Artificial targeting of the NEIL1 DNA glycosylase to the mitochondria
Accepted by: R. Sobol
Abstract
The human NEIL1 DNA glycosylase is one of 11 mammalian glycosylases that initiate base excision repair. While substrate preference, catalytic mechanism, and structural information of NEIL1's ordered residues are available, limited information on its subcellular localization, compounded by relatively low endogenous expression levels, have impeded our understanding of NEIL1. Here, we employed a previously developed computational framework to optimize the mitochondrial localization signal of NEIL1, enabling the visualization of its specific targeting to the mitochondrion via confocal microscopy. While we observed clear mitochondrial localization and increased glycosylase/lyase activity in mitochondrial extracts from low-moderate NEIL1 expression, high NEIL1 mitochondrial expression levels proved harmful, potentially leading to cell death.
1 INTRODUCTION
Everyday exogenous and endogenous damaging agents cause harmful modifications to DNA in both nuclear and mitochondrial compartments (mtDNA). This damage occurs in the form of abasic sites, base modifications (oxidation, alkylation, etc.), single-strand breaks, and double-strand breaks (Chatterjee & Walker, 2017). The Nei endonuclease VIII-like 1 (NEIL1) is one of 11 DNA glycosylases identified in humans that initiate the base excision repair (BER) process ensuring genomic integrity and cellular homeostasis (Prakash et al., 2012; Saki & Prakash, 2017). As a bifunctional DNA glycosylase with dual glycosylase and lyase activities, NEIL1 can excise oxidized DNA bases, which results in an abasic site, and subsequently cleaves the phosphodiester bond thereby generating a single-stranded break in the DNA backbone (Doublie et al., 2004; Hazra & Mitra, 2006; Prakash et al., 2012; Vik et al., 2012; Wallace, 2013). More specifically, NEIL1 catalyzes a β,δ-elimination reaction through the secondary amine of a highly conserved N-terminal proline (Pro2) residue. An adjacent glutamic acid (Glu3) is also essential for the reaction, serving as a proton donor. Additionally, mutagenesis studies have implicated Lys54 and Arg339 as crucial residues involved in the β- and δ-elimination reactions (Vik et al., 2012; Wallace, 2013). In both nuclear and mitochondrial compartments, downstream BER factors coordinate the repair of the break in a stepwise process via interactions with NEIL1 (Dianov et al., 2001; Prakash et al., 2012; Prakash & Doublie, 2015).
Enzymes that perform mitochondrial BER are encoded by nuclear genes and translocated to the mitochondria via regulated localization mechanisms (Omura, 1998). A prevalent mechanism of translocation involves the utilization of an N-terminal mitochondrial targeting sequence (MTS). Although certain BER enzymes possess a discernible MTS with a known sequence, others lack this feature (Audebert et al., 2002; Griffiths et al., 2009; Li et al., 2010). To facilitate the investigation of BER enzymes within the mitochondrion, several laboratories have explored introducing DNA glycosylases into cells, either through recombinant expression or by transgenic expression via the addition of an external MTS such as that from the manganese superoxide dismutase (MnSOD) protein. These studies consistently show improved cellular function in response to oxidative stressors (Dobson et al., 2000; Hashizume et al., 2014; Rachek et al., 2004). However, a common limitation in these studies is the use of the MTS from known mitochondrial proteins to direct the specific repair enzyme to the mitochondrial matrix. This method has several shortcomings including signal redundancy, off-target effects, and potential changes in mitochondrial homeostasis that could result from enzyme overexpression. In this work, we utilize our previously developed computational framework (Daly et al., 2021; Johnsten et al., 2022) to enhance the endogenous mitochondrial localization of the NEIL1 DNA glycosylase such that its localization to the mitochondrion is enhanced while retaining its nuclear expression. We also observed a measurable increase in NEIL1 activity in cells with increased mitochondrial NEIL1 expression.
2 MATERIALS AND METHODS
2.1 Generation of a novel MTS
We created a training set comprising 100 randomly selected nuclear-localized protein sequences sourced from DeepLoc-2.0 (Thumuluri et al., 2022) and 50 randomly selected mitochondrial matrix-localized protein sequences from the SM424-18 dataset (UniprotKB/SwissProt release 2018_02; Savojardo et al., 2020). The selected amino acid sequences were truncated to the first 86 amino acids to match the length of NEIL1's predicted MTS (Figure 1a). We generated multiple instances of the computationally engineered NEIL1 MTS using our computational tool and evaluated each generated sequence using the online tools, MULocDeep (Jiang et al., 2021) and DeepMito (Savojardo et al., 2020), to predict their cellular localization.

