Changes in lactate concentration are accompanied by opposite changes in the pattern of fat oxidation: Dose–response relationship
Abstract
It is unknown whether changes in lactate concentration produced by different situations (e.g., glycogen depletion or heat) modify fat oxidation. If confirmed, we could determine a dose–response relationship between lactate and fat. The aim of this study was to determine whether changes in lactate concentration (due to glycogen depletion or heat) alter fat oxidation during exercise. 11 males and eight females performed an incremental exercise test under three situations: control, glycogen depletion, and heat. At rest, in the last minute of each step and immediately post-exhaustion, lactate was analyzed and fat oxidation was estimated by indirect calorimetry. Lactate concentration was inversely associated with fat oxidation in the three aforementioned situations (r > 0.88 and p < 0.05). The highest lactate concentration was found in the heat situation, followed by the control situation, and finally the glycogen depletion situation (all p < 0.05). The opposite was found for fat oxidation, with the highest fat oxidation found in the glycogen depletion situation, followed by the control situation, and finally the heat situation (all p < 0.05). There is no association between the changes in lactate concentration between situations at each intensity and the changes in fat oxidation between situations at each intensity in males or females (p > 0.05). In conclusion, lactatemia is strongly and inversely associated with fat oxidation under the three different situations. Furthermore, the lowest lactate concentrations were accompanied by the highest fat oxidations in the glycogen depletion situation, whereas the highest lactate concentrations were accompanied by the lowest fat oxidations in the heat situation.
Highlights
-
It was found that a very strong inverse association between lactatemia and fat oxidation in females and males despite changes in both substrates between situations. Hence, assessing blood lactate alone could be an effective way to indirectly assess fat oxidation when the situation is the same. Importantly, lactate-fat relationship is different between situations, indicating to extrapolate fat oxidation from lactate, or vice versa, this relationship needs to be previously determined in each specific situation.
-
Since the same blood lactate concentration does not imply the same fat oxidation in different situations, a fixed blood lactate concentration may not reflect the same metabolic response, even in the same individual, if he/she is exposed to different situations.
-
Changes in lactate kinetics, due to heat or glycogen depletion, are accompanied by opposite changes in the fat oxidation pattern. However, changes in blood lactate concentration between situations at each intensity are not associated with changes in the fat oxidation between situations at each intensity. Therefore, these results suggest an absence of a dose–response relationship between both variables indicating that lactate may not be a major regulatory factor of changes in fat oxidation across different situations during exercise.
1 INTRODUCTION
Lactate has different functions in the body: (1) it is an energy source, (2) it is the major gluconeogenic precursor and, (3) it is considered a signaling molecule with autocrine-, paracrine-, and endocrine-like effects, being called “lactormone” (Brooks, 2018). This last function has gained more attention in recent years, in areas of intermediary metabolism, redox biology, mitochondrial biogenesis, neurobiology, gut physiology, appetite regulation, or nutrition (Brooks et al., 2023).
One of the main signaling functions of lactate during exercise is the regulation of muscle metabolism in an autocrine and paracrine way (Brooks et al., 2023). In 1962, Issekutz et al. were the first to describe that there was an inverse association between lactacidemia and free fatty acids in exercising dogs when infusing lactic acid (Issekutz & Miller, 1962). More than 10 years later, the pioneer study in humans by Boyd et al. showed that infusion of D, L-sodium lactate during exercise decreased free fatty acid and glycerol levels in the blood. They suggested that a high lactate concentration during exercise produces a direct inhibition of lipolysis (Boyd et al., 1974). Thereafter, at the beginning of this century, Achten et al. described a clear relationship between the increase of blood lactate concentration above baseline levels and the onset of a decrease in fatty acid oxidation (Achten & Jeukendrup, 2004). Specifically, they found that the intensity corresponding to the first rise in lactate concentration during incremental exercise was correlated with the intensity, which elicited maximal fat oxidation rates (Fatmax). Moreover, they found that the intensity corresponding to the inflection point of the lactate curve during an incremental test was correlated with the intensity at which fat oxidation rates become negligible (Fatmin). Recently, San-Millán et al. went one step further observing that lactate concentration was positively correlated with carbohydrate oxidation and negatively correlated with fat oxidation across participants with different metabolic characteristics (elite endurance athletes, moderately active individuals, and patients with metabolic syndrome). Interestingly, those subjects with a faster increase in blood lactate concentration presented a lower fat oxidation rate and vice versa (San-Millán & Brooks, 2018).
