14
The study participants fulfilled all of the inclusion criteria: (1) magnetic resonance imaging (MRI) or computed tomography-confirmed diagnosis of unilateral perinatal stroke; (2) birth at gestational age ≥36 weeks; (3) clinical follow-up of at least 18 months. The exclusion criteria were: (1) structural disease other than stroke affecting the central nervous system (hypoxic–ischemic encephalopathy, central nervous system's infectious disease, tumor, cortical malformation, congenital anomaly); (2) specific disease-causing gene variant or copy number variant suggested to be pathogenic for epilepsy, or developmental delay; (3) absence of neuroimaging-confirmed stroke.
2.2 Clinical data
Clinical information of the study patients about pregnancy, delivery and the neonatal period, as well as about the time of the first epileptic seizure, the time of epilepsy diagnosis, and the clinical features of epilepsy was collected from medical records. At the last follow-up for Kaplan–Meier estimation, the presence of epilepsy was ascertained from the digital national Health Portal which provides patients' health and prescriptions' data in case the on-site visit is not possible. Clinical outcome was evaluated by one of the two child neurologists at the follow-up visit (UV, RL). Pediatric Stroke Outcome Measure was used for evaluation of global outcome.18
2.3 Neuroimaging
All radiological images (cerebral ultrasonography, computed tomography and MRI) are stored in the population-based Estonian Picture Archive. The images of the patients in the Estonian Pediatric Stroke Database were independently reviewed by a radiology resident (No. I) and two neuroradiologists (PI and DL) who were blinded to the clinical outcome of the patients. By consensus agreement the patients were classified as having the vascular syndrome based on previous classifications.2 MRI for this study was performed in the chronic stage of perinatal stroke without anesthesia at the age of 6–18 years (n = 66). A 3T Philips Achieva MRI scanner was used with a 8-channel SENSE head coil (Philips Medical Systems, Best, The Netherlands). In children who were not able to complete this investigation, earlier MRI (n = 14) or computed tomography (n = 2) images done at the age of 1 month or older for clinical purposes were used, except in the case of one child whose MRI investigation from the age of 6 days was used to confirm stroke.
2.4 Classification of perinatal stroke according to the time of diagnosis and vascular syndrome
Neonatal ischemic stroke is diagnosed after birth, before or on the 28th postnatal day. Presumed perinatal ischemic stroke is diagnosed in infants >28 days of age after the normal neonatal period if neuroimaging reveals signs of chronic infarction and it is presumed that ischemic event has occurred between the 20th week of fetal life through the 28th postnatal day.1, 3
According to the vascular type, ischemic stroke was classified as AIS or PVI. According to the vascular syndrome, ischemic stroke comprises: (1) PMI compromising both lateral lenticulostriate arteries with infarction in the basal ganglia and the distal MCA territory; (2) DMI involving the distal MCA; (3) AT occlusion of the distal MCA compromising the frontal lobe anteriorly of the central sulcus and the anterior temporal lobe; (4) PT occlusion of the distal MCA compromising the parietal lobe posteriorly of the central sulcus and the posterior temporal lobe; (5) LLS infarction with involvement of the basal ganglia and the posterior limb of the internal capsule; (6) PVI as infarction of the periventricular white matter in the medullary venous territory.2
2.5 Epilepsy and EEG
Epilepsy was diagnosed if it met one of the following conditions: (1) at least two unprovoked seizures occurring >24 h apart; (2) one unprovoked seizure with high recurrence risk.19 High recurrence risk was defined if a single seizure occurred at least 1 month after stroke and was accompanied with structural changes on MRI and epileptiform changes on electroencephalography (EEG).19 All epilepsy diagnoses were reviewed and confirmed by a child neurologist and an EEG specialist (UV).
