Boosting algal lipids: Diurnal shifts in temperature exceed the effects of nitrogen limitation
Abstract
Algal lipids have been observed to increase during autumn conditions (low light, low mean temperature, and diurnal shift in temperature), in large-scale outdoor photobioreactors. In this paper, we tested the effect of diurnal shifts in temperature (DS) and nitrogen (N) limitation on algal BODIPY lipid fluorescence cell−1 (BPF). We show that DS increased BPF in algal biomass up to 28% more compared with N limitation, the standard stressor to boost neutral lipids (NL) in commercial production. Biomass yield was constant, regardless the DS range (6°C-12°C). A combination of both stressors had an additive effect on algal BPF. A polyculture from an outdoor photobioreactor was cultivated under controlled conditions at different regimes of light, temperature, and N limitation. DSs were mimicking autumn conditions with a difference of 6°C, 10°C, and 12°C between day and night. Biomass and BPF were monitored over one to two weeks, and NLs were stained with a fluorescent marker (BODIPY) and detected with flow cytometry. Results indicate that, during autumn conditions, daily heating and cooling processes in contrast to N limitation do not challenge the trade-off between biomass production and BPF. During seasons when day temperature is still relatively high, DSs are rapid BPF boosting stressors, while N limitation could be applied to boost BPF further during other seasons.
1 INTRODUCTION
For more than half a century, microalgae have been proposed as a source of food, feed, and energy for commercial applications.1 Today, the issue is more pressing than ever due to global challenges such as resource depletion, eutrophication, and climate change associated with a growing and developing world population. Microalgal lipids have received considerable attention due to their potential as raw material for biofuels and food supplements that could increase the profit of microalgal cultivation. Microalgae contain lipids with different cellular function such as structural polar lipids (eg, phospholipids and glycolipids), commonly found in membranes, as well as neutral lipids (NLs) (eg, monoglycerides, diglycerides, and triglycerides) that act as intracellular storage. The main target for biofuel production is neutral triglycerides (TAGs) that can be transformed into biodiesel through transesterification processes.2 NLs like TAGs usually increase under environmental stress. Nitrogen (N) limitation is the most traditional lipid boosting stressor and can increase lipid content in several taxa.3-6 The suggested underlying mechanisms are (1) the rearrangement of cellular components such as a decrease in protein content7 and rearrangement of membrane lipids or (2) as a way for cells to maintain redox homeostasis under stress by producing lipids as an electron sink to protect the cell from reactive oxygen species (ROS).8 However, when exposed to N limitation, the production of functional biomass (ie, protein, chlorophyll, DNA, and RNA) is compromised.9 A two-step cultivation process is often needed to compensate for the loss of biomass growth, where biomass production is facilitated in the first step followed by a lipid boosting stressor in the second step.3 Olofsson et al5 suggested a trade-off threshold of N:P 5:1 to increase lipids without a major decrease in productivity. Hence, this ratio was used for the N limited treatments in the current study.
Other than N limitation, temperature and light are important factors to predict lipid yields throughout the year in large-scale outdoor cultivation systems.10 Light and temperature explained 50% of the variation in total lipids (TLs) in a study focusing on the seasonal variation of lipids and fatty acids in Nannochloropsis oculata (Eustigmatophyceae) grown in outdoor large-scale photobioreactors in Algarve, Portugal. Lipid content peaked in autumn when both light and temperature were decreasing. It was hypothesized that the peak was due to diurnal temperature variations between day and night combined with high irradiance during day.11 A similar pattern with a pronounced peak of lipids during autumn was observed in a photobioreactor at higher latitude, southeast coast of Sweden, indicating the same trend independent of latitude.12 Previous studies have shown that diurnal temperature shifts (DSs) increase lipid content in commercial algal strains with contrasting effect on cell biomass, from no effect5, 13-15 to decreased cell growth.13 To what extent can environmental stress, ie, DSs decrease biomass productivity (BP), while changing lipid content is unclear.
The mechanism inducing lipids during temperature and light stress is suggested to be a strategy of energy storage, as a protection from oxidative stress or as a result of lower growth rates10 but less is known about how lipids are affected by short-term fluctuations in temperature. DSs occur naturally during spring-autumn in cold temperate latitudes, and may have a large impact on the algal production yield over the year since temperature affects the rate of all enzymatic and electron transport reactions within the algal cell.16 N limitation has however remained the standard lipid boosting stressor but could be problematic when wastewater is introduced as a sustainable nutrient source or medium. Outdoor algal cultivation is also very dynamic in terms of temperature fluctuations, and they are constantly forced to adapt to large variations in temperature. These thermal adaptations will give perspective to the variation of lipid production.17 Therefore, more insight into how daily heating and cooling processes affect lipid content is needed, especially to investigate if DSs have benefits compared with the current paradigm of using N limitation as a lipid boosting stressor. Additionally, combinations of stressors should also be researched more since they have potential to give a synergistic output on lipid content but may also result in harmful stress levels with negative effects for the cell.
