MYT1L variant inherited by a mosaic father in a case of severe developmental and epileptic encephalopathy
Abstract
The MYT1L gene plays a critical role in brain development, promoting the differentiation and proliferation of cells, important for the formation of brain connections. MYT1L is also involved in regulating the development of the hypothalamus, which is a crucial actor in weight regulation. Genetic variants in the MYT1L are associated with a range of developmental disorders, including intellectual disability, autism spectrum disorder, facial dysmorphisms, and epilepsy. The specific role of MYT1L in epilepsy remains elusive and no patients with developmental and epileptic encephalopathy (DEE) have been described so far. In this study, we report a patient with DEE presenting with severe refractory epilepsy, obesity, and behavioral abnormalities. Exome sequencing led to the identification of the heterozygous variant NM_001303052.2: c.1717G>A, p.(Gly573Arg) (chr2-1910340-C-T; GRCh38.p14) in the MYT1L gene. This variant was found to be inherited by the father, who was a mosaic and did not suffer from any neuropsychiatric disorders. Our observations expand the molecular and phenotype spectrum of MYT1L-related disorders, suggesting that affected individuals may present with severe epileptic phenotype leading to neurocognitive deterioration. Furthermore, we show that mosaic parents may not display the disease phenotype, with relevant implications for genetic counseling.
Abbreviations
-
- DEE
-
- developmental and epileptic encephalopathy
-
- EEG
-
- electroencephalogram
-
- MYT1L
-
- myelin-transcriptor-factor-1-like
-
- NGS
-
- next generation sequencing
-
- WES
-
- whole exome sequencing
1 INTRODUCTION
The myelin-transcriptor-factor-1-like (MYT1L) gene encodes for the homonymous protein MYT1L which belongs to the family of myelin transcription factors, together with MYT1 and ST18 genes.1 Its expression is highest during fetal development, when it plays a pivotal role in neurogenesis. MYT1L allows transformation from neuronal stem cells to mature neurons and promotes the differentiation and proliferation of oligodendrocytes, that are necessary for myelination and remyelination in the central nervous system.2 Moreover, MYT1L gene has been suggested to be involved in regulating the development of the neuroendocrine hypothalamus, thus having a possible role in the predisposition to overweight and obesity.3, 4
In humans, the reported MYT1L variants have been associated with neurodevelopmental conditions and consist of microdeletions, missense variants, and de novo truncating single nucleotide change.4 Clinical phenotypes are heterogeneous and are mainly characterized by intellectual disability, neurodevelopment disorders, overweight/obesity, facial dysmorphisms, and epilepsy.3, 5 Although an association with seizures is reported, no specific characterization of the epilepsy phenotype has been provided so far. Coursimault et al. reported 9/40 patients with several forms of seizures, but no details concerning the epilepsy severity. All these patients were on monotherapy, suggesting that they were most likely not drug-resistant.3 To the best of our knowledge, no association of MYT1L variants with developmental and epileptic encephalopathy (DEE) has been described in the literature.
Here, we report the case of an 8-year-old girl harboring a heterozygous variant in the MYT1L gene, inherited from the mosaic father not displaying the disease phenotype, and presenting with severe form of DEE.
2 CASE REPORT
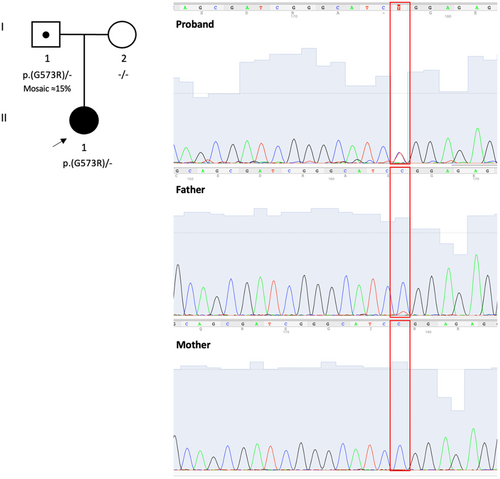
This is an 8-year-old female, firstborn of non-consanguineous parents (Figure 1). She was born at 40 weeks gestation by cesarean section due to premature rupture of membranes, with normal Apgar scores and auxologic parameters. During the first days of life, the patient experienced self-resolving sucking difficulties. Follow-up clinical examinations revealed mild psychomotor delay. The child reached independent walking by 23 months of age. Speech development was also impaired. She was toilet trained without difficulties. The patient also had mild dysmorphism: hypoplasic earlobes, hyperlordosis, and flat foot.