To classify and describe the course of epilepsy, we used a modified version of the Engel classification: class 0—seizure free and off antiseizure medication for >6 months; class 1—seizure free for at least 6 months while on medication or seizure free off medication for <6 months; class 2—on medication, fewer than one seizure a month; class 3—one to four seizures a month; class 4—five to 30 seizures a month; and class 5—>30 seizures a month.20 Status epilepticus was defined as a condition leading to prolonged seizures, using the time point of 5 min for bilateral tonic–clonic status epilepticus and 10 min for focal status epilepticus with impaired consciousness.21 Spike-wave activation in sleep was defined as an EEG pattern which consists of continuous spike-and-slow-waves during sleep, affecting ≥85% of sleep.22, 23 Drug resistant epilepsy was defined as failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drug schedules to achieve sustained seizure freedom.24 We defined complicated epilepsy: (1) modified Engel class ≥3; (2) history of status epilepticus; (3) history of spike-wave activation in sleep; or (4) drug resistant epilepsy.
Standard EEG in the postneonatal period included the awake and sleep (daytime nap) periods. An international full 10–20 system was adopted for electrode placement.25 Focal interictal epileptiform discharges (IED) were defined as transient epileptiform activity manifesting itself up to two electrodes consistent with the same anatomical location. Regional IEDs were defined if IED appeared in more than two electrodes, but in the same hemisphere, and in case bilateral IED epileptiform activity was represented in both hemispheres. For classifying localization of background activity changes, the same definition was used as for IED. All EEG investigations from 2005 to 2011 were stored on digital video discs and from 2011 onward in the neurophysiology database of Tartu University Hospital.
2.6 Statistical analysis
The statistical package SAS version 9.4 (SAS Institute, Cary, NC) and RStudio were employed for statistical analysis. The Shapiro–Wilk test was used for assessment of normality. Continuous data were summarized as means with the 95% confidence interval (CI) or medians with the interquartile range (IQR), and categorical data, as absolute counts and percentages. Differences between the groups were analyzed with the Student's t-test or by the nonparametric Mann–Whitney U test for continuous variables. To compare the proportions, the chi-square test, Fisher's exact test (when expected values were less than five) and Firth's logistic regression were used. Odds ratio (OR) and the 95% CI were used to estimate the measure of association.
We applied the Kaplan–Meier estimation of the proportion of subjects at any point during follow-up. Age at the first epileptic seizure was used for calculating cumulative incidence. An indicator of the length of follow-up was the median follow-up time whose calculation was based on the reverse Kaplan-Meier estimator.26 The log-rank statistic and the Cox proportional hazards regression were used to assess differences between the survival curves. All P values were two-sided.
2.7 Ethics
The study was approved by the Research Ethics Committee of the University of Tartu. Written informed consent was provided by all individual participants older than 7 years who were able to read, as well as by their parents.
3 RESULTS
3.1 Population
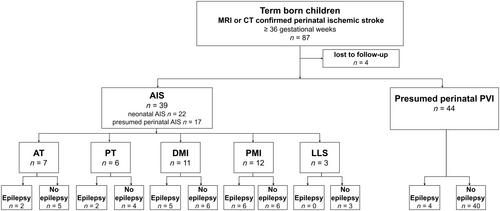
Eighty-seven patients from the Estonian Pediatric Stroke Database met the criteria of perinatal ischemic stroke and had been born at a gestational age of ≥36 weeks. Four patients were lost to outcome studies. The final study group consisted of 83 patients with perinatal stroke: 39 (47%) with AIS (patients with AT or PT occlusion n = 13 [15.7%]; patients with PMI or DMI occlusion n = 23 [27.7%]; patients with LLS infarction n = 3 [3.6%]), and patients with presumed perinatal PVI n = 44 (53%). There were 22/39 patients with neonatal AIS and 17/39 patients with presumed perinatal AIS. The flow chart of the study population is presented in Figure 1.
During a median follow-up of 15.1 years (95% CI: 12.4–16.5 years) for the study group epilepsy developed in 22.9% (19/83) of the patients. In 58% (11/19) of the patients epilepsy was diagnosed after the first seizure. The median age at follow-up for patients with epilepsy was higher compared to patients without epilepsy, 19.8 years [IQR: 14.0–23.1] and 14.9 years [IQR: 9.2–17.3], p = .004, respectively.