Lipid content also varies between different species and communities of microalgae. Monocultures are often used in commercial applications due to specific traits such as high growth rate or high lipid content but clonal strains are also more susceptible to environmental fluctuations and contaminations such as viruses, predators/grazers, and parasites.18 It may be beneficial to apply a multispecies approach in outdoor cultivation systems since a more diverse community tends to be more resilient to grazing and changing environmental conditions that otherwise may alter the productivity.19-23 Polycultures of microalgae, native and adapted to an area, could allow different functional groups to coexist alongside each other and therefore maximize the use of available resources.20 Therefore, we use a microalgal polyculture from the Baltic Sea cultivated in outdoor photobioreactors (project ALGOLAND, Linnæus University) located in the southeast coast of Sweden, Öland.
In this study, we investigate the response of algal NLs in terms of BODIPY lipid fluorescence cell−1 (BPF) to autumn conditions, featuring light, temperature, and DSs, against the standard stressor N limitation. We first tested the direct response of BPF to single factors N limitation and DSs (experiment 1) using settings mimicking autumn conditions (high and low average light and temperature levels). Since BPF responded positively to both N limitation and DSs, we then tested for possible synergistic/additive effects of both factors (experiment 2).
2 RESULTS AND DISCUSSION
2.1 Algal biomass and species identification
Microalgal polycultures from the Baltic Sea, cultivated in outdoor photobioreactors using brackish Baltic Sea water as medium, were used as test systems. Experimental microcosms contained polycultures of co-adapted microalgae, protozoans and bacteria, where the green algae Monoraphidium convolutum (Chlorophyceae) was dominant in all treatments in both experiments (1 and 2) and throughout the incubations (data not shown). Experiment 1 was run for 5 days, and end points were compared between single factor treatments of: N limitation, DSs in autumn conditions (high (HL) and low (LL) average light and temperature levels (6°C and 12°C)). Experiment 2 was run for 14 days and single and multiple factors were compared over time: N limitation and DSs. The N limitation treatments had an N:P ratio of 5:1, whereas other treatments had an N:P ratio of 16:1.
In the current study, biomass yield was not affected by any of the stressors in experiment 1 (Multiple linear regression, R2 = 0.35, Supporting information supplementary Figure S1) nor in experiment 2 (Two-way ANOVA, p > 0.05, see Supporting information supplementary Figure S2). Productivity (measured in experiment 2, Supporting information supplementary Figure S3) was not affected by the stressors either (Two-way ANOVA, p > 0.05). The level of stress might have played a role in the lack of effect on biomass yield and productivity. Microalgal strains belonging to the Chlorophyceae class, such as Monoraphidium convolutum that dominated in both experiments in the current study, often have characteristics such as tolerance to a wide set of environmental condition. Bogen et al24 found that Monoraphidium neglectum (Clorophyceae) had a high lipid content (21%) and stable growth under N starved conditions and could therefore have potential as biofuel. There was no effect of light on biomass yield in the current experiment, which could be due to light niche complementarity effects in the polyculture.25 Different Dunaliella (Chlorophyceae) strains can for example differ in their response to light stress due to differences in sensitivity to light intensities. In high light, Dunaliella cells can use the carotenoid synthesis pathway as a protective mechanism against photodamage.26
Stressors used for microalgal cultivation aimed at lipid production would, in the best case scenario, not influence BP negatively since both biomass production and lipid content contribute to TL productivity. However, stressors such as N limitation have different optima for growth and lipid production.27 Olofsson et al5 showed that this trade-off was minimal at an N:P ratio of 5:1, where biomass production was as high as for N sufficient (N:P 20:1) but lipid production the double. Hence, an N:P ratio of 5:1 was chosen in the present study. N limitation (N:P 5:1) did not show any negative effect on biomass yield nor productivity in the current study but could potentially have both negative and neutral effects on the biomass yield and productivity. Breuer et al9 found productivity to decrease as a result of N deficiency after around 4 days, whereas Olofsson et al5 observed similar productivities in N limited (N:P 5:1) and N sufficient (N:P 20:1) cultures. Therefore, negative effects of N limitation may depend on the severity of nitrogen stress.
