Association of temporomandibular disorders with pain sensitivity: A cohort study
Funding information
Jarno Knuutila was supported with a grant from the Finnish Association for the Study of Pain (FASP). The authors have no financial relationships relevant to this article to disclose.
Abstract
Background
Pain related to temporomandibular disorders (TMD) can be linked with multiple site pain (MSP), and may associate with increased pain sensitivity, more frequently among women than men. The aim of the study was to examine the associations of pressure pain threshold (PPT) and tolerance (PPTo) with TMD and associated MSP in the Northern Finland Birth Cohort 1966 (NFBC1966) study.
Methods
Altogether 1961 NFBC1966 subjects attended clinical medical and dental examination at the Institute of Dentistry, University of Oulu in 2012–2013. Clinical examinations were carried out using a modified Diagnostic Criteria for TMD protocol (DC/TMD). MSP was defined based on questions regarding body pain sites. Additionally, PPT and PPTo were assessed using algometer measurements. Mann–Whitney U-test and Tobit regression models were used to analyse associations between TMD sub-diagnoses, MSP, PPT and PPTo, stratified by sex. Further models were adjusted with anxiety and depressive symptoms, which were assessed using Hopkins Symptom Checklist-25 (HSCL-25) and two-way interaction terms.
Results
Among females, lower PPT and PPTo were associated with myalgia and arthralgia. Among males, lower PPT and PPTo were associated with MSP-linked TMD. Tobit regression analysis showed significantly lower PPT and PPTo values in the myalgia and arthralgia subgroups among female TMD subjects. Among females, disc displacement with reduction had an inverse association with PPT and PPTo. Among males, lower PPTo was associated with degenerative joint disease and MSP-linked TMD.
Conclusions
The pain regulatory mechanisms behind TMD act differently between the genders as local TMD among females and MSP-linked TMD among males were associated with pain sensitivity.
Significance
The study shows that there are differences in the associations of painful TMD with pressure pain tolerance, pressure pain sensitivity and MSP between male and female subjects.
1 INTRODUCTION
Temporomandibular disorders (TMD) is a term used to describe a variety of dysfunctions and pain in the masticatory system (Okeson, 2013). Common subjective TMD symptoms and signs include clicking and crepitus of the temporomandibular joint (TMJ), masticatory muscle pain and mandibular movement limitations. Facial pain, headache and ear symptoms (e.g. tinnitus) can sometimes also be linked to TMD (Buergers et al., 2014; Okeson, 2013; Scrivani et al., 2008). It has been reported that TMD-related pain is one of the most common forms of musculoskeletal pain in all age groups (Ohrbach et al., 2011; Schiffman, 2010). The prevalence of clinical TMD signs has been found to be 34%–38% among Finnish adults, being more prevalent in women than men (Jussila et al., 2017; Rutkiewicz et al., 2006).
Widespread pain has been associated with painful TMD and lack of treatment response, and subjects with a chronic pain condition quite commonly experience comorbid chronic pain conditions (Maixner et al., 2016; Pagé et al., 2018). It has been suggested that also TMD may be part of a broader generalized pain condition (Greenspan et al., 2013). Sipilä et al. (2005) presented that subjects with facial pain were more likely to report pain and have more muscular tenderness in other areas of the body. Individuals with painful TMD also seem to be more sensitive to experimental pain stimuli, with lower thermal and ischemic pain threshold values as well as lower tolerance values than non-TMD subjects (Greenspan et al., 2013; Maixner et al., 1995).
Enhanced pain sensitivity is presented to be a first-onset TMD risk factor and it has been proposed that first-onset TMD lowers an individual’s pressure pain threshold (PPT). The threshold will recover to some extent, once the clinical TMD has resolved, but the PPT will remain lowered in cases of chronic TMD (Greenspan et al., 2013; Maixner et al., 2016; Slade et al., 2014, 2016). This suggests that the increased pain sensitivity found in chronic TMD patients could to some extent be a result of an abnormal central nervous system (CNS) reaction to nociceptive information, affecting an individual’s emotional, physiological and neuroendocrine responses to factors inflicting physical or emotional stress (Maixner et al., 1995, 2016). A state of hyperalgesia can also be linked to other chronic pain conditions (e.g. fibromyalgia, migraine headaches). In addition, increased pain sensitivity has been associated with genetic factors as well as psychological disorders, such as depression, stress and anxiety, which too have been linked to chronic TMD (Jussila et al., 2018; Maixner et al., 2016; Sipilä et al., 2013; Smith et al., 2011). Studies have also shown that men and women differ in their responses to pain, with increased pain sensitivity and risk for clinical pain commonly being observed among women (Bartley & Fillingim, 2013).