There were no differences in gender, gestational age, Apgar score, need for emergency cesarean section, presence of neonatal seizures or side of stroke between patients who developed epilepsy and those without epilepsy (Table 1). Pediatric Stroke Outcome Measure score was higher in the group of children with epilepsy compared to patients without epilepsy (p = .018).
| Epilepsy (n = 19) | Without epilepsy (n = 64) | p Value | OR (95% CI) | |
|---|---|---|---|---|
| Male gender, n (%) | 6 (31.6) | 32 (50.0) | .16 | 2.2 (0.7–6.4) |
| Gestational weeks at birth, median [IQR] | 40 [39–40] | 40 [38–40] | .22 | |
| Apgar score at 1 min, median [IQR] |
8 [7–8] NA = 1 |
8 [7–9] NA = 3 |
.37 | |
| Apgar score at 5 min, median [IQR] |
8 [8–9] NA = 1 |
9 [8–9] NA = 3 |
.26 | |
| Emergency cesarean section, n (%) | 4 (21.1) |
19/63 (30.2) NA = 1 |
.44 | 1.6 (0.5–5.5) |
| Neonatal seizures, n (%) | 5 (26.3) | 11 (17.2) | .51 | 1.7 (0.5–5.8) |
| Side of stroke left, n (%) | 15 (79.0) | 37 (57.8) | .095 | 2.7 (0.8–9.2) |
| Small for gestational age <3 percentiles, n (%) | 1 (5.3) |
5/63 (7.9) NA = 1 |
>.99 | 1.6 (0.16–77) |
| AIS, n (%) | 15 (79.0) | 24 (37.5) | .0015 | 6.3 (1.9–21) |
| Neonatal AIS | 8 (42.1) | 14 (21.9) | .079 | 2.6 (0.9–7.7) |
| Presumed perinatal AIS | 7 (36.8) | 10 (15.6) | .057 | 3.2 (0.8–11) |
| PVI | 4 (21.1) | 40 (62.5) | ||
| Vascular syndromes of AIS, n (%) | ||||
| AT + PT | 4 (26.7) | 9 (37.5) | 0.23 | |
| LLS | 0 (0) | 3 (12.5) | ||
| PMI + DMI | 11 (73.3) | 12 (50.0) | ||
| Age (median) at follow-up for epilepsy, years, [IQR] | 19.8 [14.0–23.1] | 14.9 [9.2–17.3] | .004 | |
| PSOM score, median [IQR] | 2.5 [2.0–4.0] | 2.0 [1.0–2.8] | .018 | |
- Abbreviations: AIS, arterial ischemic stroke; AT, anterior trunk of the distal middle cerebral artery (MCA) infarction; CI, confidence interval; DMI, distal M1 MCA infarction; IQR, interquartile range; LLS, lenticulostriate arteries infarction; NA, not available; OR, odds ratio; PMI, proximal M1 MCA infarction; PSOM, Pediatric Stroke Outcome Measure; PT, posterior trunk of the distal MCA infarction; PVI, periventricular venous infarction.
3.2 Development of epilepsy in different vascular syndromes
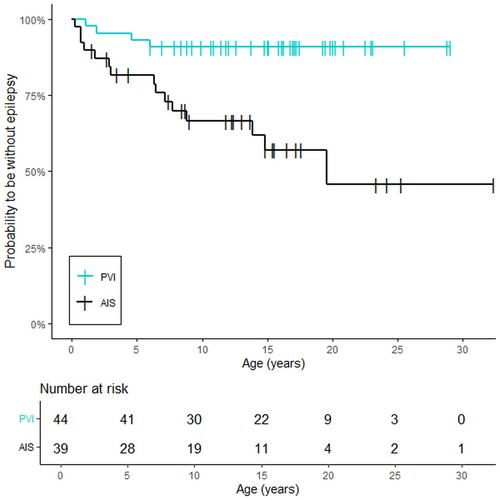
The probability of having epilepsy was higher in the group of patients with AIS compared to patients with PVI (hazard ratio [HR] 5.3; 95% CI: 1.9–18.6, p = .0009) (Figure 2).