The present study did not observe any major effects of DSs on biomass yield nor productivity. Similarly, Yang et al15 studied the impact of DSs on growth and lipid accumulation in three Chlorella (Chlorophyceae) strains from three different regions and found that DSs did not have a negative effect on the biomass concentration. The arctic strain of Chlorella even grew more strongly under DSs than at constant temperature. When Nannochloropsis oculata was subjected to a DS of 5°C, there was no negative effect on growth either.14 Since photosynthesis is one of the most temperature sensitive processes in plants and algae,28 aquatic microalgae are constantly forced to adapt to large variations in temperature such as seasonal and diel cycles. These adaptations can determine how biomass and lipid productivity are affected by temperature changes.17 Shifts in temperature can however cause shorter lag phases in growth. Lynch et al13 observed a lag period when photosynthetic efficiency declined when cells were shifted from 30°C to 12°C, but after 96 hours, the growth rate stabilized to normal again.
It is however problematic to compare results of different studies. Productivity is for example highly dependent on other specific culture conditions (volume, cultivation vessel, light, aeration, etc) and productivities found in laboratory-scale experiments cannot be directly extended to productivities in outdoor photobioreactors but can be used as indications of stressors effect on productivity in general.9
2.2 Direct response of neutral lipids (BPF) to single stressors: Experiment 1
The first experimental setup was designed to test the direct response of microalgal BPF to conditions of HL and LL, high (18°C) and low (12°C) temperature and DSs of 6°C (DS 12–6) or 12°C (DS 18–6) compared with N:P ratio of the growth media (16:1 or 5:1). The experiment was run for 5 days and monitored at start and end.
The overall variation in BPF could be explained to 72% by the model (multiple linear regression, R2 = 0.72), where DSs had an effect on BPF (p < 0.05), but light and temperature conditions alone did not (p > 0.05). Although light alone did not give any effect on BPF, the treatments with LL in combination with DS (Tukey's post hoc, p < 0.001), increased BPF in both 12°C and 18°C compared with control (see Figure 1). However, NLs are known to increase under HL conditions (400 μmol photon m−2 s−1) and lipid productivity was three-fold compared with LL (40 μmol photon m−2 s−1) in green microalgae such as Chlorella sp and Monoraphidium sp.29 In an outdoor study by He et al,30 they noticed a similar pattern of increasing NL under HL intensity where polar lipids declined simultaneously. The differences in the effect of HL in the current experiment could be a result of the shorter experimental time period of 5 days compared with 12 days.
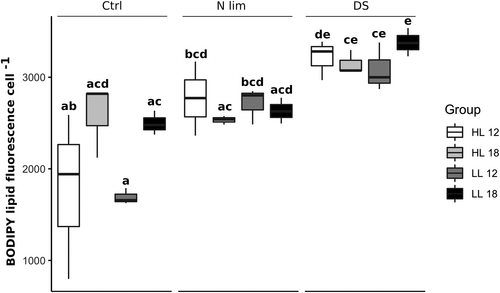
Temperature is another major factor that affects growth and lipid production in microalgal cultivation.31, 32 The response to high or low temperature can however differ from species to species.33 Wang et al34 have confirmed that low temperatures can also have negative effects on lipid production and growth, which could raise the cost of microalgal production. There was no difference in BFP, in the current study, between high temperature and low temperature treatments (see Figure 1). This could be due to species-specific differences in temperature tolerance range and its effect on lipid yield.35, 36 Yang et al15 compared the temperature tolerance range of three different Chlorella strains from different temperature regions (desert, arctic, and temperate) and found a wider temperature tolerance range in temperate and desert strains compared with polar strains due to region-specific adaptations. The polyculture isolated from the Baltic Sea used in the current study could have complex underlying dynamics where different adaptations and temperature optima may be present in different strains.
Compared to control treatments, DS increased BPF in all treatments (Tukey's post hoc, p < 0.01) except for in HL and high temperature in combination (HL18). In comparison to the standard stressor, N limitation, DSs increased BPF more in treatments with high temperature and LL (Tukey's post hoc, p < 0.05) as shown in Figure 1. The mechanism for lipid induction at lowered night temperature (DS) could be related to the cells maintenance of membrane fluidity, which is a metabolic adaptation influencing growth and photosynthesis at low temperature.37 One of the most characteristic adaptations to low temperature is an increase of the degree of unsaturated membrane fatty acids, leading to greater membrane fluidity and protection of the photosynthetic machinery from photoinhibition.38 This is however coupled to polar lipids such as phospholipids and glycolipids. In general, low temperature also means low metabolic activity when storage compounds, such as NL oil bodies in the cytoplasm, are formed.37 Others have reported that the proportion of lipids in polar algae increased with moderately warm temperatures, and that at low temperature, protein is the main product of photosynthesis.15, 39 In general, high daytime temperatures promote photosynthesis while low night temperatures inhibit cellular respiration, which enables glucose to be accumulated. However, since most cells have a limit in storage capacity for glucose, they convert excess carbon into lipids.40
On the other hand, DS resulted in the largest increase in BPF compared with constant temperature when cultures were kept at low day temperature (multiple linear regression, p < 0.05). N limitation also compensated for the decrease in BPF at constant low temperature (multiple linear regression, p < 0.05). The response in lipid production can be a result of different mechanisms that may be related to their adaptation to the environment such as light, salinity, nutrients and other environmental factors.37 If NL synthesis occurs as a result of environmental stressors, it is ultimately controlled by gene regulation of genes related to NL synthesis such as the Diacylglycerolacyltransferase (DGAT) gene.41 Previous studies have not fully established whether different stressors result in specific cellular mechanisms of lipid accumulation. Menon et al42 found that intracellular lipid content increased irrespective of the type of stress in Chlorella vulgaris and correlated to ROS levels, which indicate a similar response independent of stressor. However, in the current study, DSs increased BPF the most compared with other stressors tested.