Based on previous studies, it can be hypothesized that individuals with TMD, especially those with TMD and multiple site pain (MSP), have increased pain sensitivity and lower pain thresholds in relation to those without TMD or MSP. It may also be hypothesized, that females are more sensitive to pain compared to males. The aim of the study was to examine the associations of pain sensitivity and pain tolerance with TMD and MSP-linked TMD in Finnish subjects in the Northern Finland Birth Cohort 1966 (NFBC1966).
2 MATERIALS AND METHODS
2.1 Participants
The study is part of the NFBC1966, which is an epidemiological longitudinal study covering the subjects’ entire life cycle and is aimed at improving the well-being and health. The whole dataset consists of 12,058 subjects born in 1966 in Oulu and Lapland, both located in Northern Finland (University of Oulu - Infrastructure for Population Studies).
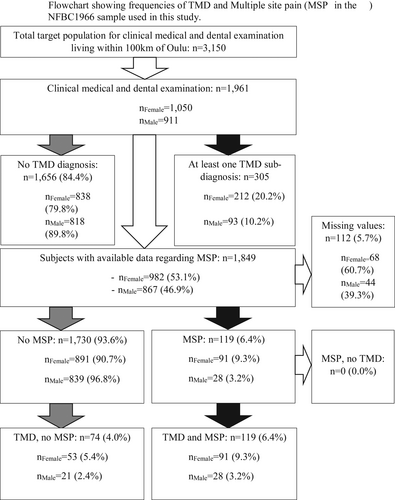
Between 2012 and 2013, subjects included in the NFBC 1966 who lived within 100 km from the city of Oulu (n = 3,150) were invited to a follow-up study consisting of clinical medical and dental examinations. In total 1,961 subjects aged 46–47 years attended the clinical examination at the Institute of Dentistry, University of Oulu, and gave their consent to use the data (response rate ~62.3%). Of these 1,961 subjects 1,050 were female and 911 male. The frequencies regarding clinical TMD diagnoses are further described in Figure 1.
2.2 TMD diagnostics
Prior to the clinical examinations, the participants received a two-part questionnaire, which they could fill on paper, or on the internet. The first part included questions regarding health and health-related factors, while the second part included questions about subjects’ financial and work situation as well as mental resources (e.g. stress and optimism/pessimism). Subjects participating in the clinical dental examinations filled in a third questionnaire including questions on oral health (University of Oulu - Infrastructure for Population Studies).
The clinical stomatognathic examination was carried out using a modified DC/TMD protocol presented at the International Association for Dental Research (IADR) conference in 2010 (Jussila et al., 2017; Schiffman, 2010). Clinical examinations were performed between 2012 and 2013. The standardized clinical examinations were conducted by six calibrated examiners (dentists) and included assessment of the following clinical TMD signs: limited maximal mouth opening without assistance (<40 mm restricted, measured as the distance between the upper and lower right incisor incisal edges, including overbite), lateral and protrusive movements (<7 mm restricted), maximal assisted opening (MAO; examiner actively assisting by pushing the jaw open). Subjects were asked if they felt pain during the motions and if this was ‘familiar pain’ (pain similar to pain sensations felt during the past 30 days). Clicking in the TMJs, crepitus in the TMJs during opening, closing, lateral and protrusive motions were also registered, as well as pain on palpation of the masticatory muscles, and pain on palpation in the TMJs.