2.2 Generation of lentivirus
Lentiviral plasmids (pLV) containing the endogenous (en) MTS and the engineered MTS, referred to as 339 MTS, sequences driven a mutated thymidine kinase promotor (TKTSC) were procured from Vector Builder. To incorporate alternative expression promoters, a cytomegalovirus (CMV) promoter was cloned from the enMTS NEIL1 pCDNA3.1 vector (FWD 5′ gatgcggctagcTAGTTATTAATAGTAATCAATTACGGGG 3′ and RVS 5′ gacattctcgagGGGTTCTCTAGTTAGCCAGAGAGC 3′), while the mouse phosphoglycerate kinase 1 promoter (mPGK) promoter was obtained from the resistance gene of the pLV vector (FWD 5′ gacattgctagcTTCTACCGGGTAGGGGAGGCG 3′ and RVS 5′ gacattctcgagCGAAAGGCCCGGAGATGAGGAAG 3′). Promoter cloning was performed using 5′ NheI and 3′ HincII sites.
To generate lentivirus, HEK293FT cells were cultured in a 60 mm dish (37°C, 5% CO2) until reaching 50%–60% confluency, using 5.5 mL of antibiotic-free Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). In a separate tube, 375 μL of Opti-MEM was combined with 10 μL of XtremeGENE HP transfection reagent (1 μL reagent:1 μg DNA), 2 μg of each packaging vector (pMD2.G, pRSV-Rev, pMDLg/pRRE), and 4 μg of the lentiviral expression plasmid containing the MTS sequence. This mixture was incubated at room temperature for 25 min. Subsequently, the DNA/XtremeGENE HP mixture was added dropwise to the cultured HEK293FT cells and incubated at 37°C for 48 h. After incubation, the culture media containing lentivirus particles was collected, filtered using a 0.45 μm Steriflip filter, and stored at −80°C for future use.
2.3 Lentiviral transduction
U2OS, LN428, and HEK293 cells (2.2 × 105) were seeded in 6-well plates with DMEM supplemented with 10% FBS. After 18 h of culture, the cells were incubated with 50% viral culture supernatant (prepared as described above) and 10 μg/mL polybrene. Cells were incubated for 72 h.
2.4 Immunostaining and microscopy
Transduced cells were seeded into 35-mm fluorodishes and incubated for 18 h. The cells were subsequently incubated in incomplete media (FBS-free, antibiotic-free) containing 50 nM MitoTracker Red CMXRos and Hoechst for 15 min at 37°C. The dye-containing media were replaced with fresh media, and the cells were further incubated at 37°C for 1 h. After the incubation period, cells were fixed with 4% paraformaldehyde in PBS for 15 min on ice and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature. Following permeabilization, samples were blocked in 1× PBS, 5% goat serum, and 0.3% Triton X-100 for 60 min at room temperature before incubation with an anti-cMyc tag antibody (1:1000 dilution in 1× PBS, 1% bovine serum albumin [BSA], and 0.3% Triton X-100) at 4°C overnight.
After the primary antibody incubation, cells were treated with an anti-mouse secondary antibody (1:1000 dilution in 3% milk in PBS) for 1 h at room temperature in the dark. Subsequently, cells were rinsed three times in PBS for 10 min each and covered with a drop of Prolong Gold Anti-fade reagent before the application of a coverslip. The mountant was allowed to cure overnight at room temperature in the dark. All localization experiments were performed using a Nikon A1r confocal microscope with a 60× oil objective.
2.5 Mitochondrial isolation
Mitochondria were extracted from cells using the ThermoFisher mitochondrial isolation kit following the manufacturers' recommended instructions. Purity of the mitochondrial extracts was confirmed via western blot, probing for COXIV (0.5 μg/mL; 5% milk in Tris-buffered saline with Tween 20 [TBST]) and α-Tubulin (0.1 μg/mL; 4% milk in TBST).