Different situations can modify lactate concentration during exercise. For example, exercise in the heat in non-acclimatized individuals results in a greater reliance on carbohydrate metabolism (M. Febbraio et al., 1994; Hargreaves, 2008). Since lactate is the end product of glycolysis (Rogatzki et al., 2015), the greater reliance on glucose in a hot environment will lead to increased lactate production (De Barros et al., 2011; M. Febbraio et al., 1994; M. A. Febbraio et al., 1996; Hargreaves, 2008). Studies of metabolic reactions to exercise and heat have shown a higher muscle glycogen use and a higher glycolysis resulting in a higher lactate concentration (M. A. Febbraio, 2001). In fact, a relationship between muscle temperature and muscle lactate has been observed (Kozlowski et al., 1985). On the other hand, several studies have modified previous glycogen levels by combining diet and exercise, showing lower lactate concentrations with low glycogen levels (Busse et al., 1991; McLellan & Gass, 1989; Prusaczyk et al., 1992). However, it has not yet been established whether these changes in lactate concentration produced by the different situations modify fat oxidation. If confirmed, we could determine a dose–response relationship between lactate concentration and fat oxidation that would help to elucidate the interaction between fat oxidation and blood lactate concentration during exercise. In addition, it would be possible to estimate fat oxidation from lactate concentration, and vice versa, regardless of the situation. This would avoid having to establish the blood lactate–fat oxidation relationship in each situation.
Therefore, the aim of this study was to assess whether changes in lactate concentration, due to glycogen depletion or heat, alter the pattern of fat oxidation during a maximal incremental cycle ergometer test in healthy young active females and males. It was hypothesized that blood lactate concentration was inversely correlated with fat oxidation in the different situations. Additionally, we hypothesized that the lowest lactate concentrations observed in the glycogen depletion situation will be accompanied by increased fat oxidation. In contrast, the highest lactate concentrations in the heat situation will be accompanied by less fat oxidation.
2 MATERIAL AND METHODS
2.1 Participants
Eleven males and eight naturally menstruating females participated in this study. All of them were healthy and endurance trained (McKay et al., 2022; Table 1). Inclusion criteria required all subjects to (a) be healthy and between 18 and 40 years of age; (b) undertake endurance training more than 3 h per week; and (c) be a nonsmoker; and for women to (d) not be users of hormonal contraceptives; (e) not be pregnant; (f) have natural and regular menstrual cycles (Elliott-Sale et al., 2021) and; (g) not have abundant menstrual bleeding (<80 mL; Magnay et al., 2020).
| Age (years) | Males | 22 ± 3 |
| Females | 22 ± 2 | |
| Height (cm) | Males | 176.2 ± 4.0 |
| Females | 165.1 ± 5.3 | |
| Weight (kg) | Males | 68.4 ± 4.9 |
| Females | 60.8 ± 4.5 | |
| Fat (kg) | Males | 6.80 ± 2.14 |
| Females | 14.06 ± 2.51 | |
| Fat free mass (kg) | Males | 59.42 ± 4.86 |
| Females | 43.82 ± 5.37 | |
| Fat (%) | Males | 10.3 ± 3.1 |
| Females | 24.4 ± 4.7 | |
| Peak oxygen uptake (mL/min) | Males | 3581 ± 369 |
| Females | 2767 ± 433 | |
| Peak oxygen uptake (mL/min/kg) | Males | 52.30 ± 3.64 |
| Females | 45.33 ± 5.37 |
- Note: Data are shown as mean ± standard deviation.
Ethical authorization for this study was obtained from the research ethics committee of the university where the research was conducted (ID: DATOS-20220524-RCC-LacFat). Subjects were informed of the risks and benefits of the study prior to participation and written informed consent was obtained from each subject before their inclusion.