Clicking and crepitus were observed by ear at a distance of approximately 15 cm from the TMJ. The masticatory muscles palpated were the temporalis muscle (anterior, middle and posterior regions) and the masseter muscle (origin, deep and insertion regions). Masticatory muscle and TMJ palpations for familiar pain around the lateral pole of the TMJ were done with a force of 1.0 kg, and on the lateral pole of the TMJ with a force of 0.5 kg. All palpations were conducted bilaterally, and the forces were calibrated before measurements using a digital postage scale placed near the dental unit. Pain on palpation was recorded if the subject expressed a pain sensation verbally or via protective reflex (Jussila et al., 2017).
Examiners were blinded to the participants’ questionnaire answers, except for questions regarding TMD symptoms (Jussila et al., 2017). The modified DC/TMD protocol was chosen as the current DC/TMD criteria (International Association for Dental Research (IADR), 2014) were not available at the time of the clinical examination, and the RDC/TMD criteria was seen as too time-consuming for the large population sample.
- Myalgia: reported pain during the last 30 days in the areas of the face, jaws, temples, ears/behind the ears, pain modified by movement and familiar pain in the masticatory muscles during jaw movements and/or familiar pain on palpation at previously mentioned muscle palpation sites.
- Arthralgia: reported pain in areas of the face, jaws, temples, ears or behind the ears and pain modified by movement during the prior 30 days, and familiar pain in the TMJs during jaw movement and/or pain on palpation (familiar pain) in the right or left TMJ (around the lateral pole or laterally).
- DD w/R: reported history of clicking noises in the TMJ, and TMJ clicking recorded by the examiner during opening and closing movements, or during opening or closing movements and in either protrusive or right/left lateral movement.
- DD w/o R: self-reported jaw locking in closed position and restricted MAO.
- Degenerative JD: self-reported history of noises in the TMJ and TMJ crepitus recorded by the examiner.
Frequencies regarding TMD signs and symptoms are presented in a previous study by Jussila et al. (2017) using the same dataset used in this study. Frequencies regarding sub-diagnoses may be seen in Table 1 and in the Table S1. Referred pain and headache attributed to TMD, which are included in the modern DC/TMD protocol, were not included in the modified DC/TMD protocol used in this study.
| Sub-diagnosis (n) | Male | pPPTo | Sub-diagnosis (n) | Female | pPPTo | ||||
|---|---|---|---|---|---|---|---|---|---|
| PPT median (95% CI), kPa | pPPT | PPTo Median (95% CI), kPa | PPT median (95% CI), kPa | pPPT | PPTo median (95% CI), kPa | ||||
| Myalgia (911) | Myalgia (1,044) | ||||||||
| Yes (20) | 674 (669–678) | 1,075 (1,070–1,079) | Yes (77) | 569 (561–578) | 887 (878–895) | ||||
| No (891) | 784 (755–813) | 0.208 | 1,155 (1,126–1,184) | 0.132 | No (967) | 594 (564–624) | 0.010* | 920 (890–950) | 0.044* |
| % Differenceb | −14.0% | −6.9% | % Differenceb | −4.2% | −3.6% | ||||
| Arthralgia (899) | Arthralgia (1,040) | ||||||||
| Yes (18) | 679 (675–683) | 1,108 (1,104–1,112) | Yes (85) | 567 (558–576) | 878 (870–887) | ||||
| No (881) | 788 (759–816) | 0.150 | 1,158 (1,129–1,187) | 0.274 | No (955) | 594 (564–624) | 0.008** | 921 (891–951) | 0.014* |
| % Differenceb | −13.8% | −4.3% | % Differenceb | −4.6% | −4.7% | ||||
| DD w/R (905) | DD w/R (1,042) | ||||||||
| Yes (49) | 784 (777–791) | 1,136 (1,129–1,142) | Yes (88) | 620 (611–629) | 935 (926–944) | ||||
| No (856) | 780 (752–809) | 0.701 | 1,155 (1,126–1,183) | 0.418 | No (954) | 589 (559–619) | 0.064 | 914 (884–944) | 0.064 |
| % Differenceb | +0.5% | −1.7% | % Differenceb | +5.0% | +2.3% | ||||
| DD w/o R (910) | DD w/o R (1,047) | ||||||||
| Yes (1) | 833 (832–834) | 945 (944–946) | Yes (3) | 443 (441–445) | 753 (751–754) | ||||
| No (909) | 782 (752–811) | 0.777 | 1,154 (1,125–1,184) | 0.202 | No (1,044) | 591 (559–622) | 0.140 | 916 (885–948) | 0.152 |
| +6.1% | −18.1% | % Differenceb | −25.0% | −17.8% | |||||