2.6 NEIL1 mRNA and protein expression analysis
Cells were collected by cell scraping and pelleted by centrifugation. Proteins were extracted from cell pellets by incubation with CelLytic M and 1 mM phenylmethylsulfonyl fluoride for 15 min at 4°C. Lysate was clarified by centrifugation at 20,000× g at 4°C. Whole cell and mitochondrial extracts were probed for NEIL1 (0.4 μg/mL; 5% milk in TBST), COXIV (0.5 μg/mL; 5% milk in TBST), and PCNA (0.134 μg/mL; 5% BSA in TBST) protein expression.
NEIL1 mRNA expression was evaluated using the ThermoFisher Gene Expression Cells-to-Ct kit utilizing an NEIL1 and GAPDH TaqMan probes (Table 1).
| Reagent | Source | Cat no. |
|---|---|---|
| HEK 293FT | ThermoFisher | R70007 |
| U2OS | ATCC | HTB-96 |
| HEK293 | ATCC | CRL-1573 |
| 60 mm tissue culture dish | Corning | 353,002 |
| DMEM media | Life Technologies | 11-995-065 |
| OptiMEM media | Fisher Scientific | 11058021 |
| FBS | Atlanta Biologics | S11150 |
| pMDLg/pRRE | Addgene | 12,251 |
| pRSV-Rev | Addgene | 12253 |
| pMD2.G | Addgene | 12259 |
| X-tremeGENE HP transfection reagent | Millipore-Sigma | 6366236001 |
| 0.45 μm Steriflip filters | Millipore-Sigma | SE1M003M00 |
| Polybrene | Millipore-Sigma | TR-1003-G |
| Puromycin | Gibco | A11138-03 |
| NucBlue Live ReadyProbes Reagent (Hoechst 33342) | Life Technologies | R37605 |
| MitoTracker Red CMXRos | MitoTracker Red CMXRos – Special Packaging | M7512 |
| 35 mm fluorodishes | World Precision Instruments (WPI) | FD35-100 |
| 4% Paraformaldehyde | Fisher Scientific | AAJ61899AK |
| Triton X-100 | Fisher Scientific | BP151-100 |
| Goat serum | ThermoFisher | 31,873 |
| Glycine | Fisher Scientific | G48-212 |
| PBS | Fisher Scientific | 14190250 |
| Bovine serum albumin | VWR | T9784 |
| Blotting-grade blocker | Bio-Rad | 1706404 |
| Prolong gold anti-fade | Life Technologies | P10144 |
| cMyc-tag antibody | Cell Signaling Technologies | 2276S |
| Alexafluor-488 | Fisher Scientific | A11029 |
| Mitochondrial isolation kit | ThermoFisher | 89874 |
| TaqMan gene expression cells-to-Ct kit | ThermoFisher | AM1728 |
| NEIL1 TaqMan probe | Life Technologies | 4331182 (Hs00226327_m1) |
| GAPDH TaqMan probe | Life Technologies | 4331182 (Hs02758991_g1) |
| Gels | BioRad | 4568094 |
| Transfer pack | BioRad | 1704156 |
| COXIV antibody | abcam | ab16056 |
| α-Tubulin antibody | Calbiochem | CP06 |
| NEIL1 antibody | ProteinTech | 12145-1-AP |
| PCNA antibody | Cell Signaling Technologies | 13110S |
| Anti-mouse secondary | Cell Signaling Technologies | 7076S |
| Anti-rabbit secondary | Cytiva | NA934-1ML |
| CelLytic M | Fisher Scientific | C2978-50ML |
| Phenylmethylsulfonyl fluoride | Sigma-Aldrich | P7626-25 g |
| DC Protein Estimate Kit | BioRad | 5000112 |
| Advansta Western Blot Detection Kit | Fisher Scientific | NC0896372 |
| KCl | Fisher Scientific | P217-3 |
| Tris HCl | Fisher Scientific | BP153-1 |
| TBS | BioRad | 170–6435 |
| Tween-20 | Fisher Scientific | BP337-100 |
| Primers | Sequence (5′–>3′) | |
| mPGK FWD | gacattgctagcTTCTACCGGGTAGGGGAGGCG | |
| mPGK RVS | gacattctcgagCGAAAGGCCCGGAGATGAGGAAG | |
| CMV FWD | gatgcggctagcTAGTTATTAATAGTAATCAATTACGGGG | |
| CMV RVS | gacattctcgagGGGTTCTCTAGTTAGCCAGAGAGC | |
2.7 Activity assay
The glycosylase/AP lyase cleavage assay was performed as previously described (Jacobs et al., 2013), with varying concentrations of purified NEIL1 (purification previously described Sharma et al., 2018), BSA, and mitochondrial extracts. Fluorescence was acquired on a Spark (Tecan, Mannedorf, Switzerland).