2.2 Study design
This crossover study consisted of four situations carried out on five visits to the laboratory on five different days (Figure 1). All the tests were performed by males in less than 5 weeks, whereas females performed all the tests in less than 10 weeks due to the control of the menstrual cycle. The first visit included informed consent, an assessment of body composition by total body dual-energy X-ray absorptiometry (DXA), and individualized nutritional plan. Afterward, in the following appointments, the same maximal incremental tests were performed in three different experimental situations (control, glycogen depletion, and heat). Tests were performed at the same time of day (±2 h). In the case of females, all tests occurred during the early-mid follicular phase of the menstrual cycle (day 4 ± 1.67). The first test was in all cases a maximal incremental test in the standard situation (control situation). The order of the two remaining incremental tests (glycogen depletion or heat situations) was randomized. In order to prepare for the glycogen depletion test, 24 h before the incremental test, an exercise protocol was performed to completely deplete muscle glycogen stores. In addition, from breakfast on the same day of the glycogen depletion exercise protocol until the maximal incremental test, a low-carbohydrate diet was followed. On the contrary, in control and heat situations, from breakfast of the previous day of the tests, participants followed a high-carbohydrate diet. During both control and glycogen depletion situations, the ambient temperature was maintained at ∼21°C and a relative humidity at ∼40%. Finally, during the heat situation, participants were placed in a heat chamber designed to maintain an ambient temperature between 36°C and 38°C and a relative humidity at ∼40% for 30 min prior the incremental test, and the incremental test was performed at the same environmental conditions (Maunder et al., 2021). In all the situations, body weight loss was less than 2%.
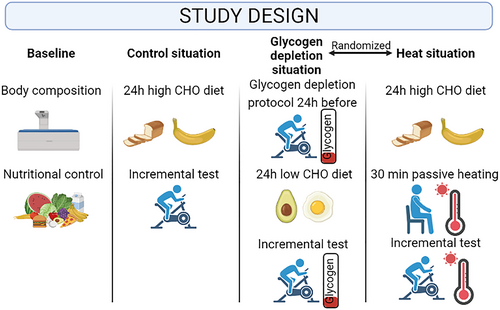
Overview of the study design. CHO, carbohydrate.
2.3 Body composition assessment
Body composition was measured by a DXA scan (Version 6.10.029GE Encore 2002, GE Lunar Prodigy; GE Healthcare) to obtain fat mass and fat-free mass (FFM). All participants performed the test wearing light sportswear (e.g., top and shorts). In addition, they must be with their body and hands in a supine position and their feet joined by a strap. Participants were instructed not to move or speak while the scanner performed the measurements. The scan was calibrated every other day using the phantom supplied by the manufacturer.
2.4 Nutritional intervention
2.4.1 High-energy and high-carbohydrate diet
Participants followed a diet at least 24 h before the incremental test in the control and heat situations to ensure adequate energy and carbohydrate intake to avoid performance limitations during testing. The macronutrient distribution was standardized for all participants at an approximate ratio of 65% carbohydrate, 15% protein, and 20% fat. Energy and carbohydrate intake were determined for all participants in relation to their FFM (energy: 60 kcal·kg·FFM−1 and CHO: 9.8 g·kg·FFM−1; Burke et al., 2021; see Figure S1 for example in Supplementary Material).
2.4.2 High-energy, low-carbohydrate, and high-fat diet
This diet matched the energy intake of the high carbohydrate diet (60 kcal·kg−1·FFM·day−1). However, carbohydrate intake was severely restricted (<50 g·day−1) and protein and fat intake were 20% and 75%–80%, respectively (Burke et al., 2021). The most difficult nutritional objective to achieve was to reach the previously established daily caloric intake without exceeding the carbohydrate limit. Therefore, a medium-chain triglycerides supplementation was used to achieve this energy objective without contributing to increase carbohydrates (see Figure S1 for example in Supplementary Material).
2.5 Exercise protocol
2.5.1 Incremental exercise test
The incremental test performed in the three different situations was carried out on a cycle ergometer (Lode Excalibur Sport). The cadence throughout the test was constant, remaining between 70 and 90 rpm. The protocol started with 1 min of rest followed by 5 min of warm-up at 30 W. Subsequently, power was increased by 30 W every 3 min until maximum effort was reached, considering this when a reduction in cadence of 10 rpm was observed.