| Degenerative JD (907) | Degenerative JD (1,042) | ||||||||
| Yes (34) | 777 (771–782) | 1,138 (1,133–1,144) | Yes (66) | 616 (608–624) | 926 (918–934) | ||||
| No (873) | 782 (754–811) | 0.733 | 1,155 (1,126–1,183) | 0.094 | No (976) | 589 (559–619) | 0.885 | 915 (885–946) | 0.405 |
| % Differenceb | −0.6% | −1.5% | % Differenceb | +4.4% | +1.2% | ||||
| MSP + TMDa (867) | MSP + TMDa (982) | ||||||||
| Yes (28) | 708 (703–713) | 1,079 (1,074–1,084) | Yes (91) | 574 (564–583) | 908 (899–918) | ||||
| No (839) | 787 (759–815) | 0.239 | 1,158 (1,130–1,186) | 0.008** | No (891) | 590 (561–619) | 0.201 | 916 (887–945) | 0.348 |
| % Differenceb | −10.0% | −6.8% | % Differenceb | −2.7% | −0.9 | ||||
- Abbreviations: pPPT, pressure pain threshold p-value; pPPTo, pressure pain tolerance p-value.
- a Subjects with multiple site pain and a TMD pain diagnosis.
- b The percentual difference in PPT/PPTo median values comparing subjects with TMD versus subjects without TMD.
- * p < 0.05; **p < 0.01 (p-values calculated via Mann–Whitney U-test).
2.3 Pain measurements
MSP was defined as self-reported pain in at least one upper extremity, one lower extremity and either the neck, back or chest (White et al., 1999). Subjects with MSP were identified using the dichotomous (yes/no) pain-related questions on pain sites from the second NFBC1966 questionnaire. These questions inquired if the subjects had experienced pain or ache during the last 12 months in other areas of the body. A body outline drawing was used to help identify the areas (Sipilä et al., 2006). MSP was examined as a comorbidity in subjects diagnosed with TMD. It should also be noted that the criteria presented by White et al. (1999) were modified to accommodate the NFBC1966 dataset. The timeframe and exact definition regarding MSP differ, as the timeframe used in the NFBC1966 questionnaires was 12 months instead of 3 months used by White et al., and the side of the body in which the pain sensation occurred was not inquired in the NFBC1966 questionnaires.
The clinical data regarding pain sensitivity and pain tolerance was examined via PPT and pressure pain tolerance (PPTo). The data were collected from NFBC 1966 subjects using an algometer (Somedic AB) with a 10 mm contact head. During the clinical data collection, the contact head of the algometer was placed on the subjects’ skin perpendicularly, with a small piece of paper placed between the contact head and the skin to minimize the effect of the sharp plastic borders of the contact head during the measurements. The pressure was steadily increased from 0 kPa at a rate of 50 kPa/s. The participants were informed about the gradually increasing pressure and they were instructed to press down on a button at the point at which subject starts to experience the pressure as uncomfortable, and to release the button once the increasing pressure becomes intolerable. The point at which the button was pressed down was recorded as PPT and the point at which the button was released was recorded as PPTo. The pressure was terminated at a safety maximum of 1,200 kPa at the latest.
- Shoulder (the mid-belly of the upper trapezius muscle, participant in the prone position)
- Shin (the mid-belly of the tibialis anterior muscle, supine position)
- Wrist (the dorsal aspect of the wrist joint line, supine position)
- Lower back (the L5/S1 interspinous space, prone position)
The higher value of the two measurements was used in the analysis, as the focus was on examining subjects’ maximal pressure pain endurance. It was also considered that for various possible reasons the subjects may overreact to the increasing pressure during the first measurement, which may understate their actual PPT and PPTo values. The exact anatomical point was changed slightly between measurements to prevent nociceptor sensitization at contact. Slight changes in areas of the measurements may have occurred between subjects. The anatomical points were chosen, and the measurements were conducted in a manner similar to other cohorts (Slater et al., 2015). Masticatory muscles were not included in the measurements as they were used to specifically examine pain in other areas of the body.