2.3 Response to multiple stressors: Experiment 2
The stressors that explained the variation in NL in experiment 1 (DSs and N limitation) were tested both individually and in combination in a second setup (experiment 2). The BPF was monitored over a 14 day period in cultures with medium light (ML) and constant temperature (18°C) in full medium (N:P 16:1) compared with experimental treatments of N limitation (N:P 5:1), DSs (10°C shift), and a combination of both stressors.
2.3.1 Neutral lipids over time
The stress-induced increase in BPF occurred in early stationary phase after 8 days (two-way ANOVA, p < 0.01), as shown in Figure 2. Before 5 days, cells were likely in exponential growth when lipids usually do not accumulate as much (two-way ANOVA, p > 0.05). In experiment 2, the overall trend was that DS resulted in higher BPF than both N limitation and constant temperature on both days 8 (Tukey's post hoc, p < 0.001), 11 (Tukey's post hoc, p < 0.05), and 14 (Tukey's post hoc, p < 0.001). Temperature generally impacts the physiology of the cell by, eg, the rate of chemical reactions and stability of different cellular components.43 It was clear that DSs gave a faster response in BPF compared with N limitation since an increase was seen after 8 days (Tukey's post hoc, p < 0.001) compared with N limitation that did not show any effect on BPF compared with constant temperature until day 11 (Tukey's post hoc, p < 0.05). In commercial scale cultivation, lipid content could be kept at a high concentration using a semicontinuous cultivation strategy once the peak has been reached. N limitation does however not always take a long time to show an effect in NL content. Fan et al44 found TAG content and TLs to increase after only 4 days of N limitation. That was however under total N depletion, which decreased biomass density instead.
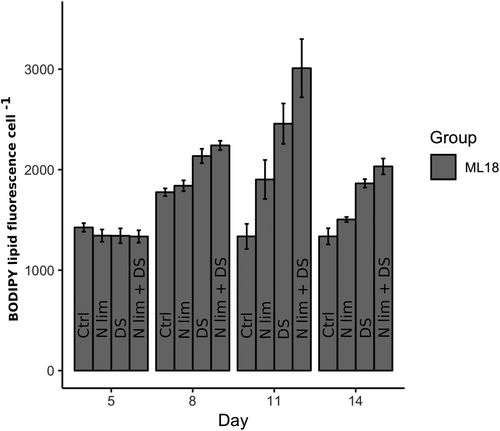
The BPF did not increase as much when exposed to N limitation compared with DSs (see Figure 2), especially in the shorter experiment 1 (see Figure 1). Possibly, the internal N reservoir could sustain the cells the first days of N limitation and hence give a delayed response in BPF as seen by Fan et al44 N is the most abundant element of intracellular components and is the primary constituent of most macromolecules.44 Once the cells have become depleted in N, they can utilize other compounds rich in nitrogen such as chlorophyll that is easily accessible to support further growth.45 Therefore, to reach a faster response in NL, N starvation (instead of N limitation) could be an option but will challenge BP.
2.3.2 Diurnal shifts compared with nitrogen limitation
N limitation has for many years been considered the norm stressor to boost lipid content in microalgae. Limitation of N can increase the formation of acetyl-CoA rapidly, which is the precursor of NL in terms of triglycerides. In the present study, an N limitation (N:P ratio 5:1) was applied. In comparison to N limitation, cultures at constant day temperature and light decreased in BPF (%) in all groups except for HL18 (see Figure 3).