At 5 years, she had a first epileptic seizure consisting of a generalized tonic–clonic episode immediately after awakening. The standard wake electroencephalogram (EEG) performed soon after this episode showed focal epileptiform abnormalities over the right temporal region with secondary diffusion. Brain magnetic resonance (MRI) and genetic tests (karyotype and array-CGH) were normal. Antiseizure medication with levetiracetam was started. Six months later, seizures recurred with repeated head drops in the morning, sometimes with fall to the ground. Topiramate was added, with discrete clinical improvement. The first neuropsychological assessment performed at 6 years of age showed an intelligence quotient in the normal range (IQ 89 at the Leiter Scale), while the Bells test revealed attention deficit. The patient was addressed to neuropsychomotor rehabilitation. Seizures persisted in the form of frequent drop attacks, absences, and rare generalized convulsions. EEG at 6.5 years of age showed severe increase of the epileptic paroxysmal activity during sleep, with medium-voltage polyspikes in right frontal-temporal region and generalized high-voltage spike–waves. Clobazam was added to topiramate, and levetiracetam was stopped.
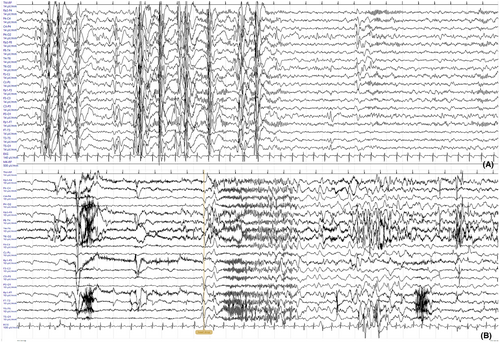
At 7 years of age, she presented at our Institute with drug-resistant epilepsy, developmental impairment, hyperactive behavior, and attention deficit. She had poor tolerance to frustrations and behavioral dyscontrol, with inappropriate bizarre and happy behavior in social contexts. Seizures mostly occurred in the morning, with massive jerks or sudden psychomotor arrest (absences) with slow loss of tone, sometimes preceded by laugh. The EEG during wake displayed irregular background activity with spike–wave and sharp wave on right occipital-temporal. Sleep EEG showed generalized spike–wave and polyspikes–wave abnormalities in bursts lasting for 10–15 s. An electroclinical seizure was also recorded during wake (Figure 2). Auxologic parameters were within normal ranges. At 7 years and 3 months, a follow-up neuropsychological assessment with Weschler Scale for Intelligence (WISC-IV) revealed a total IQ < 40, supporting diagnosis of severe intellectual disability. The patient scored below the low level for language, memory, and learning tasks at the NEPSY-II battery. Diagnosis of DEE of infancy was posed, with myoclonic, absences and generalized tonic and tonic–clonic seizures. Valproic acid was added resulting in mild improvement, but the patient suddenly deteriorated with the attempt to decrease topiramate, showing prolonged tonic–clonic episodes. Therapy was changed again raising topiramate and adding ethosuximide that allowed a significant reduction of absences and drop attacks.
The patient was investigated through trio whole exome sequencing (WES) according to local protocols. Variant analysis was performed as previously reported.6 WES led to the identification of the heterozygous variant NM_001303052.2: c.1717G>A, p.(Gly573Arg) (chr2-1910340-C-T; GRCh38.p14) in the MYT1L gene (Figure 1). Although the variant appeared to be de novo according to WES data (coverage across the target regions was >20× and average coverage was 200,0), Sanger sequencing showed instead that it was present with ≈15% mosaicism in the blood of the unaffected father. This variant is absent in the gnomAD database, affects a conserved residue (GERP score = 5.55), and it is predicted pathogenic according to in silico tools (CADD score = 29.9; Mutation Taster score = 1; Polyphen-2 score = 1). Furthermore, according to the American College of Medical Genetics and Genomics (ACMG) guidelines, the variant is classified as pathogenic (PM1, PM2, PM5, PP3, and PP5 criteria). This variant is reported in ClinVar as a variant of unknown significance (submitted interpretation 2017), pathogenic significance (submitted interpretation 2021), and likely pathogenic significance (submitted interpretation 2022; https://www.ncbi.nlm.nih.gov/clinvar/variation/521634/). The alternative variant p.(Gly573Glu) affecting this same residue is classified as likely pathogenic by ClinVar (submitted interpretation 2020; https://www.ncbi.nlm.nih.gov/clinvar/variation/984650/).