2.5.2 Glycogen depletion protocol
The glycogen depletion protocol consisted of a 60-min continuous cycling exercise at the power associated with 60% of VO2peak calculated from the power-VO2 ratio obtained during the maximal incremental cycling test performed in the control situation. The pedaling cadence was set at 70 rpm. After that, six 30-s sprints were performed at the maximum possible intensity with a 7.5% body weight load with a 4-min recovery between bouts. This protocol has been carried out in previous studies with the same objective, having already demonstrated its efficacy in the depletion of glycogen stores (Cheng et al., 2020).
2.6 Data analysis
Whole blood lactate concentration was measured during the three different incremental tests. Capillary blood was drawn from the fingertip at rest, in the last 30 s of each step and immediately after the moment of maximum effort, and these samples were analyzed with a lactate analyzer (EKF Diagnostic GmbH). The difference in lactate concentration (ΔLactate concentration) and fat oxidation (ΔFat oxidation) at each power output between situations was calculated.
Moreover, heart rate was analyzed beat by beat throughout the three different incremental tests and averaged every 15 s through the Polar H10 (Polar Electro Oy). This band was connected via Bluetooth to a cell phone that received the signal and where the data were stored. Throughout the tests, central temperature with a tympanic thermometer (Braun IRT6520), rate of perceived exertion with 0–10 and 6–20 scales, and ambient temperature and humidity (Dienmern) was controlled.
Two males and two females were excluded from the final analyses because their gas-exchange data reflected abnormalities (due to excessive hyperventilation) during the control situation that precluded the correct assessment of fat oxidation. In the first steps of the control test, the RER in these participants was very close to 1 coinciding with lactate concentrations similar to baseline, indicating individuals were ventilating above what would be appropriate for the intensity they were performing (Keir et al., 2022). Given that fat oxidation is obtained from respiratory variables, hyperventilation prevents us from being able to determine fat oxidation correctly.
2.7 Statistical analysis
Data are presented as mean ± standard deviation. The initial sample size was determined through a pilot study with 6 subjects, and a priori power analysis was conducted to determine the final sample size using the statistical software (G*Power v. 3.1.9.2). One way ANOVA repeated measures within factors was selected using power output at the lactate threshold (OBLA 4) in the three different situations of the study with the following criteria: effect size F = 2.015, α = 0.05, power = 0.95, number of groups = 3, number of measurements = 3, correlation among repeated measures = 0.5, and nonsphericity correction = 1. Based on these inputs, at least six participants were required. Using the statistical software (G*Power v. 3.1.9.2), a post hoc power analysis using a repeated measures ANOVA was performed with the following inputs from lactate concentration: effect size f = 47.54 in males and 18.15 in females; α = 0.05; sample size = 11 in males and 8 in females; groups = 3; measurements = 8 in males and 6 in females; correlation = 0.5; and nonspherecity correction = 1. In all cases, statistical power was very close to 1. The normal distribution was confirmed by the Shapiro–Wilk test. A mixed linear model was used to compare the individual response (random effect) of lactate or fat oxidation between situations (fixed effect) at the different protocol intensities (fixed effect). Bonferroni correction was applied for post hoc analyses. Pairwise comparisons effect size was calculated by Cohen's d considering d < 0.5 as small, d between 0.5 and 0.8 as moderate, and d > 0.8 as large (Hojat & Xu, 2004). The association between different variables was calculated using Pearson's correlation. Statistical analysis was carried out using the Jamovi V1.6 statistical software (Jamovi). The significant level was set at p < 0.05.
3 RESULTS
In males and females, a very strong inverse association was found between lactate concentrations and fat oxidation in control, glycogen depletion, and heat situations (Figures 2 and S2).
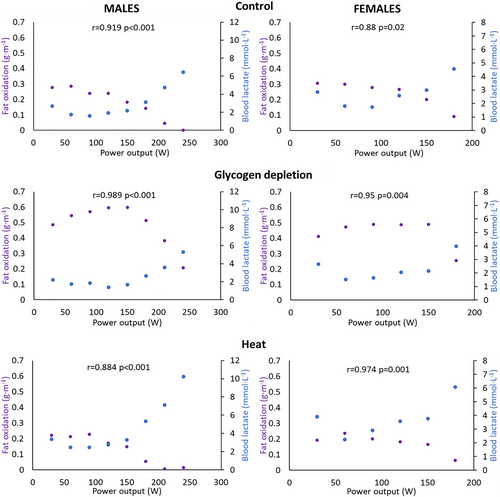
Relationships between the average blood lactate concentrations and fat oxidation as a function of exercise power output in control, glycogen depletion, and heat situations in males and females.