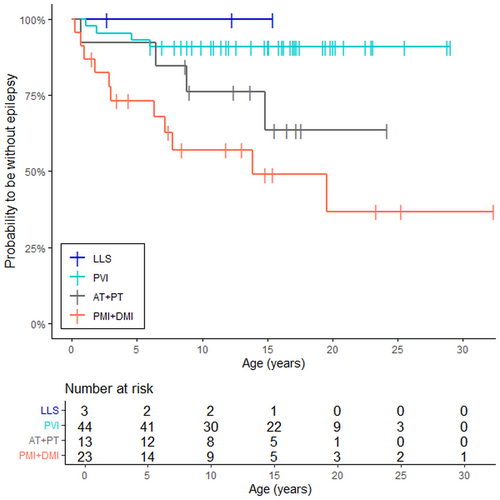
According to pairwise comparison, for patients with PMI or DMI the probability to have epilepsy was significantly higher compared to patients with PVI (HR 7.2; 95% CI: 2.5–26, p = .0007) (Figure 3). There was no significant difference in the probability to have epilepsy between the group of AT + PT compared to the group of PVI (HR 3.6; 95% CI: 0.89–15, p = .069) or between the group of AT + PT compared to the group of PMI + DMI (HR 2.0; 95% CI: 0.68–7.2; p = .24). None of the patients with LLS developed epilepsy during the follow-up period.
3.3 Cumulative survival function for epilepsy at different ages
The cumulative probability to be without epilepsy for the whole study group was 87.8% at 5 years (95% CI: 81.0–95.2), and 75.4% at the median age of 15 years (95% CI: 65.8–86.4) (Figure 4, Table 2).
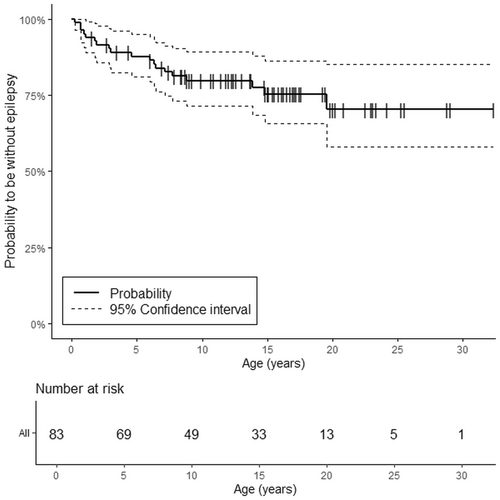
| Year | Whole study group (n = 83) | AIS (n = 39) | PVI (n = 44) | AT + PT (n = 13) | PMI + DMI (n = 23) |
|---|---|---|---|---|---|
| Cumulative survival % (95% CI) | |||||
| 0 | 100 | 100 | 100 | 100 | 100 |
| 1 | 95.2 (90.7–99.9) | 89.7 (80.7–99.8) | 100 | 92.3 (78.9–100) | 82.4 (68.1–99.7) |
| 2 | 91.5 (85.7–97.7) | 87.1 (77.2–98.3) | 95.5 (89.5–100) | 92.3 (78.9–100) | 82.4 (68.1–99.7) |
| 3 | 89.1 (82.6–96.1) | 81.7 (70.2–94.9) | 95.5 (89.5–100) | 92.3 (78.9–100) | 73.2 (57.0–94.1) |
| 4 | 89.1 (82.6–96.1) | 81.7 (70.2–94.9) | 95.5 (89.5–100) | 92.3 (78.9–100) | 73.2 (57.0–94.1) |
| 5 | 87.8 (81.0–95.2) | 81.7 (70.2–94.9) | 93.2 (86.0–100) | 92.3 (78.9–100) | 73.2 (57.0–94.1) |
| 6 | 86.5 (79.4–94.3) | 81.7 (70.2–94.9) | 90.9 (82.8–99.8) | 92.3 (78.9–100) | 73.2 (57.0–94.1) |
| 7 | 83.9 (76.3–92.3) | 75.8 (63.2–91.0) | 90.9 (82.8–99.8) | 84.6 (67.1–100) | 68.0 (50.9–90.9) |
| 8 | 81.2 (73.1–90.3) | 69.9 (56.4–86.6) | 90.9 (82.8–99.8) | 84.6 (67.1–100) | 57.1 (39.1–83.4) |
| 9 | 79.8 (71.4–89.2) | 66.6 (52.6–84.2) | 90.9 (82.8–99.8) | 76.2 (55.8–100) | 57.1 (39.1–83.4) |
