Boosting oxygen evolution reaction by activation of lattice-oxygen sites in layered Ruddlesden-Popper oxide
Funding information: Government of Western Australia; Australian National Computational; Australian Research Council, Grant/Award Number: DE190100005
Abstract
Emerging anionic redox chemistry presents new opportunities for enhancing oxygen evolution reaction (OER) activity considering that lattice-oxygen oxidation mechanism (LOM) could bypass thermodynamic limitation of conventional metal-ion participation mechanism. Thus, finding an effective method to activate lattice-oxygen in metal oxides is highly attractive for designing efficient OER electrocatalysts. Here, we discover that the lattice-oxygen sites in Ruddlesden-Popper (RP) crystal structure can be activated, leading to a new class of extremely active OER catalyst. As a proof-of-concept, the RP Sr3(Co0.8Fe0.1Nb0.1)2O7-δ (RP-SCFN) oxide exhibits outstanding OER activity (eg, 334 mV at 10 mA cm−2 in 0.1 M KOH), which is significantly higher than that of the simple SrCo0.8Fe0.1Nb0.1O3-δ perovskite and benchmark RuO2. Combined density functional theory and X-ray absorption spectroscopy studies demonstrate that RP-SCFN follows the LOM under OER condition, and the activated lattice oxygen sites triggered by high covalency of metal-oxygen bonds are the origin of the high catalytic activity.
1 INTRODUCTION
Research into the oxygen evolution reaction (OER) has been of particular interest, as it plays a critical role in multiple electrochemical energy conversion and storage technologies such as regenerative fuel cells, rechargeable metal-air batteries, water splitting, and CO2 reduction.1-3 Nevertheless, the sluggish kinetics of OER limits the overall efficiency of these electrochemical systems due to complex four-electron oxidation process, hence calling for efficient electrocatalysts to accelerate.3 Currently, the benchmark OER electrocatalysts are precious metal-based oxides (ie, RuO2 and IrO2); however, the problems of high cost and low abundance of these metals make them unsuitable for commercial scale application. Great efforts have been thus devoted to the search for efficient alternative OER catalysts composed of low-cost and earth-abundant elements.
As an important family of functional materials, perovskite-type metal oxides with a general formula of ABO3 (A = alkaline-earth or rare-earth metals; B = transition metals) have emerged as promising OER electrocataysts during the past few decades.4-7 Traditionally, the OER catalytic activity of perovskite oxides is dominated by the cationic redox centers (ie, the B-site transition metal cations are regarded as active sites). In view of this, some descriptors related to the electronic structure of B-site transition metals, such as eg orbital occupancy,8 d electron number,9 and magnetism,10 have been well correlated with the OER activity. As such, some well-known perovskites with high intrinsic OER activity have been developed, for example, Ba0.5Sr0.5Co0.8Fe0.2O3-δ8 and SrCo0.7Fe0.2Nb0.1O3-δ (SCFN).11 However, this conventional mechanism concerning cation redox was recently challenged by few experimental and theoretical studies.12-17 A new OER mechanism involving lattice-oxygen participation for perovskite oxides was proposed and increased metal-oxygen covalency was found to be critical to promote the OER kinetics.14-17 By advanced in-situ 18O isotope labeling mass spectrometry, Grimaud et al gave a direct experimental evidence that the generated O2 during OER on some highly covalent perovskite oxides comes from lattice oxygen.17 These findings highlight the significance of surface lattice oxygen in OER catalysis and open an exciting opportunity for designing highly active oxide-based catalysts. However, up to date, catalysts via lattice-oxygen oxidation mechanism (LOM) for OER are still very limited. Therefore, it is highly desirable but challenging to develop highly active electrocatalysts with lattice-oxygen active sites.