In males, a main effect of the situation was found in blood lactate concentration (F (2,220) = 47.45 and p < 0.001). Pairwise comparisons showed that blood lactate concentration was higher in heat compared to control (p < 0.001 and d = 0.473) and glycogen depletion situations (p < 0.001 and d = 0.728). In addition, blood lactate concentration was higher in control compared to glycogen depletion situation (p = 0.037 and d = 0.255). On the other hand, fat oxidation was different between situations (F (2,182) = 216.24 and p < 0.001). Pairwise comparisons showed that fat oxidation was lower in heat compared to control (p = 0.047 and d = −0.236) and glycogen depletion situations (p < 0.001 and d = −2.038). Moreover, fat oxidation was lower in control compared to glycogen depletion situation (p < 0.001 and d = −1.801; Table 2).
| Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity (W) | Situation | N | Lactate (mmol·L−1) | N | Fat oxidation (g·min−1) | N | Lactate (mmol·L−1) | N | Fat oxidation (g·min−1) |
| 30 | Heat | 11 | 3.40 ± 1.18 | 9 | 0.221 ± 0.099 | 8 | 3.48 ± 2.073 | 6 | 0.192 ± 0.136 |
| Control | 11 | 2.59 ± 0.94 | 9 | 0.277 ± 0.144 | 8 | 2.79 ± 0.809 | 6 | 0.305 ± 0.141 | |
| Depletion | 11 | 2.27 ± 0.74 | 9 | 0.486 ± 0.096 | 8 | 2.41 ± 1.124 | 6 | 0.410 ± 0.091 | |
| 60 | Heat | 11 | 2.44 ± 0.93 | 9 | 0.212 ± 0.099 | 8 | 2.16 ± 0.981 | 6 | 0.236 ± 0.198 |
| Control | 11 | 1.81 ± 0.57 | 9 | 0.285 ± 0.130 | 8 | 1.96 ± 0.915 | 6 | 0.299 ± 0.189 | |
| Depletion | 11 | 1.66 ± 0.96 | 9 | 0.544 ± 0.082 | 8 | 1.52 ± 0.602 | 6 | 0.472 ± 0.144 | |
| 90 | Heat | 11 | 2.58 ± 0.78 | 9 | 0.228 ± 0.154 | 8 | 2.59 ± 1.620 | 6 | 0.199 ± 0.208 |
| Control | 11 | 1.73 ± 0.40 | 9 | 0.238 ± 0.173 | 8 | 1.84 ± 1.153 | 6 | 0.279 ± 0.206 | |
| Depletion | 11 | 1.81 ± 0.82 | 9 | 0.569 ± 0.094 | 8 | 1.61 ± 0.728 | 6 | 0.489 ± 0.227 | |
| 120 | Heat | 11 | 2.88 ± 0.58 | 9 | 0.172 ± 0.130 | 8 | 3.44 ± 2.002 | 6 | 0.181 ± 0.243 |
| Control | 11 | 2.13 ± 0.81 | 9 | 0.238 ± 0.175 | 8 | 2.65 ± 1.577 | 6 | 0.265 ± 0.214 | |
| Depletion | 11 | 1.65 ± 0.78 | 9 | 0.596 ± 0.134 | 8 | 1.85 ± 1.277 | 6 | 0.488 ± 0.260 | |
| 150 | Heat | 11 | 3.57 ± 1.08 | 9 | 0.148 ± 0.159 | 7 | 3.51 ± 1.629 | 5 | 0.165 ± 0.200 |
| Control | 11 | 2.52 ± 1.10 | 9 | 0.182 ± 0.190 | 7 | 2.89 ± 1.306 | 6 | 0.200 ± 0.163 | |
| Depletion | 11 | 1.94 ± 0.91 | 9 | 0.598 ± 0.221 | 7 | 1.98 ± 0.978 | 5 | 0.490 ± 0.162 | |
| 180 | Heat | 11 | 5.78 ± 2.21 | 9 | 0.054 ± 0.100 | 7 | 5.61 ± 2.410 | 5 | 0.061 ± 0.138 |
| Control | 11 | 3.61 ± 1.68 | 9 | 0.142 ± 0.167 | 7 | 4.39 ± 1.270 | 5 | 0.091 ± 0.089 | |
| Depletion | 11 | 3.00 ± 1.57 | 9 | 0.513 ± 0.251 | 7 | 3.50 ± 1.971 | 5 | 0.254 ± 0.184 | |
| 210 | Heat | 10 | 7.25 ± 3.31 | 9 | 0.007 ± 0.023 | ||||
| Control | 11 | 5.33 ± 2.40 | 9 | 0.045 ± 0.076 | |||||
| Depletion | 11 | 4.37 ± 2.45 | 9 | 0.383 ± 0.269 | |||||
| 240 | Heat | 6 | 10.24 ± 3.76 | 8 | 0.018 ± 0.051 | ||||
| Control | 10 | 6.75 ± 3.22 | 9 | 0.000 ± 0.000 | |||||
| Depletion | 8 | 5.29 ± 2.53 | 8 | 0.232 ± 0.248 | |||||
| Average | Heat | 4.40 ± 2.92 | 0.135 ± 0.137 | 3.42 ± 2.03 | 0.176 ± 0.185 | ||||