| 10 | 79.8 (71.4–89.2) | 66.6 (52.6–84.2) | 90.9 (82.8–99.8) | 76.2 (55.8–100) | 57.1 (39.1–83.4) |
| 11 | 79.8 (71.4–89.2) | 66.6 (52.6–84.2) | 90.9 (82.8–99.8) | 76.2 (55.8–100) | 57.1 (39.1–83.4) |
| 12 | 79.8 (71.4–89.2) | 66.6 (52.6–84.2) | 90.9 (82.8–99.8) | 76.2 (55.8–100) | 57.1 (39.1–83.4) |
| 13 | 79.8 (71.4–89.2) | 66.6 (52.6–84.2) | 90.9 (82.8–99.8) | 76.2 (55.8–100) | 57.1 (39.1–83.4) |
| 14 | 77.6 (68.6–87.9) | 61.8 (46.9–81.5) | 90.9 (82.8–99.8) | 76.2 (55.8–100) | 48.9 (30.1–79.4) |
| 15 | 75.4 (65.8–86.4) | 57.0 (41.5–78.4) | 90.9 (82.8–99.8) | 63.5 (39.5–100) | 48.9 (30.1–79.4) |
| 16 | 75.4 (65.8–86.4) | 57.0 (41.5–78.4) | 90.9 (82.8–99.8) | 63.5 (39.5–100) | 48.9 (30.1–79.4) |
| 17 | 75.4 (65.8–86.4) | 57.0 (41.5–78.4) | 90.9 (82.8–99.8) | 63.5 (39.5–100) | 48.9 (30.1–79.4) |
| 18 | 75.4 (65.8–86.4) | 57.0 (41.5–78.4) | 90.9 (82.8–99.8) | 63.5 (39.5–100) | 48.9 (30.1–79.4) |
| 19 | 70.4 (58.1–85.3) | 57.0 (41.5–78.4) | 90.9 (82.8–99.8) | 63.5 (39.5–100) | 48.9 (30.1–79.4) |
| 20 | 70.4 (58.1–85.3) | 45.6 (26.6–78.4) | 90.9 (82.8–99.8) | 63.5 (39.5–100) | 36.7 (17.4–77.3) |
- Abbreviations: AIS, arterial ischemic stroke; AT, anterior trunk of the distal middle cerebral artery (MCA) infarction; CI, confidence interval; DMI, distal M1 MCA infarction; PMI, proximal M1 MCA infarction; PT, posterior trunk of the distal MCA infarction; PVI, periventricular venous infarction.
The odds to develop epilepsy persisted throughout childhood and young adulthood until the age of 20 years in the group of PMI + DMI when the probability of being epilepsy free was 36.7% (95% CI: 17.4–77.3). In the group of AT + PT the survival function reached the lowest level at the age of 15 years when 63.5% (95% CI: 39.5–100) of the patients were without epilepsy. For patients with PVI, the survival function declined until the age of 6 years when it was 90.9% (95% CI: 82.8–99.8) and remained at this level during the whole follow-up period.