As psychosocial factors impact on experimental pain perception (Dickens et al., 2003), the presence of anxiety and depressive symptoms was used as a possible confounding factor in the analyses, and they were evaluated using the Hopkins Symptom Checklist-25 (HSCL-25). The HSCL-25 is a globally widely used instrument designed for the screening of common psychiatric symptoms (Nettelbladt et al., 1993).
2.4 Statistical analysis
Statistical analysis was implemented by examining associations between TMD sub-diagnoses, TMD with MSP and PPT and PPTo. Variable ‘MSP-linked TMD’ was set when there was at least one TMD sub-diagnosis and pain in the areas of at least one upper extremity (shoulders, arms/elbows, wrists/hands/fingers), one lower extremity (hips, thighs, knees/calves, ankles/feet), and either the neck/occiput, back, or chest.
The medians of the PPT and PPTo with 95% CI were calculated and compared between subjects with TMD sub-diagnoses versus those without sub-diagnoses stratified by gender using Mann–Whitney U-test. Because of the truncated nature of the data, further analyses were conducted using the Tobit regression models. In crude models, the PPT and PPTo were the response variables, the separate sub-diagnoses and MSP-linked TMD were used as explanatory variables, and the further models were adjusted with anxiety and depressive symptoms (HSCL-25), as well as two-way interaction terms (p < 0.2). The HSCL25-variable was formed by calculating the mean value of the sum of all 25 HSCL question answers and dividing the results into two categories (<1.55 and ≥1.55). The Tobit regression models were carried out by sex.
The limit for statistical significance was set at p < 0.05. Data were analysed using Rstudio (2009–2019 RStudio Inc.) and IBM SPSS Statistics version 24.0.
3 RESULTS
The medians of PPT and PPTo were lower among those with myalgia and arthralgia as compared to those without these diagnoses, the differences being statistically significant among females (Table 1). Among males, those having MSP-linked TMD showed statistically significantly lower values of PPTo as compared to those without this condition. Concerning joint-related TMD diagnoses, no statistically significant differences were found in PPT or PPTo between those with versus those without diagnosis.
Based on Tobit regression analysis, PPT and PPTo showed statistically significantly lower values in the myalgia and arthralgia subgroups among TMD-positive female subjects. These associations remained significant after adjustment. In the DD w/R subgroup among TMD-positive female subjects, PPT as well as PPTo values were significantly higher than the values of females without TMD, and the PPT value remained statistically significant after adjustment. Among males, lower PPTo associated with degenerative JD and MSP-linked TMD (Table 2). Additional descriptive statistics regarding the sub-diagnoses and MSP can be found in the Supplementary Table, as well as in Figure 1. At total, 119 subjects had MSP + TMD and 74 subjects had TMD, but no MSP. No subjects were present with MSP, but no TMD.
| Pain threshold (kPa)* | Pain tolerance (kPa)* | |||
|---|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | |||
| Crude | Adjusted** | Crude | Adjusted** | |
| Female | ||||
| Myalgia | −62.2e (−107, −17.2) | −54.9d (−103, −6.83) | −52.7d (−101, −4.76) | −66.5a,d (−128, −5.37) |
| Arthralgia | −62.0e (−106, −18.2) | −59.0d (−106, −11.7) | −62.6e (−109, −16.1) | −54.2d (−104, 4.00) |
| DD w/R | 50.1e (7.43, 92.7) | 51.1d (7.37, 94.9) | 48.0d (2.11, 93.8) | 44.2 (−2.77, 91.2) |
| DD w/o R | −171 (−391, 49.2) | −165 (−384, 53.7) | −150 (−384, 84.4) | −145 (−377, 88.0) |
| Degenerative JD | 0.98 (−47.9, 49.9) | 3.36 (−47.7, 54.4) | 16.5 (−35.9, 68.9) | 14.7 (−39.9, 69.2) |
| MSP + TMDc | −25.1 (−67.3, 17.2) | −20.4 (−63.9, 23.2) | −26.0 (−71.3, 19.2) | −21.3 (−67.9, 25.3) |
| Male | ||||
| Myalgia | −48.9 (−149, 51.8) | −24.8 (−133, 83.8) | −61.7 (−140, 16.8) | 47.4a (−63.1, 12.6) |
| Arthralgia | −60.0 (−165, 45.9) | −63.1 (−171, 45.2) | −67.8 (−150, 14.4) | 8.29b (−94.7, 111) |
| DD w/R | 6.43 (−59.8, 72.7) | −4.77 (−72.0, 62.5) | −16.8 (−69.6, 35.9) | −25.3 (−78.7, 28.2) |
| DD w/o R | 40.7 (−406, 488) | 38.7 (−405, 482) | −182 (−522, 158) | −189 (−528, 150) |
| Degenerative JD | −15.2 (−95.5, 65.2) | −27.6 (−113, 57.5) | −62.3 (−125, 0.42) | −89.4e (−155, −23.4) |
| MSP + TMDc | −48.8 (−136, 38.2) | −50.1 (−138, 38.1) | −77.7d (−145, −10.1) | −85.4d (−154, −16.9) |
- a Average pressure pain threshold/tolerance of wrist, shoulder, low back and leg.