3 RESULTS AND DISCUSSION
The overall low-cellular expression combined with undetectable (via microscopy) endogenous expression levels of mitochondrial NEIL1, led us to develop a novel computational method (described in detail in Johnsten et al., 2022) to generate an engineered MTS (339), referred to as 339-NEIL1, to target NEIL1 to the mitochondria, while retaining its nuclear localization. Briefly, this method is based on the multilayer vector space (MLVS) model (Johnsten et al., 2022). The MLVS model represents DNA or protein sequences as combinations of specific pairs of elements (in our case amino acids) that are separated by a certain number of positions in the sequence. Each pair is described by the distance between them, referred to as the step size. The maximum step size is one less than the length of the sequence. A “BB” (which could be thought of as a “building block”) is defined by these pairs and their step size, where the first element is considered the anchor and the second element the tail. In this model, biological sequences that do not achieve the desired function or localization (in our case specifically to the mitochondria) can be computationally re-engineered. This is done by replacing specific building blocks within the sequence that are likely impeding localization with others that are predicted to improve the desired outcome (Daly et al., 2021; Johnsten et al., 2022).
Here, we accomplished our primary objective to test the framework in the laboratory by transforming the endogenous MTS of NEIL1 to enhance its localization to the mitochondria. The optimal computationally engineered MTS, referred to as 339-NEIL1, was selected based on its high predicted localization to the mitochondrial matrix (Figure 1a,b) and its low number of modified amino acids (73% identity to the endogenous NEIL1 MTS [enMTS]).
Given the low endogenous expression of the NEIL enzymes, we and others commonly resort to overexpression systems to visualize the NEIL enzymes via confocal microscopy. For this purpose, we designed pLV vectors with varying promoter strengths (Figure 1c) to generate stable isogenic human cell lines. While we continue to employ the widely utilized and robust CMV promoter, known to dramatically increase protein expression, we have also chosen two alternative promoters associated with lower expression levels. In contrast to the CMV promoter, the mPGK promotor has been demonstrated to induce relatively consistent and moderate protein expression in mammalian cells (Qin et al., 2010). For achieving low expression levels, more closely resembling that of endogenous NEIL1, we selected the TKTSC promotor, which consistently yields low expression levels across multiple cell lines, while generating detectable protein levels via confocal microscopy (Ali et al., 2018).
Confocal analysis revealed distinct nucleolar expression of NEIL1 (Figure 1d; shown in green) harboring the enMTS (En-NEIL1) with the three different promoters (TKTSC, mPGK, and CMV). In contrast, NEIL1 harboring the 339-MTS maintains nucleolar expression while exhibiting a notable increase in mitochondrial localization (Figure 1d; merged yellow). Additionally, we observed an expected increase in NEIL1 expression with an increase in promotor strength.
We consistently observed the 339-MTS driving expression to the mitochondrion when driven by the TKTSC (low expression) and mPGK (moderate expression) promoters, which bolsters the validity and efficacy of our computational framework in a wet-lab setting. We anticipated the sustained nuclear localization of 339-NEIL1, given the retention of the NEIL1 C-terminal nuclear localization signal (NLS; Figure 1d). Curiously, our expectation for the CMV promoter to drive the highest levels of NEIL1 mitochondrial expression was confounded when we observed no detectable NEIL1 expression above background. Despite multiple attempts, we were unable to capture even a single cell expressing the 339-NEIL1 with the CMV promoter. We postulate that the high levels of CMV-promoted 339-NEIL1 directed to the mitochondria may be leading to cell death before the cells can be fixed and visualized via microscopy. These results were reproducible across multiple cell lines (U2OS [Figure 1], HEK-293 and LN428 [data not shown]). This finding carries significant implications; simply put, excessive levels of a DNA repair enzyme within the mitochondria could prove toxic to cells, leading to cell death.