3.4 Features of poststroke epilepsy and EEG
The detailed clinical and neurophysiological characteristics of children with epilepsy according to the vascular syndrome are presented in Table 3.
| Epilepsy, n = 19 | PVI with epilepsy, n = 4 | AIS with epilepsy, n = 15 | AT + PT with epilepsy, n = 4 | PMI + DMI with epilepsy, n = 11 | AIS vs. PVI, p value, OR (95% CI) | AT + PT vs. PMI + DMI vs. PVI, Overall p | |
|---|---|---|---|---|---|---|---|
| Age (median) of first seizure, years, [IQR] | 4.6 [1.1–7.8] | 3.3 [1.5–5.3] | 6.4 [0.9–8.8] | 7.6 [3.6–11.8] | 3.1 [0.9–7.8] | .52 | .57 |
| Complicated epilepsy, n (%) | 14 (73.7) | 4 (100) | 10 (66.7) | 1 (25) | 9 (81.8) | .53 | .075* |
| Status epilepticus or SWAS, n (%) | 7 (36.8) | 4 (100) | 3 (20.0) | 0 (0) | 3 (27.3) | .009, 32 (1.2–870) | .0093** |
| Polytherapy, n (%) | 9 (47.4) | 3 (75) | 6 (40.0) | 1 (25.0) | 5 (45.5) | .30 | .41 |
| Modified Engel class ≥3, n (%) | 11 (57.9) | 3 (75) | 8 (53.3) | 1 (25) | 7 (63.6) | .60 | .45 |
| EEG only focal slowing, n (%) | 12 (63.2) | 2 (50) | 10 (66.7) | 1 (25) | 9 (81.8) | .60 | .13 |
| EEG bilateral slowing, n (%) | 2 (10.5) | 1 (25) | 1 (25) | 0 (0) | 1 (9.1) | .39 | .68 |
| EEG only focal IED, n (%) | 2 (10.5) | 0 (0) | 2 (13.3) | 1 (25) | 1 (9.1) | >.99 | .68 |
| EEG regional IED, n (%) | 10 (52.6) | 2 (50) | 8 (53.3) | 1 (25) | 7 (63.6) | >.99 | .58 |
| EEG bilateral IED, n (%) | 7 (36.8) | 2 (50) | 5 (33.3) | 2 (50) | 3 (27.3) | .60 | .54 |
| EEG hypsarrhythmia, n (%) | 2 (10.5) | 0 (0) | 2 (13.3) | 0 (0) | 2 (18.2) | >.99 | >.99 |
- Abbreviations: AIS, arterial ischemic stroke; AT, anterior trunk of the distal middle cerebral artery (MCA) infarction; CI, confidence interval; DMI, distal M1 MCA infarction; IED, interictal epileptiform discharges; IQR, interquartile range; OR, odds ratio; PMI, proximal M1 MCA infarction; PT, posterior trunk of the distal MCA infarction; PVI, periventricular venous infarction; SWAS, spike–wave activation in sleep ≥85% of it.
The median age of the first epileptic seizure was 4.6 years [IQR: 1.1–7.8] for all patients who developed epilepsy.
Complicated epilepsy occurred in 14/19 (73.7%) of all patients with epilepsy. In the case of complicated epilepsy, the first seizure occurred at 7 years or earlier in 12/14 (85.7%) of the cases compared to 1/5 (20%) in the uncomplicated cases (OR = 24; 95% CI: 1.2–1276, p = 0.017). Status epilepticus occurred in five (26.3%) patients and spike-wave activation in sleep in two (10.5%) patients. Four patients (21%) presented with status epilepticus at the time of epilepsy diagnosis. For two of them, detailed history revealed previous focal seizures that had not been recognized earlier.
Patients with PVI (4/4, [100%]) had significantly more often status epilepticus or spike-wave activation in sleep compared to the AIS group (3/15, [20%], OR = 32; 95% CI: 1.2–870, p = .009) and compared to the AT + PT group (0/4 [0%], OR = 81; 95% CI: 1.3–5046, p = .029). In patients with PMI or DMI status epilepticus or spike-wave activation in sleep presented in 3/11 (27.3%) patients and 7/11 (63.6%) had frequent seizures. In this group two patients (18.2%) had hypsarrhythmia and epileptic spasms.
There were no significant differences in the neurophysiological characteristics between the different vascular syndromes.