- b Adjusted with anxiety and depressive symptoms (HSCL25).
- c Adusted also with myalgia*HSCL-25.
- d Adjusted also with arthralgia*HSCL-25.
- e Subjects with multiple site pain and a TMD pain diagnosis.
- * p < 0.05
- ** p < 0.01.
4 DISCUSSION
In this study, significant associations between pain sensitivity and pain tolerance with TMD and MSP-linked TMD were found, and subjects with TMD and MSP-linked TMD were found to be more sensitive to pain stimuli when compared to those without TMD. Among males, lower PPTo associated with degenerative JD and MSP-linked TMD. Among female subjects, associations were found with more localized pain, that is, with TMD myalgia or arthralgia. The differences in PPT and PPTo between males and females are in line with other studies, except for DD w/R, as lower pain thresholds and pain tolerances are seen among females as compared to males (Borzan & Fuchs, 2006; Bulls et al., 2015; Ostrom et al., 2017). However, this a posteriori observation is not directly related to the aims of this study. Previous studies have also had similar results, suggesting that individuals with TMD, especially those with MSP, have increased sensitivity to pressure pain stimuli and that their PPT is lower when compared to individuals without TMD (Sipilä et al., 2005; Slade et al., 2016). It was also noted that all subjects who had MSP also had TMD, although not all subjects with TMD had MSP. It has previously been proposed that MSP is a risk factor for TMD, and this finding is in line with the previous studies (LeResche et al., 2007; Monaco et al., 2017; Plesh et al., 1996).
The present findings regarding the association of pain sensitivity with TMD may be linked to central sensitization (CS). This originates from alterations in neurons, nociceptive pathways and functions of the CNS, causing pain to be uncoupled from noxious stimuli (Baroni et al., 2020; Latremoliere & Woolf, 2009). CS can induce an exaggerated reaction to stimuli that would normally not lead to pain and has been reported to have a role in several conditions, including rheumatoid arthritis, osteoarthritis, TMD, fibromyalgia and neuropathic pain (Baroni et al., 2020; Woolf, 2011). CS may sometimes be triggered by a peripheral injury or dysfunction, such as chronic TMD, with muscular/TMJ-dysfunctions working as peripheral triggers. In the present study, however, the chronicity of TMD remained unknown, and thus further studies are needed to evaluate the role of TMD pain in the process of CS.
The pain sensitivity studies here can be related to endogenous pain modulation circuits which can alter the perceived magnitude of afferent noxious stimuli, reinforcing or attenuating it via wide dynamic range (WDR) neurons within the dorsal horns of the spine (Nir & Yarnitsky, 2015). With TMD patients, nociceptive-specific (NS) neurons are presumed to primarily transmit noxious information in the receptive fields conducting chronic pain instead of WDR neurons. This way, the inhibition of WDR neurons is left untriggered by the conditioning stimuli, possibly causing impairment of the pain inhibition system. In TMD patients, one study found this impairment particularly at chronic pain sites, but not at pain free sites (Oono et al., 2014). In contrast, Yang et al. (2016) reported somatosensory abnormalities both within and outside the primary painful region. This was confirmed in the present study, as sites beyond the masticatory structures were used in pain sensitivity measurements, thus indicating that CS may have a role in painful TMD conditions.