Apart from the simple perovskite oxides, complex metal oxides with special crystal structure have been widely used in various fields by virtue of their fascinating physical-chemical properties.18-22 Diverse crystal structures of complex oxides can trigger some novel geometrical, electronic, and conductive properties, consequently promoting the electrocatalytic performance. Among various complex oxides, Ruddlesden-Popper (RP) type oxides (formula of An + 1BnO3n + 1 or equivalently [AO] (ABO3 ± δ)n), wherein n ABO3 perovskite layers are sandwiched between two AO rock-salt layers, have received considerable attention due to their chemical flexibility, layered structure, wide element accommodation, and labile lattice oxygen.23-26 The RP phase possesses preferential formation of oxygen vacancies at the apical O-site pointing toward the rock-salt layers,26, 27 which provides high possibility of lattice oxygen-participated OER in RP structure.24
Herein, we report that the lattice-oxygen sites of RP structure can be effectively activated by designing a suitable composition to achieve the exceptionally high OER activity. In particular, RP Sr3(Co0.8Fe0.1Nb0.1)2O7-δ (RP-SCFN) is synthesized as an RP catalyst to demonstrate this new strategy. When tested on glass carbon electrode in 0.1 M KOH solution, the RP-SCFN material achieves the 10 mA cm−2 at an extremely low overpotential of 334 mV, outperforming the benchmark RuO2 catalyst and nearly any metal oxides reported to date. Moreover, the RP-SCFN exhibits much higher mass activity (MA) and specific activity (SA) than the simple SrCo0.8Fe0.1Nb0.1O3-δ perovskite (P-SCFN). First-principle calculations reveal that the OER on the RP-SCFN proceeds via the LOM pathway with a low theoretical thermodynamic overpotential of 0.22 V, which originates from the strong metal-oxygen covalency as confirmed by advanced soft X-ray absorption spectroscopy (sXAS) technique. This work suggests the potential of RP oxides as efficient and economic OER catalysts involving lattice oxygen sites.
2 RESULT AND DISCUSSION
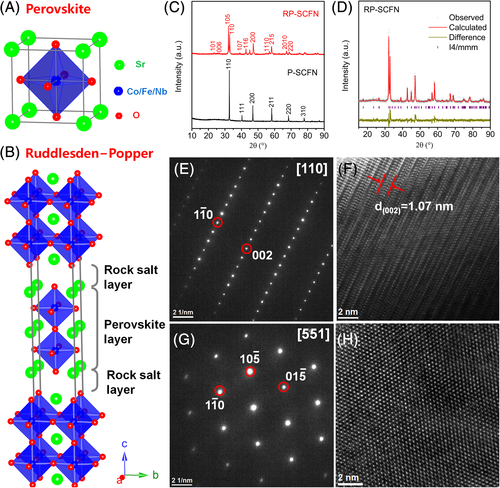
2.1 Crystal structure characterization
In the idea cubic-symmetry ABO3 perovskite structure, larger A-site cations (eg, Sr) are 12-fold oxygen coordination and smaller B-site cations (eg, Co/Fe/Nb) are sixfold oxygen coordinated (Figure 1A). RP structure with the chemical formula of A3B2O7 is a layered structure, which consist of alternative perovskite ABO3 layer and rock-salt AO layer along the c-direction (Figure 1B). SrCo0.8Fe0.1Nb0.1O3-δ perovskite (denoted as P-SCFN) and RP Sr3(Co0.8Fe0.1Nb0.1)2O7-δ oxide (denoted as RP-SCFN) were successfully synthesized using a facile and scalable sol-gel method, and their crystal structures were initially verified by X-ray diffraction (XRD) (Figure 1C). Rietveld refinement of the XRD pattern demonstrates that the RP-SCFN adopts a pure tetragonal structure with a space group of I4/mmm and unit cell parameters of a = b = 3.8531(2) Å, c = 20.144(1) Å (Figure 1D). The crystal structure of RP-SCFN was further confirmed by two selected-area electron-diffraction patterns along the [110] and [551] zone axes (Figure 1E,G) and the corresponding high-resolution transmission electron microscopy images (Figure 1F,H). Scanning electron microscopy images show that the P-SCFN and RP-SCFN powders are both composed of micron-sized particles (but slightly smaller size is observed for RP-SCFN) (Figure S1), suggesting the bulk nature of the as-prepared powders. In addition, in order to investigate the effect of composed elements (eg, Nb and Fe) on the crystal structure of RP-SCFN, we also attempted to synthesize Sr3Co2O7-δ (RP-SC), Sr3(Co0.9Fe0.1)2O7-δ (RP-SCF), and Sr3(Co0.9-xFexNb0.1)2O7-δ with different Fe substitutions (x = 0, 0.2, 0.3, 0.4, and 1) under the same conditions. In Figure S2, pure RP phase was observed in Sr3(Co0.9-xFexNb0.1)2O7-δ while Nb-free RP-SC and RP-SCF could not form pure RP structure, implying the key role of Nb5+ on stabilizing the RP structure as also reported elsewhere.28, 29