3.5 Outcome of epilepsy
At the last follow-up 10/19 (52.6%) patients were seizure free and off medication over 6 months (Engel class 0). Detailed data of these patients is presented in the Table 4. Their mean follow-up time after the last seizure was 147.1 months (95% CI: 93.5–200.7). The mean time of active seizures was 32.7 months (95% CI: 5.6–59.8), but three patients had only single seizure.
| Patient's number | Age (months) at first seizure | Age (months) at last seizure | Engel class (maximum) | Age (months) at last follow-up | Period of active seizures (months) | Period of follow-up after last seizure (months) | Vascular syndrome |
|---|---|---|---|---|---|---|---|
| 1 | 9 | 9 | 1 | 306 | 0 | 297 | AT + PT |
| 2 | 24 | 139 | 3 | 322 | 115 | 183 | PVI |
| 3 | 55 | 63 | 1 | 264 | 8 | 201 | PVI |
| 4 | 77 | 131 | 2 | 288 | 54 | 157 | PMI + DMI |
| 5 | 77 | 146 | 3 | 218 | 69 | 72 | AT + PT |
| 6 | 86 | 119 | 3 | 277 | 33 | 158 | PMI + DMI |
| 7 | 93 | 93 | 1 | 275 | 0 | 182 | PMI + DMI |
| 8 | 106 | 144 | 1 | 237 | 38 | 93 | AT + PT |
| 9 | 166 | 166 | 1 | 224 | 0 | 58 | PMI + DMI |
| 10 | 178 | 188 | 2 | 258 | 10 | 70 | AT + PT |
- Abbreviations: AT, anterior trunk of the distal middle cerebral artery (MCA) infarction; DMI, distal M1 MCA infarction; PMI, proximal M1 MCA infarction; PT, posterior trunk of the distal MCA infarction; PVI, periventricular venous infarction.
4 DISCUSSION
This study provides detailed assessment of the development of epilepsy in different vascular syndromes of perinatal ischemic stroke. We found significant differences in the probability of the development and course of epilepsy among children with different vascular syndromes.
Our study showed that the risk of having epilepsy was significantly higher in patients with AIS compared to patients with PVI, and particularly in patients with PMI or DMI compared to patients with PVI. Previous studies have reported data about epilepsy development after ischemic stroke without distinguishing between the different vascular syndromes.5, 7, 8, 11 Only a few studies have stressed that vascular classification can predict motor and non-motor outcome and epilepsy development.2, 10 We speculate that the underlying anatomical mechanism could be the simultaneous involvement of both the basal ganglia and the cortex in the case of PMI or DMI and the relative sparing of the basal ganglia and the cortex in case of PVI.2 Different studies of childhood and adult epilepsies and poststroke epilepsy have revealed changes in the thalamus and the basal ganglia, but also in cortical involvement.10, 12, 13, 27
We found that the risk for having epilepsy persists high until young adulthood only in patients with PMI or DMI. In patients with PVI the risk is high until the age of 6 years and in patients with AT or PT until the age of 15 years. A previous nationwide cohort study established a similarly elevated risk for epilepsy in ischemic stroke until the early twenties, but the authors were unable to differentiate between the vascular syndromes.7 Radiological classification of the vascular type and the exact vascular syndrome is essential for the neurologist to evaluate epilepsy risk and to plan follow-up. Why the risk of epilepsy remains high for many years after perinatal stroke is not unambiguously known, but the vulnerability of the developing brain due to a mismatch between γ-aminobutyric acid-mediated inhibition and glutamatergic excitation, as well as neuroinflammation are the main processes associated with childhood epileptogenesis.28, 29 There were differences in the clinical features of epilepsy according to the vascular syndrome. In children with PVI status epilepticus or spike-wave activation in sleep occurred significantly more often compared to patients with AT or PT or compared to the whole AIS group. Although we failed to establish a statistically significant difference in the occurrence of status epilepticus or spike-wave activation in sleep between the PMI + DMI and the AT + PT groups, none of the patients with AT or PT had status epilepticus or spike-wave activation in sleep in our cohort. Also, we found that in the case of complicated epilepsy, the first seizure appeared before the age of 7 years in 85.7% of the patients.