It has also been presented that multiple system domain dysregulation, especially dysregulation of sensory and psychological domains, is associated with increased probability of painful TMD. Dysregulation of individual domains (i.e. sensory, autonomic, inflammatory, psychological) may also increase the chance of having comorbid chronic pain conditions in addition to TMD (Chen et al., 2013; Maixner et al., 2016). Chen et al. (2013) have also suggested that heterogeneous multisystem dysregulation patterns may be present within painful TMD subgroups with varying numbers of comorbid pain conditions. Interestingly, the association between PPT and PPTo with TMD seemed to differ between sexes; in females, they associated with local pain-related TMD, whereas in males, they associated with MSP-linked TMD. These associations were significant even after adjusting for psychological symptoms. Sarlani and Greenspan (2002) reported differences between sexes in temporal summation of mechanically invoked pain, with females providing higher pain ratings than males. The authors also noted that temporal summation of mechanical pain seemed to be more dependent on the frequency of the stimulus and may not rely on sensitization of primary afferent nociceptive neurons. Ostrom et al. (2017) also reported female subjects showing greater sensitivity in 29 of 34 pain sensitivity measurements, including measurements from temporal and masseter muscles and TMJs, but also from other areas of the body (trapezius, forearm). In addition to pressure pain, mechanical cutaneous pain and heat pain were examined. These previous findings and the present findings could be interpreted as meaning that females are more prone to react to more localized pain conditions when the stimulus is of a higher frequency, whereas in males, a more widespread condition may be required.
The differences in pain sensitivity and their association with TMD between sexes can partially be explained by hormonal factors as testosterone and estrogen influence pain sensitivity. Testosterone has been suggested to reduce chronic pain (Gupta et al., 2011) and has been associated with a higher pain tolerance (Borzan & Fuchs, 2006). Estrogen, on the other hand, has been associated with conditions such as TMD, and fibromyalgia, as well as a lower tolerance to pain (Bulls et al., 2015; Gupta et al., 2011). It has been found that TMD is more common in females than males, being most common in females 20–50 years of age, with a decrease in prevalence after menopause (Jussila et al., 2017; Okeson, 2013; Scrivani et al., 2008; Wang et al., 2008). Estrogen has also been suggested to contribute to the degeneration of TMJ cartilage and associate with pathological subchondral bone findings in TMJ osteoarthritis (Orajärvi et al., 2012; Talwar et al., 2006; Ye et al., 2018; Yu et al., 2020). Estrogen also affects the limbic system, increasing the sensitivity to pain (Wang et al., 2007). This implies a link between TMD and hormonal factors, though the etiology of TMD is multifactorial.
Another possible explanation for the differences between males and females could be found by examining social structures and the differences in societal gender roles. In many cultures, masculinity is associated with a high pain tolerance while femininity is seen as the opposite (Defrin et al., 2009). This could to some extent be behind the statistically significant differences in the male MSP subgroup. It can be speculated that this could be caused by the symptoms being worse in male subjects when compared to the female group, or that stress induced by pain may lower testosterone levels, thus lowering PPTo. In addition, there may be differences in the expression of feelings and sensations between sexes. These may be caused by an individual’s upbringing and views on gender roles, but difficulties in differentiating emotional and physical states (alexithymia) may also play a role (Defrin et al., 2009; Sipilä et al., 2001). This could also offer a possible explanation for the differences between sexes in the study, especially regarding the association found between lower PPTo and MSP-linked TMD in male subjects.
Surprisingly, contrary findings were also noted. Median PPT values in the male and female DD w/R groups, male DD w/o R and female degenerative JD groups, as well as the PPTo values in male and female DD w/R and female degenerative JD groups were higher in subjects with TMD than in those without TMD (Table 1). This could be explained by the broad scale of possible factors causing DD w/R. These conditions may also exhibit little to no pain, with lesser effects regarding sensitization to pain. However, the actual cause behind this is unclear and further research is needed. TMJ-related conditions, such as degenerative JD, may also be chronic in nature and include periods of pain fluctuation, possibly including pain sensitization. These findings were interesting, yet not statistically significant. Also, associations regarding DD w/o R could not be examined reliably, as the number of subjects with TMD within the subgroup was low, only including one male and three female subjects (Table 1), also requiring further research.
