PD-1 regulation of pathogenic IL-17-secreting γδ T cells in experimental autoimmune encephalomyelitis
Abstract
The PD-1-PD-L1 immune checkpoint helps to maintain self-tolerance and prevent the development of autoimmune diseases. Immune checkpoint inhibitors are successful immunotherapeutics for several cancers, but responding patients can develop immune-mediated adverse events. It is well established that PD-1 regulates CD4 and CD8 T-cell responses, but its role in controlling the activation of pathogenic γδ T cells is less clear. Here we examined the role of PD-1 in regulating γδ T cells in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. We found that PD-1 was highly expressed on CD27− Vγ4 γδ T cells in the lymph node (LN) and CNS of mice with EAE. Treatment of mice with anti-PD-1 significantly augmented IL-17A-producing CD27− Vγ4 γδ T cells in the LN and CNS and enhanced the severity of EAE. The exacerbating effect of anti-PD-1 on EAE was lost in Tcrd−/− mice. Conversely, ligation of PD-1 suppressed Il17a and Rorc gene expression and IL-17A production by purified Vγ4 γδ T cells stimulated via the TCR, but not with IL-1β and IL-23. Our study demonstrates that PD-1 regulates TCR-activated CD27− Vγ4 γδ T cells, but that cytokine-activated IL-17A producing γδ T cells escape the regulatory effects of the PD-1-PD-L1 pathway.
Introduction
Immune checkpoints, including PD-1 interaction with PD-L1 and CTLA-4 interaction with CD80/CD86, are key regulators in the immune system, preventing activation of autoreactive T cells and the development of autoimmune diseases. Immune checkpoint inhibitors (ICIs), especially monoclonal antibodies that target PD-1, PD-L1, or CTLA-4 have shown considerable efficacy in the treatment of cancer, through their ability to remove the brake on T cell responses and thereby enhance antitumor immunity. ICIs have been approved for the treatment of several cancers, including metastatic melanoma and non-small cell lung cancer (NSCLC) [1]. However, treatment with ICIs commonly causes immune-related adverse events in cancer patients [2]; the most common of which are present in the skin, GI tract, and endocrine system [3] [4]. ICIs have caused neurological side effects in certain patients with melanoma, glioblastoma, and Hodgkin's lymphoma [5]. Treatment with ICIs has also been associated with relapses in multiple sclerosis (MS) patients, which resulted in rapid neurologic progression and even death [6]. Treatment of NSCLC with anti-PD-1 was shown to promote development of MS in a patient with prior history of white matter brain lesions, but no previous neurological symptoms [7]. Furthermore, a single nucleotide polymorphism in the PD-1 gene (Pdcd1) has been associated with disease progression in MS [8]. This single nucleotide polymorphism reduced the inhibitory function of PD-1 on CD4 T cell activation and cytokine production. PD-L1 expression has been identified in CNS tissue from both healthy controls and MS patients [9].
Studies on the role of PD-1-PD-L1 interactions in promoting immune responses to tumors and preventing autoimmune diseases have largely focused on conventional αβ T cells. PD-1 signaling suppresses T cell receptor (TCR)-mediated activation, proliferation, and cytokine production by CD4 T cells [10]. However, the role of PD-1 in regulating γδ T cell function has received less attention. PD-1 is highly expressed by murine skin-resident Scart1+Vγ6+ γδ T cells [11]. In a murine model of metastatic breast cancer, lung-resident Vγ6 γδ T cells expressed much higher PD-1 than Vγ4 γδ T cells [12]. Edwards et al. [12] reported that recombinant PD-L1 suppressed IL-1β and IL-23-induced IL-17A production by lung CD3+ T cells, and suggested that PD-1 suppressed Vγ6 γδ T cells via activation of FOXO1.
Human γδ T cells can be activated via direct binding of phosphoantigens to their TCR [13, 14]. Vγ9Vδ2 γδ T cells are activated by butyrophilin (BTN) 3A1, which induces TCR signaling [14]. Similarly, human Vγ4 γδ T cells can be activated via direct binding of BTN2A1 or BTN3A1 to the TCR [15]. Direct activation of γδ T cells via their TCRs suggests that immune checkpoint expression by these cells can modulate their function. Vγ9Vδ2 γδ T cells in the bone marrow of multiple myeloma patients have high PD-1 expression and are anergic [16]. PD-L1 expression is increased in most myeloma cells and anti-PD-1 treatment partly rescued the reactivity of Vγ9Vδ2 γδ T cells to phosphoantigens [16]. Treatment with anti-PD-1 enhances IFN-γ production by phosphoantigen-activated Vγ9Vδ2 γδ T cells co-cultured with leukemia cell lines [17]. Furthermore, anti-PD-1 treatment improved adoptive cell therapy with Vγ9Vδ2 γδ T cells in a humanized mouse model of prostate cancer [18].