The long-term outcome of poststroke epilepsy in our study group was favorable: half of the patients were seizure free and off antiseizure medication at the last follow-up. Patients who were in remission more than 12 years of follow-up after the last seizure had had no epilepsy recurrence. Generally, symptomatic etiology and abnormal neuroimaging are important predictors for unfavorable outcome of epilepsy.30-32 Status epilepticus has also been associated with seizure recurrence.31 However, according to our data, remission was achieved in children with status epilepticus as well.
A strength of our study is the long follow-up period, a median of 15 years. According to earlier studies, the risk for having epilepsy is the highest in the first years of life and remains high until early adulthood. However, how long the risk persists in the case of different vascular syndromes is not well established.7, 10 Another strength of the study is the consistent assessment of radiological diagnosis: all neuroimages for this study were reviewed by three of the authors to confirm the diagnosis of stroke and establish an accurate classification of the vascular syndrome.
We have to acknowledge some limitations of our study. Some subgroups, particularly the group of LLS, was too small for making statistical conclusions. It is hard to say if this group is truly free from epilepsy risk, compared to the other vascular syndromes, or if this finding was occasional. Also, we noted some differences between the groups of AT + PT and PMI + DMI, for example, later time of the first seizure and less complications in the group of AT + PT, but these differences were not statistically significant. Additionally, from the age of 20 years, the subset of the patients is quite small and Kaplan–Meier estimates should be interpreted with caution. Until 2003 patients were included in the Estonian Pediatric Stroke Database retrospectively and only thereafter was the data collected prospectively. We were able to review all neuroimages of the study group, however, the clinical data of the patients included in the database during the first years were mainly based on previous case histories and may be incomplete.
Our study provides valuable information about epilepsy development in the case of different vascular syndromes after perinatal stroke, which can help guide clinicians in decision making. Vascular classification is feasible already in early postneonatal radiological evaluation. Radiological diagnosis should comprise not only the diagnosis of AIS or PVI, but also the exact vascular syndrome. AT, PT, PMI, DMI, or LLS occlusion should be implemented to obtain a reliable estimation of prognosis for children with perinatal stroke. All children with perinatal ischemic stroke should be carefully monitored for epilepsy until school age. In children with PVI the risk of epilepsy decreased by school age, but the risk remains high in patients with AT or PT until teenage, as well as in children with PMI or DMI even in young adulthood. In a quite large number of patients the course of epilepsy was complicated, but remission was attained in more than half of the children. Larger prospective studies are needed in the future to evaluate the outcome of epilepsy focusing on different vascular syndromes of perinatal stroke.
5 CONCLUSION
The risk for having epilepsy after perinatal stroke is higher in children with AIS, particularly with the PMI or DMI vascular syndrome. In patients with PVI epilepsy develops before school age, but in patients with PMI or DMI the risk remains high until young adulthood. In patients with AT or PT, there was less status epilepticus or spike-wave activation in sleep compared to patients with PVI. Our study focuses on classifying the vascular syndrome of perinatal stroke and targeted follow-up of patients for epilepsy until young adulthood.
ACKNOWLEDGMENTS
This study has been financially supported by Estonian Research Council PRG1912 and the Tartu University Hospital PR-143/22.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflicts of interest to disclose.
REFERENCES
Test yourself
-
How many patients developed epilepsy during a median follow-up time of 15 years after perinatal stroke?
- 13%
- 23%
- 33%
-
Which vascular syndrome has the highest probability of developing poststroke epilepsy?
- Periventricular venous infarction
- Lateral lenticulostriate arteria infarction
- Proximal or distal M1 middle cerebral artery infarction
-
Which vascular syndrome carries the highest risk of status epilepticus or spike–wave activation in sleep ≥85% of it?
- Periventricular venous infarction
- Proximal or distal M1 middle cerebral artery infarction
- Anterior or posterior trunk of the distal middle cerebral artery infarction
Answers may be found in the supporting information.