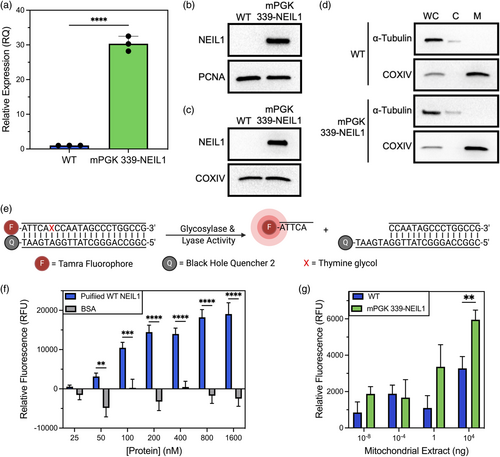
To further investigate the functional implications of mitochondrial expression of the engineered 339-NEIL1, we assessed the activity of stable cell lines expressing 339-NEIL1, using mPGK-driven expression as a representative model. To accomplish this, we first clonally expanded HEK-293 mPGK 339-NEIL1 cells and utilized reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) and western blot analyses to verify that NEIL1 expression was increased at both the mRNA and protein levels (Figure 2a–d). Next, to show that the stable expression of 339-NEIL1 enhances glycosylase activity within the mitochondria, we performed a glycosylase activity assay with purified mitochondrial extracts. For this, we utilized a fluorescently labeled (TAMRA), double-stranded DNA substrate (22-nt in length) harboring a single thymine glycol (Tg, Figure 2e), one of the favored substrates for NEIL1. For proof of concept, we performed this assay initially with purified recombinant NEIL1 and observed a concentration-dependent increase in NEIL1 activity as measured by the release of the cleaved fluorophore-containing DNA (Figure 2f) compared with a negative control, BSA, which displayed no activity against the Tg-containing substrate. Next, we performed the assay with mitochondrial extracts prepared from wild-type nontransfected cells and mPGK-339-NEIL1 expressing cells. We note a consistent increase in activity with the mPGK-339-NEIL1 mitochondrial extracts, with increasing significance observed with the highest dose of extract. Collectively, our data demonstrate that we can successfully engineer NEIL1 expression to be elevated within the mitochondrial compartment, resulting in enhanced activity against Tg, one of NEIL1's preferred substrates.
The delicate balance of DNA repair enzyme expression is crucial for the maintenance of cellular homeostasis. Overexpression or under-expression of enzymes essential for key cellular processes such as DNA repair, can disrupt mitochondrial function, resulting in cellular toxicity and death. Therefore, maintaining this balance is essential for preserving cell viability and function. This work underscores the importance of fine-tuning DNA repair enzyme levels to avoid detrimental effects on cellular health. Interestingly, while several single-nucleotide polymorphisms (SNPs) within the NEIL enzymes are documented in the COSMIC and dbSNP databases, there are currently no known SNPs within the endogenous NEIL1 MTS. However, known SNPs exist within the NLS that could potentially alter the subcellular localization of the enzyme. Despite this, no current studies have directly addressed this issue, leaving a gap in our understanding of how these SNPs might impact NEIL1's function and subcellular dynamics. Currently, we are in the process of utilizing isogenic clonal cell lines for future investigations aimed at elucidating the impact of NEIL1 expression within the mitochondria on cellular health. Furthermore, this work demonstrates the efficacy of our computational framework (Daly et al., 2021; Johnsten et al., 2022) for engineering functional MTSs.
AUTHOR CONTRIBUTIONS
AP conceived and designed the study. TJ developed the computational framework and performed the computational experiments. MKT and MHE performed the wet laboratory experiments. MKT and AP analyzed the data and drafted the article. MKT, RGB, TJ, and AP edited the article. AP and TJ obtained the funding for the study.
FUNDING INFORMATION
MKT, MHE, and AP are supported by a grant from the National Institutes of Environmental Health Sciences (NIEHS) Outstanding New Environmental Scientist (ONES) R01 grant no. R01ES030084 to AP. Startup funds provided to AP by the University of South Alabama Health Mitchell Cancer Institute are also acknowledged. This work was also supported by startup funds from the School of Computing that were used to purchase some of the materials.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