3 DISCUSSION
We describe the electroclinical phenotype of a young girl harboring a pathogenic variant in MYT1L gene, presenting with a severe and drug-resistant DEE. Compared to the previously reported cases with MYT1L variants, our patient showed common signs and symptoms such as motor and speech delay, later diagnosed as intellectual disability of a moderate/severe degree.3, 7 She had no significant dysmorphic features but was overweight since the age of 3 years with a hyperphagic attitude, as reported in the literature.4, 8-10 She showed hyperactivity with extremely cheerful behavior, disinhibition, and bizarre happy demeanor in social context. These manifestations have been occasionally reported in MYT1L patients.7, 11
Epilepsy occurs in <30% of subjects with MYT1L variants, starting in the neonatal period or during childhood with febrile seizures, absences, focal, and/or tonic–clonic seizures.3 To date, no electroclinical characterization has been provided, except for one male patient harboring a 376-kb deletion including MYT1L. This subject suffered from epilepsy with frequent “frontal absences and generalized secondary seizures,” and he was treated with two antiepileptic drugs.12 Our patient suffered from a severe epilepsy, with polymorphic seizures (absences, myoclonic, tonic–clonic, and later tonic; Figure 2B), drug resistance, and cognitive deterioration after epilepsy onset. This clinical course is highly suggestive of DEE and has never been reported in association with genetic variants in the MYT1L gene. Topiramate resulted the most effective drug in controlling mostly tonic–clonic seizures.
Epileptic encephalopathies are disorders in which the epileptiform activity itself contributes to severe cognitive and behavioral impairments beyond that expected from the underlying pathology alone. The term “epileptic encephalopathy” in the 2010 ILAE classification proposal was modified to “Developmental and Epileptic Encephalopathy” (DEE) in the 2017 formal revision of the classification since variants in many of the genes causing epileptic encephalopathies may adversely impact neurodevelopment regardless of the uncontrolled epileptic activity.13, 14 The definition of DEE fits our case, since MYT1L variants can affect neurodevelopment regardless of the epileptic disorder. The clinical progression of our patient is highly suggestive of MYT1L-related DEE. In the past two decades Next generation sequencing (NGS) techniques have proven to be an efficient tool for the identification of underlying genetic causes in DEE, allowing to achieve early diagnosis even in complex cases.15 These recent advancements have shown that the pathophysiology of DEEs can be particularly heterogeneous and complex, supporting the importance of accurate genetic counseling in the diagnostic process.15
In our patient, we detected the heterozygous variant NM_001303052.2: c.1717G>A, p.(Gly573Arg) in MYT1L through exome sequencing. The variant is localized within a mutational hot spot in the third Zinc Finger domain of C2HC type, in proximity to other variants associated with heterogeneous neurodevelopmental phenotypes, thus not suggesting evident genotype–phenotype correlations.3 Although the p.(Gly573Arg) variant appeared to be de novo from NGS data, Sanger sequencing showed that this change was inherited from the unaffected father, who was a carrier in a mosaic state (≈15% mosaicism). This finding is particularly interesting for two reasons. First, it supports the role of Sanger sequencing as a confirmation procedure for variants detected through NGS-based techniques, even in the case of well-covered variants. The presence of the variant in a mosaic healthy parent has relevant impact on genetic counseling. Second, it supports the idea that MYT1L variants in mosaic parents can be considered potentially pathogenic. In particular, mosaicism is a crucial factor affecting penetrance in genetic conditions and the presence of a variant in mosaic state in an unaffected parent should not exclude the pathogenic potential of the genetic defect.
MYT1L variants are associated with a neurodevelopmental disorder with behavioral abnormalities and seizures. DEE has never been associated with MYT1L so far, suggesting that the phenotype spectrum of MYT1L-related disorder may be wider than expected based on the current literature. The identification of the same variant in mosaic state in the unaffected father of the proband suggests that apparently de novo variants in MYT1L gene should be carefully examined to allow an accurate genetic counseling.
REFERENCES
Test yourself
-
When is Myelin-transcriptor-factor-1-like (MYT1L) gene mainly expressed?
- Fetal development
- Childhood
- Adulthood
- Elderly age
-
Which drug, in our case, best controlled tonic–clonic seizures?
- Valproate
- Levetiracetam
- Topiramate
- Clobazam
-
Why does our case allow us to expand the knowledge of MYT1L-related disorder?
- The patient presents a drug-resistant epilepsy
- The patient carries a pathogenic variant of the MYT1L gene inherited from father as a mosaicism
- The patient presents cognitive deterioration after epilepsy onset
- All answers are correct
Answers may be found in the supporting information.