| Control | 3.27 ± 2.30a | 0.176 ± 0.171a | 2.71 ± 1.40a | 0.245 ± 0.177a | |||||
| Depletion | 2.66 ± 1.87a, b | 0.494 ± 0.213a, b | 2.12 ± 1.29a, b | 0.438 ± 0.192a, b | |||||
- Note: data are shown as mean ± SD.
- a Significantly difference from heat.
- b Significantly difference from control.
In females, we observed similar results. Blood lactate concentration varied between situations (F (2,112) = 18.15 and p < 0.001). Specifically, blood lactate concentration was higher in heat compared to control (p = 0.005 and d = 0.438) and glycogen depletion situations (p < 0.001 and d = 0.809). In addition, blood lactate concentration was higher in control compared to glycogen depletion situation (p = 0.020 and d = 0.371). Regarding fat oxidation, it was significantly different between situations (F (2,80) = 48.87 and p < 0.001). A post hoc test showed that fat oxidation was lower in heat compared to control (p = 0.019 and d = −0.371) and glycogen depletion situations (p < 0.001 and d = −1.417). Finally, fat oxidation was lower in control compared to glycogen depletion situation (p < 0.001 and d = −1.045; Table 2).
On the contrary, in males or females, there is no association between the changes in lactate concentration between situations at each intensity and the changes in fat oxidation between situations at each intensity (Figure 3).
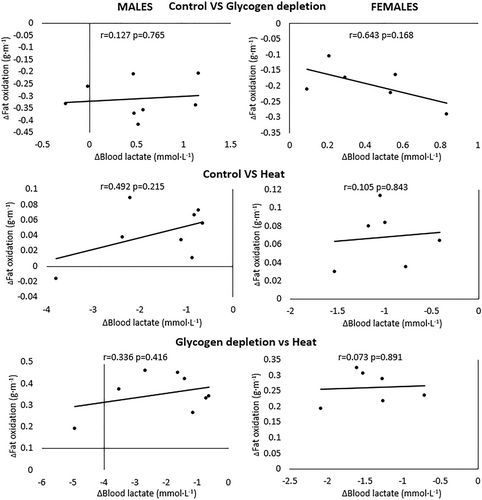
Relationship between the changes in blood lactate concentration between situations at each intensity and the changes in fat oxidation between situations at each intensity in males and females.
4 DISCUSSION
This study aimed to investigate whether changes in lactate concentration, due to glycogen depletion or heat, alter the pattern of fat oxidation during a maximal incremental cycle ergometer test in healthy young active females and males in a within-subject design. In agreement with our hypothesis, a very strong inverse association was found between lactatemia and fat oxidation in females and males in all three situations. That means measuring blood lactate concentration is an effective way to indirectly assess changes in fat oxidation when the situation is the same. Importantly, lactate concentration-fat oxidation relationship is different between the three situations (control, glycogen depletion, and heat). Therefore, as a practical application, this implies that determining fat oxidation from lactate, or vice versa, requires prior identification of the specific lactate–fat oxidation relationship in each situation. Furthermore, the lowest lactate concentrations were accompanied by the highest fat oxidations in the glycogen depletion situation, whereas the highest lactate concentrations were accompanied by the lowest fat oxidations in the heat situation. However, changes in blood lactate concentration between situations at each intensity are not associated with changes in the fat oxidation between situations at each intensity. Therefore, these results suggest an absence of a dose-response relationship between both variables. This helps to elucidate the interaction between lactate concentration and fat oxidation during exercise.