2.2 Electrocatalytic OER measurement
The OER electrocatalytic activity of RP-SCFN and P-SCFN catalysts was assessed by a typical thin-film rotating disk electrode technique.8, 30, 31 Similar measurements were also conducted on the benchmark RuO2 catalyst for comparison (Figure S3). Unless indicated otherwise, all data in our work were corrected to reversible hydrogen electrode (Figure S4) and iR-corrected for compensating the electrolyte resistance. Figure 2A shows the capacitance- and Ohmic resistance-corrected polarization curves acquired from cycle voltammetry (Figure S5) of RP-SCFN, P-SCFN, and RuO2 catalysts on glassy carbon (GC) in 0.1 M KOH solution. The electrode activity of RP-SCFN distinctly exceeds that of P-SCFN and benchmark RuO2, as indicated by the smaller onset potential of ~1.45 V and higher catalytic current. The overpotential (η) required to deliver a 10 mA cm−2 is a metric associated with solar fuel synthesis and widely used as a parameter for activity comparison.32, 33 Strikingly, the RP-SCFN displays an extremely small η of only 334 mV, much lower than that of P-SCFN and RuO2. To evaluate the activity more comprehensively, the MA normalized to the catalyst mass loading and SA normalized to real oxide surface area (as estimated from BET measurements, Table S1 and Figure S6) were calculated. As seen from Figure 2B, the RP-SCFN achieves an MA of 80.7 A g−1 at η = 0.35 V, which is ~ 81 and ~6 times higher than that of P-SCFN (1.0 A g−1) and RuO2 (12.9 A g−1). Besides the MA, the SA of RP-SCFN is ~14 times higher than that of P-SCFN, suggesting the considerable intrinsic activity enhancement by structure engineering. To examine the kinetics, Tafel plots were constructed in Figure 2C. The Tafel slope for RP-SCFN (57 mV dec−1) is smaller than that for hex-BSC (109 mV dec−1) and RuO2 (80 mV dec−1) catalysts, implying more rapid OER rates. Electrochemical impedance spectroscopy tests were further performed to examine the kinetics during OER process. Smaller charge transfer resistance (Rct) of RP-SCFN was observed than P-SCFN and RuO2 (Figure 2D), corresponding to better charge transfer abilities for OER catalysis. As a whole, all above electrochemical analyses (including low overpotential, low Tafel slope, high MA, and SA) highlight the outstanding catalytic activity of RP-SCFN for OER.
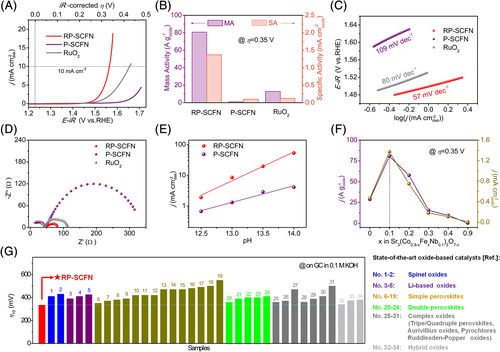
It was reported that some highly active oxides enabling lattice oxygen-oxidation typically displays pH-dependent OER activity, involving nonconcerted proton-electron transfers in the OER mechanism.17, 34 The OER activity of RP-SCFN was then examined in KOH solutions at different pH values (Figure S7). As a result, the RP-SCFN catalyst exhibits much stronger pH-dependent OER activity than P-SCFN (Figure 2E), which implies that the OER proceeds on RP-SCFN via the participation of lattice oxygen. In addition, what is noteworthy is that the Fe substitution for Co in Sr3(Co0.9-xFexNb0.1)2O7-δ (0 ≤ x ≤ 1) yields a volcano-like activity trend with 10% Fe substitution (ie, RP-SCFN) being the most active composition (Figures 2F and S8). Thus, a low level of Fe doping contributes to the further activity enhancement, which has been widely reported in previous studies due to favorable electronic modifications.31, 35-37 Figure 2G compares the OER activity (ie, η@10 mA cmgeo−2, one practical parameter to assess water-splitting devices32, 38) of RP-SCFN with present state-of-the-art oxide-based catalysts on GC in 0.1 M KOH solution, including spinel oxides, Li-based oxides, simple perovskites, double perovskites, complex oxides, and hybrid oxides (Table S2). As shown, the OER activity of RP-SCFN ranks among the highest oxide-based catalysts in alkaline media ever reported. Besides the activity, the RP-SCFN shows much better durability than the benchmark RuO2 catalyst (Figure S9), demonstrating its value for practical application. The poor stability of RuO2 is due to the oxidation of surface Ru4+ to soluble RuO42− anions under OER conditions.39