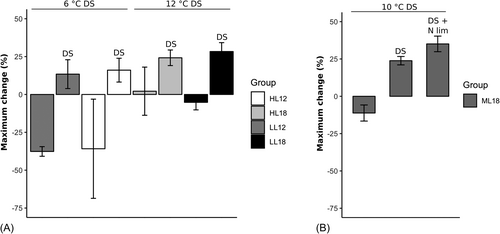
DSs increased BPF in all treatments (6°C, 10°C, and 12°C DSs) compared with N limited cultures (see Figure 3A and B). BODIPY lipid fluorescence cell−1 increased by 14% at a 6°C shift in LL and 16% in HL but increased more in the 10 and 12°C DS where BPF increased by 24% in ML (10°C DS) and HL (12°C) and 28% in LL (12°C). We show DSs to be a superior stressor to N limitation since the response is faster and induces a higher BPF. However, the effect of temperature on growth rate and lipid content is however species-specific.38 Lipids in Nannochloropsis sp and Chaetoceros sp (Coscinodiscophyceae) were observed to decrease with increasing temperature by Chaistuyakorn et al,38 whereas lipids increased with increasing temperature in Nannochloropsis oculata and Tetraselmis subcordiformis (Chlorodendrophyceae) according to Wei et al.46
Yang et al15 found an accumulation of lipids under different DSs in Chlorella sorokiniana isolated from desert and temperate climate and Chlorella sp from an arctic glacier. The highest lipid content at almost 52% was a result of a treatment of 11°C DSs, whereas 25°C and 7°C gave a smaller response. In the present study, BPF was equal at a DS of 10°C and 12°C DS, which could indicate that NL increase up to a certain level of DS after which NL content reaches a plateau. The differences in BPF as a response to DSs could depend on the temperature range in which the DS occurs and the temperature optimum/range of the strain or community. In summary, BPF increased more in treatments with a 10°C and 12°C DS than in 6°C compared with N limitation. The increase in NL in 10°C and 12°C DS was similar to N limitation cultures, but a combination of the stressors increased BPF further than DS alone.
2.4 Synergistic/additive effect of stressors: Experiment 2
Combinations of stressors were investigated in both experiments (1 and 2). In experiment 2 however, we focused on the potential synergistic/additive effect of the two strongest lipid boosting stressors, DSs, and N limitation. In outdoor cultivation systems, a single stress response is quite unlikely to occur. Instead, combinations of several stressors are more plausible due to fluctuations of environmental conditions on a diurnal as well as a longer time perspective.
In the current study, a combination of the two stressors N limitation and 10°C DS increased BPF by 35% compared with N limitation (Tukey's post hoc, p < 0.001) and 11% compared with DS alone (Tukey's post hoc, p < 0.001) (see Figure 3). It proves that DS and N limitation do not only have independent effects on BPF but may also be additive when combined. Srirangan et al47 observed an increase in TAG content when Dunaliella viridis cells were shifted from 25°C to 35°C under both continuous light and a 12:12 h light:dark cycle. They suggested both independent and additive effects of light and temperature since the increase was larger under 12:12 light:dark conditions in combination with the shift in temperature in comparison to continuous light.
The combination of the stressors increased BPF faster than N limitation alone after only 8 days (Tukey's post hoc, p < 0.001) as shown in Figure 2. After 11 days, the combination of stressors gave a higher BPF response than DS alone (Tukey's post hoc, p < 0.05) and the same pattern was consistent throughout the rest of the experiment. Therefore, the current study shows that to stressors can improve BPF in comparison to single stressors. Previous studies have confirmed that combinations of stressors such as N limitation and high salt concentrations can increase the lipid productivity by 25% to 45% compared with a single stressor.48 In contradiction, Roleda et al31 found that the combination of temperature and N stress gave no additive effect on lipid content. Nannochloropsis sp on the other hand, could increase TAG formation under high irradiance and increased salinity but decreased in both lipid content and culture biomass when N starvation was added to the stress combination.49 Thus, combinations of three or more stressors can result in negative stress and reduce the lipid productivity.48, 49
Within the limits of positive stress, photosynthetic carbon flow is directed at storage lipid formation whereas several negative stresses could result in enhanced production of ROS and thus damage to fatty acid and TAG biosynthesis enzymes,49 but a clear consensus regarding the mechanism has not been established yet. It is also difficult to predict exactly how certain stressors could affect the culture since different stressors can interplay and a response may be species specific, but the understanding of such processes is of great importance for the overall management and optimization of cultivation systems.
2.5 Conceptual model of DS impact on annual lipid content
Our results show that DSs is a powerful and rapid NL boosting stressor. The contribution of DS to lipid content could be expected to occur from spring to autumn over a cultivation season (April to December) in cold temperate latitudes. In the ALGOLAND photobioreactor on the southwestern coast of Öland, Sweden, from where the algal culture was obtained, DSs occur during spring to autumn but are usually not present in winter. During autumn (Sept-Oct 2014-2016), DS ranged 6.3°-7.6°C and peaks in lipid content have been observed during Sept-Nov (unpublished data) similar to results by previous studies.5, 11, 50 Within these periods of high DS, lipids are boosted naturally. The average yearly DSs ranged 7.5°C-10.3°C during the three years of cultivation in the reactor (2014-2016), which is in the range of DS used in the present study (6°C, 10°C, and 12°C).