It should be noted that in the cross-sectional study, no information on the chronicity of TMD pain or other pain was available. The accurate explanatory mechanisms behind the findings and the cause-relationship between variables also remain unknown, creating a need for further studies. In addition, the temporal aspect of TMD pain and MSP may not be examined in this study. The sample also consisted of 46–47-year-old adults born in Northern Finland, and thus does not represent the total adult population. The use of the modified DC/TMD protocol may also be seen as a limitation as it is not as widely acknowledged as the DC/TMD and RDC/TMD criteria. Referred pain as well as TMD related headache were also not examined in the modified DC/TMD protocol. However, this may not lead to an underestimate regarding the number of subjects with a TMD pain diagnosis, as headache attributed to TMD also requires a myalgia or arthralgia diagnosis, and referred pain is included in the diagnosis of myalgia and arthralgia. The different timeframes for MSP (12 months) and TMD pain (30 days) is also a limitation, as it is impossible to determine if the pain sensations occurred at the same point in time. This may also lead to overestimation of MSP-linked TMD.
The main strengths of this study were the large NFBC1966 cohort dataset and reliable TMD diagnostics, including a decent sized and well-executed sample of clinical pain measurements. The response rate was reasonable (62.2%), with the loss being 37.2%. This can be seen to be in line with other cohorts, for example, the assumed 30% loss to follow-up in the OPPERA study (Bair et al., 2013). The study sample may not fully represent the invited sample. Based on the attrition analyses performed for the NFBC 1966 clinical study (Nordström et al., 2021), the participants were more often females, employed and from higher social class. They were also more likely married, had children and higher education.
The findings of this study can be used to better understand the pathophysiology of TMD and to improve and optimize the treatment of TMD. However, the aim was to mainly examine and report findings regarding TMD and sensitivity to pain, and many questions remain open. The presented explanations are hypotheses, the assumed links with pain modulation/transmission and CS requiring further research. Further research is also needed regarding the associations between MSP-linked TMD, and pain sensitivity using longitudinal study settings, which may also give information on the causal relationships.
5 CONCLUSIONS
Subjects with TMD and MSP-linked TMD had increased sensitivity to pain and lower pain tolerance when compared to those without TMD and MSP-linked TMD. Among females, a lower PPT and PPTo seemed to associate with diagnosed myalgia and arthralgia, and inversely with DD w/R. Among males, a lowered PPTo is associated with degenerative JD and MSP-linked TMD. The pain regulatory mechanisms behind TMD act differently between the genders, as local TMD among females and MSP-linked TMD among males are associated with pain sensitivity. This link between TMD, MSP and pain sensitivity/pain tolerance may be caused by central sensitization and aberrations in pain modulation and/or pain transmission and may also be affected by differences in hormonal factors, as well as social factors.
CONFLICTS OF INTEREST
None to disclose.
AUTHOR CONTRIBUTIONS
Knuutila J: substantial contributions to the design and drafting of the work, as well as interpretation of the data and the final approval of the work to be published. Kivipuro J: substantial contributions to the design, revising and drafting of the work, final approval of the work to be published. Näpänkangas R: substantial contributions to the design, revising and drafting of the work, final approval of the work to be published. Auvinen J: acquisition of the data, substantial contributions to the design and revising of the work, final approval of the work to be published. Pesonen P: analysation and interpretation of the data, substantial contributions to the drafting and revising of the work. Karppinen J: acquisition of the data, substantial contributions to the design and revising of the work, final approval of the work to be published. Paananen M: acquisition of the data, substantial contributions to the design and revising of the work, final approval of the work to be published. Pirttiniemi P: substantial contributions to the design, drafting and revising of the work, final approval of the work to be published. Raustia A: substantial contributions to the design, drafting and revising of the work, final approval of the work to be published. Sipilä K: substantial contributions to the design and drafting of the work, as well as interpretation of the data and the final approval of the work to be published. All authors discussed the results and commented on the manuscript.