2.3 Electronic structure characterization
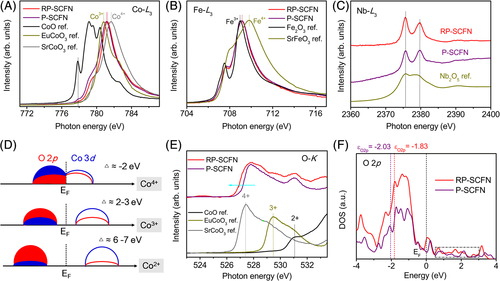
To identify the origin of the high OER activity of RP-SCFN, we resort to the advanced surface-sensitive sXAS measured with total electron yield (TEY) mode to investigate the electronic structures. sXAS in TEY mode is a well-known technique to glean the information of surface electronic structures and has a typical probing depth of ~5 nm.40-43 Figure 3A shows the Co L3-edge sXAS spectra of RP-SCFN and P-SCFN, as well as the reference materials with different valence states including divalent CoO, trivalent EuCoO3,44 and tetravalent SrCoO3.45 The Co L-edge XAS is highly sensitive to the Co valence states. The Co L3-edge peak shifts to higher energy from CoO to EuCoO3, P-SCFN, RP-SCFN, and further SrCoO3, reflecting the coexistence of Co3+/Co4+ in RP-SCFN as well as higher Co valence state of RP-SCFN relative to P-SCFN. By comparing the Co L3-edge peak position with reference samples, the average Co valence state of RP-SCFN was calculated to be ~ + 3.44, close to the optimal value of +3.4 in highly active SrCoO2.7 catalyst.14 To note, a small peak at 777.8 eV of CoO spectrum as a fingerprint of Co2+ was present for P-SCFN (but absent for RP-SCFN), indicating that some Co2+ ions are in P-SCFN and excluded for RP-SCFN. Besides, the XAS spectra of Fe-L3 and Nb-L3 depicted in Figure 3B,C display nearly the same between RP-SCFN and P-SCFN, implying no distinct change in the surface Fe and Nb oxidation state for RP-SCFN and P-SCFN. It is well known that for the later 3d transition metal oxides, the ground state can be described by Φ = αdn > +βdn + 1L_ > +γdn + 2L_2 > and the charge transfer energy (Δ) decrease by about 4 eV if the Co valence increases by one in the charge transfer models as shown in Figure 3D.42, 46-48 In this case, Δ reduces from a large positive value of 6 to 7 eV for Co2+ with a dominant 3dn configuration to a negative value for Co4+ with a dominant 3dn + 1L_ configuration. Here, L_ denotes ligand oxygen 2p hole which is originated from strong oxygen 2p and cobalt 3d covalence. This means that the lattice-oxygen oxidation for Co4+ oxides is due to its great covalency of metal-oxygen bonds.13, 17, 43, 48 Thus, high content of Co4+ renders RP-SCFN as a highly covalent oxide. The O K-edge sXAS spectra were further collected to explore the covalency between O 2p and transition metal 3d states.42, 43, 49, 50 It is known that the pre-edge peaks below ~532 eV in the O-K XAS spectra represent the unoccupied O 2p orbitals hybridized with transition metal 3d orbitals.42, 43, 49, 50 Figure 3E (bottom) shows that the O-K pre-edge peak shifts to lower energies and the spectral weight increases as the Co valence increases from Co2+ to Co3+ and further to Co4+, suggesting an increased degree of covalency between Co 3d and O 2p states. Lower O-K pre-edge energy position and higher level of spectral weight were observed for RP-SCFN relative to P-SCFN, demonstrating the enhanced metal-oxygen covalency for the former as further conformed by the calculated density of states of O 2p. The O 2p-band center was also calculated considering that it has been reported to correlate with the covalence and OER activity.17, 31, 51, 52 As seen from Figure 3F, the O 2p-band center (−1.83 eV) of RP-SCFN is closer to the Fermi level than that (−2.03 eV) of P-SCFN, implying that RP-SCFN would boost the OER. According to the above analysis, we can conclude that strong metal-oxygen covalency can be induced by the engineered RP phase, which is critical to trigger lattice-oxygen oxidation.