Environmental factors such as temperature and photosynthetic active radiation (PAR) are shown in a conceptual model of how they could be expected to influence lipid content in an outdoor cultivation system (see Figure 4). The most powerful lipid boosting stressors in the present study (N limitation and DS) could be applied at different seasons in the outdoor cultivation system to increase NL and thereby economic sustainability. During Scandinavian winter, lipids could be expected to decrease as a result of decreasing temperature and light. Olofsson et al11 found low TL content during winter in an outdoor cultivation system in Algarve, Portugal, and hypothesized that the cell demand in energy prevented lipid accumulation. Low TL content has been observed in the ALGOLAND photobioreactor during spring as well (April–May) all three years when average minimum temperature was 8.5°C compared with 13°C during autumn (unpublished data). To boost lipid content in winter and spring, N limitation could be a suitable stressor since DS are not as prominent as in autumn during this period (see Figure 4). In summer, TL are stable but light tends to reach photoinhibiting levels and an added stressor (eg, N limitation) could decrease the NL content.
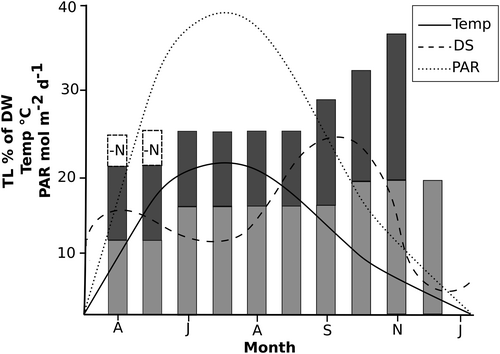
DSs do however occur in sunny arid regions as well, such as the southwestern United States where temperatures can differ as much as 26°C from day-night51 and in the tropics where microalgae grown outdoors have to adapt to a diurnal temperature range between 25°C-35°C throughout the year.33 This indicates a high prevalence and impact of DS in outdoor cultivation systems globally.
2.6 Implications
Climate change effects have increased the demands for microalgal NL production to enter the next generation of biofuels. NL production can be a slow process since it often occurs in the end of a logarithmic growth phase or induced by N limitation that can hinder the productivity. In general, cultivation temperature has to be kept stable at 20°C-30°C to gain economic benefits since lower temperatures can result in decreased productivity.2, 52 In this study, we show that DSs can boost BPF compared with more stable conditions in terms of temperature and should therefore be considered an alternative for boosting NL. In comparison to N limitation, all DS (6°C, 10°C, and 12°C) gave a higher BPF but the two stressors in combination gave the largest boost out of all factors tested.
In cultivation systems aimed at wastewater treatment, N limitation is not feasible to boost lipids. Wastewater is often rich in N compounds and Dalkrymple et al53 found that cultivation of microalgae in high strength wastewater resulted in very low lipid content (<10%) compared with the control (65%), although a DS of 13°C was present, which indicates that the strength of the DS was minimized by increasing nutrient concentration. There may therefore be several trade-offs when considering N limitation as a lipid booster, both in terms of applicability, productivity, and a delayed response in NL. The results given in this study show that it could be more economically feasible to grow native polyculture without temperature control to allow natural DS. A way to induce DSs would be to limit heating of the system during periods when high concentrations of lipids are wanted. This would also reduce costs and energy consumption and thereby increase overall sustainability of microalgal cultivation.
DSs have many advantages in comparison to N limitation since it reduces several trade-offs associated with nutrient limitation, such as reduced productivity. Combining cultivation of microalgae with wastewater treatment and CO2 sequestration is furthermore an important step towards a sustainable future. With results from this study, we illustrate the broad potential of utilizing stressors such as naturally occurring DS both alone as well as in combination with N limitation, which could overcome the drawbacks of traditional lipid boosting stressors.
3 MATERIALS AND METHODS
3.1 Culture conditions and experimental set up
Culture conditions
Microalgal suspension (40 L of microalgal polyculture) was collected from an outdoor pilot scale photobioreactor installed in the southeast coast of Sweden, Öland, (N 56° 35.2707, E 16° 40.7902). The photobioreactor belongs to the project ALGOLAND (https://lnu.se/algoland), and is a green wall panel (GWP), 1600 L, 50 m2 land area coverage, installed by Fotosintetica & Microbiologica S.r.l. In the laboratory, algal stock cultures were diluted with fresh medium on a regular basis during two weeks to obtain a stable optical density (OD range of 0.15-0.3) prior to the experiments. Medium was prepared from filtered (0.2 μm), autoclaved Baltic Sea water (7 psu) and modified f/2 nutrient solution (N:P 16:1, NO3− = 580 μM, PO34− = 36.3 μM) (54with L1 trace metals). Stock cultures were aerated and kept at 18°C and constant irradiance (200 μmol photons m−2 s−1) with a 9:15 h light:dark cycle. N and phosphorus (P) were monitored during the two weeks, and experiments 1 and 2 started upon high biomass and low nutrient concentrations.