Our results show that the increase in blood lactate concentration coincides with the decrease in fat oxidation. This phenomenon agrees with previous studies observing an inverse association between lactacidemia/lactatemia and free fatty acids after infusing lactic acid/lactate in animals (Gold et al., 1963; Issekutz & Miller, 1962; Miller et al., 1963) or humans (Boyd et al., 1974). In addition, our data are in concordance with the scientific literature showing an inverse relationship between lactatemia and fat oxidation (Achten & Jeukendrup, 2004; San-Millán & Brooks, 2018). Hence, the very strong inverse association between blood lactate concentration and fat oxidation suggests that measuring blood lactate concentration is an effective way to indirectly assess changes in fat oxidation, as previously suggested (San-Millán & Brooks, 2018), when the situation (control, glycogen depletion, and heat) is the same. However, what is novel about this study is that lactate concentration-fat oxidation relationship is different between the three mentioned situations. Therefore, as a practical application, this implies that in order to determine fat oxidation from lactate, or vice versa, requires prior identification of the specific lactate–fat oxidation relationship in each situation. In other words, we cannot extrapolate the lactate–fat oxidation relationship measured in one specific situation (usually a control situation) to a different situation such as glycogen depletion or heat.
Due to the different lactate–fat oxidation relationship between situations, the same blood lactate concentration does not imply the same fat oxidation across the different situations. It was already known that a fixed blood lactate concentration does not reflect the same metabolic situation between different individuals (Billat et al., 2003). Now, with our study, we also know that a fixed blood lactate concentration may not reflect the same metabolic response (e.g., fat oxidation) even in the same individual exposed to different situations. To the best of our knowledge, this is the first study showing this within-subject comparison.
The highest lactate concentrations coincided with the lowest fat oxidations in heat and the lowest lactate concentrations coincided with the highest fat oxidations in glycogen depletion. Interestingly, from a qualitative perspective, the lactate–fat oxidation crossover point was shifted to the left in heat and shifted to the right in glycogen depletion, both compared to control. This means that changes in lactate concentrations are accompanied by opposite changes in the pattern of fat oxidation. Several mechanisms have been proposed in the literature to explain the assumption that lactate inhibit lipolysis. On the one hand, lactate oxidation inside mitochondria increases the production of acetyl-CoA and, subsequently, malonyl-CoA. In turn, malonyl-CoA inhibits the transport of fatty acids through the mitochondrial membrane transporter Carnitine Palmitoyl Transferase 1 (CPT1; Brooks, 2018). Moreover, chronic lactate exposure reduces fatty acid transport into mitochondria decreasing the activity of CPT1 (San-Millan et al., 2022). Additionally, an increase in blood lactate concentration decreases lipolysis in adipose tissue through activation of an orphan G protein-coupled receptor (GPR81; Ahmed et al., 2010; Cai et al., 2008; Liu et al., 2009). Nevertheless, in our results, the greatest effect size in lactate concentration was found between the control and the heat situation; but on the contrary, the greatest effect size in fat oxidation was found between control and glycogen depletion. Therefore, the greatest change in blood lactate concentration does not coincide with the greatest change in fat oxidation. That means changes in lactate concentration are not proportional to changes in fat oxidation. Moreover, changes in blood lactate concentration between situations are not associated with changes in the fat oxidation between situations. Therefore, these results suggest an absence of a dose–response relationship between both variables. Hence, there may be other mechanisms which play a major role than lactate regulating fat oxidation during exercise.