2.4 Density functional theory calculations
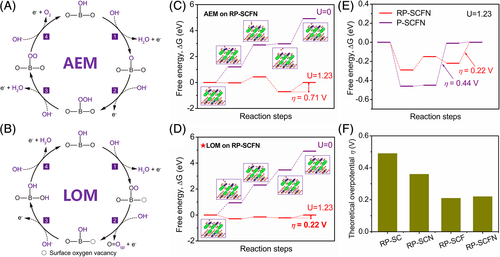
Density functional theory calculations were further performed to understand the reaction mechanism and possible active sites of RP-SCFN. Two different OER mechanisms, that is, adsorbate evolution mechanism (AEM) and LOM were considered (Figure 4A,B). The conventional AEM concerning cation redox proceeds through four concerted proton-electron transfer steps with multiple adsorbed intermediates, resulting in a minimum theoretical overpotential of ~ 0.37 V.1, 53, 54 The recently accepted LOM involves a lattice-oxygen oxidation and nonconcerted proton-electron transfer, which can bypass the limitation for AEM and potentially offer better OER activity.15, 17, 54 Figure 4C,D shows the free energy diagrams under potential U = 0 and 1.23 V of RP-SCFN via AEM and LOM. As can be seen, the theoretical thermodynamic overpotential (η) of RP-SCFN based on LOM was calculated to be 0.22 V, significantly lower than the value (0.71 V) via AEM, implying that the lattice oxygen in RP-SCFN is OER active sites. Such a theoretical η (0.22 V) for RP-SCFN is even lower than the reported state-of-the-art oxides with activated oxygen sites, such as Zn0.2Co0.8OOH (0.27 V)54 and La0.5Ba0.25Sr0.25CoO2.9-δF0.1 (0.27 V).55 The high activity of lattice oxygen in RP-SCFN could be ascribed to its strong metal-oxygen covalency, as demonstrated by aforementioned XAS analysis. As compared with the P-SCFN, the RP-SCFN also gives rise to lower η value (Figure 4E). In order to explore the role of composed elements (eg, Nb and Fe) on the activity of RP-SCFN, the free energies of Nb/Fe-free RP-SC, Fe-free RP-SCN and Nb-free RP-SCF based on AEM and LOM were also calculated (Figure S10). All RP-structured oxides generate much lower η values via LOM than AEM, addressing the dominating role of RP structure on activating lattice oxygen. Fe- containing RP-SCF and RP-SCFN samples show lower theoretical LOM η than Fe-free RP-SC and RP-SCN, respectively, which indicates that a low level of Fe doping in RP-SCFN can further enhance the activity (Figure 4F). Slight Nb5+ content in RP-SCFN is just to help stabilize the crystal structure, evidenced by the unsuccessful pure phase formation in Nb-free RP-SC and RP-SCF. This is also supported well by the fact that RP-SCF and RP-SCFN possess nearly the same theoretical LOM η. Overall, the above theoretical calculations demonstrate the lattice oxygen ions in RP-SCFN as active sites for OER and critical role of low-level Nb/Fe co-doping, which cooperatively contributes to the outstanding OER activity of RP-SCFN.
3 CONCLUSION
In summary, we have demonstrated for the first time that the lattice-oxygen in RP oxides can be activated for boosting OER. As a model catalyst, the bulk Sr3(Co0.8Fe0.1Nb0.1)2O7-δ (RP-SCFN) synthesized by the facile and scalable sol-gel method shows ultrafast OER activity (including MA and SA), which is much higher than the simple P-SCFN and benchmark RuO2 catalyst. Remarkably, the RP-SCFN achieves an extremely low overpotential of 334 mV at 10 mA cm−2 and a small Tafel slope of 57 mV dec−1 in 0.1 M KOH solution, surpassing most state-of-the-art oxide-based catalysts ever reported. The LOM was demonstrated to be proceeded on RP-SCFN by first-principle calculations, arising from its strong metal-oxygen covalency with favorable electronic structure. This work opens new avenues for developing highly active electrocatalysts for OER and other heterogeneous catalysis by unique structural design and lattice oxygen redox processes.
ACKNOWLEDGMENTS
This work was financially supported by the Australian Research Council (Discovery Early Career Researcher Award No. DE190100005). Computational work was supported by resources provided by the Pawsey Supercomputing Centre with funding from the Australian National Computational and the Government of Western Australia. We acknowledge support from the Max Planck-POSTECH-Hsinchu Center for Complex Phase Materials and the staff of Monash Center for Electron Microscopy.