Experiment 1: Response to single factors N limitation and DSs
Autumn conditions were set within the range of highest (HL) and lowest light (LL) and temperature (conditions, respectively, 215-300 (HL) and 33-50 (LL) μmol photons m−2 s−1, and 18°C and 12°C. Microalgal cultures (36 replicates) were inoculated in 3.4 L polystyrene bottles and incubated in the different conditions according to Figure 5 for a period of 5 days. The bottles were moved around daily to ensure similar light conditions in all treatments during the experiment. The cultures were diluted (30%) with fresh medium every second day. N limitation was obtained using a fresh medium deficient in N in relation to P with a ratio of N:P 5:1 (NO3− = 181.5 μM, PO34− = 36.3 μM). N replete cultures were diluted with a N:P = 16:1 medium. DSs were mimicked by lowering the temperature to 6°C during the dark period, resulting in two levels of stress (12°C and 6°C). The set of N replete cultures (12 replicates) only were moved daily to a lower temperature (6°C) during the dark period. At start and end of experiment 1, samples for BPF and dry weight (DW) were taken. Nutrient concentrations (NO3− and PO43−) were monitored at each dilution and adjusted to N:P 16:1 (N replete) or 5:1 (N limited). At the start, 50 mL of culture was filtered on a 3 μm membrane filter and frozen at −80°C with 1 mL RNAlater until DNA extraction.
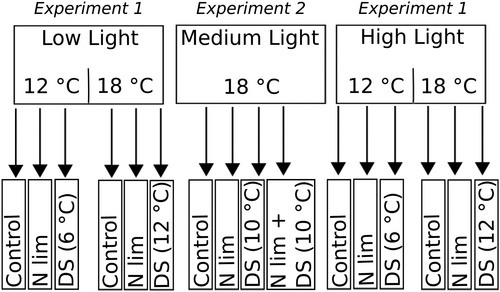
Experiment 2: Test of synergistic/additive effects
Microalgal semicontinuous cultures of 500 mL in tissue culture flasks with no aeration were set up according to Figure 5 on a shaking table to ensure mixing. Light was kept at 150-180 photons m−2 s−1 and constant temperature at 18°C. DSs were mimicked by moving cultures to a lower temperature (8°C) during the dark period. A 20% dilution was done every third day with new media. Before each dilution, samples for lipids were taken and DW was monitored. Nutrient concentrations (NO3− and PO43−) were monitored at each dilution and adjusted to N:P 16 (N sufficient) or 5 (N limited). At the start, 30 mL of culture was filtered on a 0.2 μm membrane filter and frozen at −80°C with 1 mL RNA later until DNA extraction.
3.2 Conceptual model
Data from the GWP (project ALGOLAND) on the southeast coast of Sweden, Öland, of TLs, PAR, and temperature (°C) was incorporated in the conceptual model.12 DSs were calculated from the difference between minimum and maximum temperature in the GWP 2014-2016. BODIPY lipid fluorescence cell −1 increased under DS (6°C, 10°C, and 12°C) was 22%-52% and 26% for N limitation in comparison to control (experiments 1 and 2), and the values were extrapolated from these results in the model.
3.3 Analyses methods
Algal lipid samples were fixed with formaline (37%, sterile filtered) to a concentration of 1.90%. BODIPY 505/515 (Molecular probes, D3921) was added to 3 mL fixed sample to a concentration of 5 × 103 from stock solution. After 15 minutes of incubation, samples were analyzed by flow cytometry (Partec, Cube8) according to size and fluorescence wavelength to gain mean FL1 fluorescence cell−1 (BODIPY lipid fluorescence cell −1). Average TAG content cell−1 has been proven to be highly correlated with average fluorescence of BODIPY stained cells of Chlorella vulgaris.55 DNA was extracted from filtered algal culture in experiments 1 and 2 with the PowerWater DNA isolation kit (MO-BIO Laboratories Inc, Carlsbad, CA) following the manufacturer's instructions with the modification that cells were lysed using Matrix E bead tubes (mpbio, Solon, OH) shaken twice at 60 m s−1 for 40 seconds (Fastprep-24 5G, MP Nordic biolabs). Extracted DNA yields and purity was measured using Nanodrop. The 18S ribosomal RNA gene regions were amplified (BIORAD T100 thermal cycler) using 574*F (CGGTAAYTCCAGCTCYV) and 1132R (CCGTCAATTHCTTYAART) primers56 at a concentration of 1 μM each using Illustra PureTaq Ready-to-go PCR beads. A one-step PCR procedure was applied according to the first reaction of Hugerth et al56 with 30 cycles, an initial denaturation temperature of 94°C for 10’ and final elongation at 72°C for 10’. The PCR amplicons were purified using a gel extraction kit (E.Z.N.A). The PCR products were cloned using the TOPO TA cloning kit for sequencing (Invitrogen) following the manufacturer's instructions. Plasmid DNA was isolated using the Plasmid DNA mini kit 1 (E.Z.N.A). The samples were sequenced at Eurofins genomics. The identities of the sequences were determined using NCBI Blastn. The sequences were deposited in GenBank under accession numbers MF370966-MF370971.