As any study, the present one is not exempt from limitations. Glycogen depletion was not directly measured in our study. Therefore, we cannot confirm a true glycogen depletion state of the individuals in the glycogen depletion situation. However, the changes in blood lactate concentration and fat oxidation between control and glycogen depletion strongly suggest that participants presented low glycogen levels. Furthermore, this exercise protocol (Cheng et al., 2020) and this low carbohydrate diet (Burke et al., 2021) have already been effective reducing glycogen levels. On the other hand, to control the confounding effect of the menstrual cycle in females, they performed the different tests in the early-mid follicular phase. This decision was made because previous studies suggested that menstrual cycle could affect substrate utilization and blood lactate concentration (Hackney, 2021). Since all tests could not be performed in the same menstrual cycle, most females performed all test at least one menstrual cycle apart (between two or three menstrual cycles). That means (de)training between menstrual cycles could be acting as a confounding variable in the females' results. This concern should be considered in future studies when deciding whether menstrual cycle should be controlled. Specifically, in case it is not possible to perform all measurements in the same menstrual cycle, it should be assessed whether the effect of (de)training between two menstrual cycles is greater than the effect of the menstrual cycle on the dependent variables in each specific study. Finally, sample size is relatively small and, despite obtaining a very high statistical power, future studies could confirm these findings with a larger sample size. Moreover, it should be examined if these results are found in other populations (e.g., sedentary individuals).
5 CONCLUSION
A very strong inverse association between lactatemia and fat oxidation was found in females and males in control, glycogen depletion, and heat situations. Therefore, assessing blood lactate alone could be an effective way to indirectly assess fat oxidation, or vice versa, when the situation is the same. However, lactate–fat oxidation relationship changes between situations. That means the same blood lactate concentration does not imply the same fat oxidation when the situation is different. Therefore, a fixed blood lactate concentration may not reflect the same metabolic response even in the same individual if he/she is exposed to different situations. This implies that to determine fat oxidation from lactate, or vice versa, this relationship needs to be previously determined in each specific situation. In addition, the greatest effect size in lactate concentration was found between the control and the heat situation; but on the contrary, the greatest effect size in fat oxidation was found between control and glycogen depletion. That means changes in lactate concentration are not proportional to changes in fat oxidation. Moreover, changes in blood lactate concentration between situations at each intensity are not associated with changes in the fat oxidation between situations at each intensity. Therefore, these results suggest an absence of a dose–response relationship between both variables. Hence, these results lead us to suggest that there are mechanisms apart from lactate that have a greater impact on changes in fat oxidation across different situations.
AUTHOR CONTRIBUTION
Conceived and designed research: José Antonio Benítez-Muñoz and Rocío Cupeiro; Performed experiments: José Antonio Benítez-Muñoz, Isabel Guisado-Cuadrado and María Alcocer-Ayuga; Processed data: José Antonio Benítez-Muñoz, Isabel Guisado-Cuadrado, Miguel Ángel Rojo-Tirado, María Alcocer-Ayuga, Nuria Romero-Parra; Analyzed data: José Antonio Benítez-Muñoz; Interpreted results of experiments: José Antonio Benítez-Muñoz and Rocío Cupeiro; Prepared figures: José Antonio Benítez-Muñoz; Drafted manuscript: José Antonio Benítez-Muñoz, Isabel Guisado-Cuadrado and Rocío Cupeiro; Edited and revised manuscript: José Antonio Benítez-Muñoz, Isabel Guisado-Cuadrado, Miguel Ángel Rojo-Tirado, María Alcocer-Ayuga, Nuria Romero-Parra, Ana Belén Peinado and Rocío Cupeiro; Approved final version of manuscript: José Antonio Benítez-Muñoz, Isabel Guisado-Cuadrado, Miguel Ángel Rojo-Tirado, María Alcocer-Ayuga, Nuria Romero-Parra, Ana Belén Peinado and Rocío Cupeiro.
ACKNOWLEDGMENTS
The authors would like to thank all the participants involved in the study and all the students who helped them to carry out the tests. JABM and IGC were supported by a grant provided by university of their affiliations. RC was supported by a grant for the Requalification of the Spanish University System 2021–2023 from the Spanish Ministry of Universities (RD 289/2021), funded by the European Union-NextGenerationEU.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
PATIENT CONSENT STATEMENT
Subjects were informed of the risks and benefits of the study prior to participation and written informed consent was obtained from each subject before their inclusion.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
N/A.
CLINICAL TRIAL REGISTRATION
NCT05703100.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