3.3.1 Statistics
All statistical analyses were performed using R (version 3.5.2) R studio (version 1.1.423).
Experiment 1:
Yield in DW (mg L−1) was inversely transformed to reach normal distribution (Shapiro–Wilk test of normality, W = 0.93, p = 0.0486) and equal variances (Bartlett's test for equal variances, K2 = 16.2, p > 0.05). BODIPY lipid fluorescence cell−1 data on day 5 was transformed by a Box-Cox (lambda = 2.6) transformation to achieve normal distribution (Shapiro–Wilk test of normality, W = 0.98, p > 0.05) and equal variances (Bartlett's test for equal variances, K2 = 15.4, p > 0.05). To model the relationships between explanatory variables and the response variable, a multiple linear regression was performed on both yield and NL data. A one-way ANOVA followed by a Tukey's post hoc test was then done to analyze the differences between each treatment in BPF and biomass yield.
Experiment 2:
Productivity data was normally distributed (Shapiro–Wilk test of normality, W = 0.95, p > 0.05) and had equal variances (Bartlett's test for equal variances, K2 = 0.99, p > 0.05). Yield data was also normally distributed (Shapiro–Wilk test of normality, W = 0.96, p > 0.05) and had equal variances (Bartlett's test for equal variances, K2 = 0.77, p > 0.05). A two-way ANOVA followed by Tukey's post hoc test was performed on yield and productivity data. Outliers in the BODIPY lipid fluorescence cell−1 data was removed from days 8 and 14 (Grubbs outlier test, p < 0.05), and new values were calculated from the mean of the remaining values in each treatment. BODIPY lipid fluorescence cell−1 data was normally distributed (Shapiro–Wilk test of normality, p > 0.05) and had equal variances (Bartlett's test for equal variances, p > 0.05). To distinguish significant explanatory variables, a two-way ANOVA was performed followed by Tukey's post hoc test to visualize differences between treatments on days 5, 8, 11, and 14, both in terms of lipids and biomass (yield and productivity).
ACKNOWLEDGEMENTS
This work was supported in part by Stiftelsen för Kunskaps- och Kompetensutveckling (KK- Stiftelsen, 20150219 to CL), Stiftelsen Lantbruksforskning (SLF O-15-20-559 to CL), Familjen Kamprads stiftelse (20160169 to CL), Carl Tryggers Foundation (CTS 16:270 to CL), Energimyndigheten (44677-1 to CL), The Swedish Research Council FORMAS (SFO Ecochange to CL, 2018-00692 to EL), Linnaeus University - Faculty of Health and Life Science, CementaHeidelberg AB, SMA Mineral, Kalmar Energi, and Kalmarsundsregionens renhållare (KSRR). We wish to thank Fredrik Svensson, Maurice Hirwa, Eva Sörenson, Varvara Sachpazidou, Kristoffer Bergström, Kimberly Berglöf, and Shenhong Ma for help during the experiment and with laboratory analyses and Hanna Farnelid for help and advice regarding molecular work.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Lina Mattsson, Catherine Legrand, Elin Lindehoff, and Martin Olofsson conceived and designed the experiment. Lina Mattsson performed the experiments. Lina Mattsson and Elin Lindehoff analyzed the data. Lina Mattsson, Catherine Legrand, Elin Lindehoff, and Martin Olofsson wrote the paper.
Lina Mattsson, Conceptualization-Equal, Data curation-Equal, Formal analysis-Equal, Investigation-Equal, Methodology-Equal, Project administration-Equal, Software-Equal, Visualization-Equal, Writing-original draft-Equal, Writing-review & editing-Equal; Elin Lindehoff, Conceptualization-Equal, Funding acquisition-Equal, ethodology-Equal, Project administration-Equal, Resources-Equal, Supervision-Equal, Writing-original draft-Equal, Writing-review & editing-Equal; Martin Olofsson, Conceptualization-Equal, Resources-Equal, Writing-original draft-Equal, Writing-review & editing-Equal; Catherine Legrand, Conceptualization-Equal, Funding acquisition-Equal, Methodology-Equal, Supervision-Equal,Writing-original draft-Equal,Writing-review & editing-Equal.