In the mouse model of MS, experimental autoimmune encephalomyelitis (EAE), IL-17A-producing CD27− Vγ4 γδ T cells play a key role in pathology, where they infiltrate the CNS early in disease and promote activation of IL-17A-secreting CD4 T cells (Th17 cells) [19, 20]. CD27− γδ T cells produce IL-17 in responses to IL-1β and IL-23, whereas CD27+ γδ T cells predominantly secrete IFN-γ and are thought to be more TCR dependent [20, 21]. In the EAE model, the onset of clinical signs is enhanced in Pdl1−/− mice; CD4 T cells from these mice have increased myelin oligodendrocyte glycoprotein (MOG)-specific IFN-γ and IL-17A production [9, 22]. Furthermore, PD-1 blockade early in EAE increased the severity of disease and this was associated with enhanced MOG-specific IFN-γ production [23]. However, the role of PD-1 in controlling γδ T cells in autoimmunity has not been examined.
The aim of this study was to investigate the role of PD-1 signaling in regulating γδ T cell function during EAE. We found a high frequency of PD-1-expressing CD27− Vγ4 γδ T cells at rest and this was enhanced in the lymph node (LN) and CNS during the development of EAE. Blocking studies with a PD-1 neutralizing antibody in mice with EAE demonstrated that inhibition of PD-1-PD-L1 signaling enhanced production of IL-17A, GM-CSF, TNF, and IFN-γ by CD27− Vγ4 γδ T cells and exacerbated the clinical signs of EAE in wildtype (WT), but not in Tcrd−/− mice. Furthermore, the PD-1 agonist PD-L1-Fc suppressed IL-17A production by CD27− Vγ4 γδ T cells when activated via the TCR but not when activated with IL-1β and IL-23 alone. Our study demonstrates that PD-1 regulates TCR-activated CD27− Vγ4 γδ T cells and suggests that ICI treatment may exacerbate autoimmune diseases by removing the brake on antigen-activated but not innate IL-17A-producing γδ T cells.
Results
CD27− Vγ4 γδ T cells express high levels of PD-1, which is augmented during EAE
γδ T cells have an established pathogenic role in certain autoimmune diseases, including EAE, where they provide an early source of IL-17A and other inflammatory cytokines [20]. CD27+ γδ T cells are predominantly IFN-γ producers, whereas CD27− γδ T cells secrete IL-17A and are the pathogenic γδ T cells in EAE [21]. Here we examined the role of PD-1 in regulating γδ T cells during the development of EAE.
An examination of T cells from the LNs of naïve mice revealed that CD27− and CD27+ γδ T cells expressed high levels of PD-1 compared with CD4 and CD8 T cells (Fig. 1A). Expression of PD-1 was significantly enhanced on CD27− but not CD27+ γδ T cells during the development of EAE (Fig. 1A). Expression of PD-1 was also enhanced on CD4, but not CD8, T cells during EAE. In terms of absolute numbers, the highest number of T cell-expressing PD-1 were the CD4 subpopulation (Fig. 1B). An examination of PD-1 expression on Vγ1, Vγ4, and Vγ6-expressing CD27− γδ T cells demonstrated that there was a significantly higher frequency of Vγ4 when compared with Vγ1 or Vγ6, CD27− γδ T cells expressing PD-1 in the LN of naïve mice or in mice with EAE (Fig. 1C–E). There was also significant expansion in the absolute number of PD-1-expressing Vγ4 CD27− γδ T cells in the LN during the development of EAE (Fig. 1D and E).
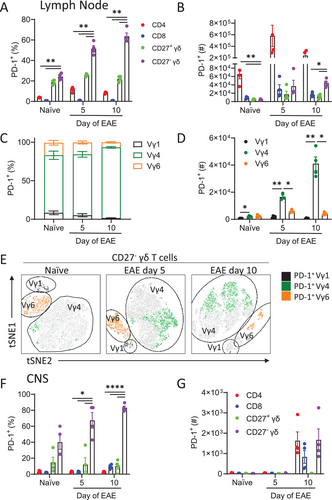
An examination of PD-1 expression in the CNS revealed a high frequency of PD-1-expressing γδ T cells infiltrating the spinal cord (Fig. 1F and G) and brain (Supporting information Fig. S1) during EAE. The vast majority of PD-1-expressing γδ T cells in the CNS were CD27−. The absolute number of PD-1-expressing CD27− γδ T cells in the CNS was similar to that of PD-1-expressing CD4 T cells (Fig. 1G). These findings demonstrate that PD-1 is highly expressed on Vγ4 CD27− γδ T cells and that these cells are expanded in the LN and CNS during EAE.
Anti-PD-1 enhances IL-17A production by CD27− γδ T cells and the severity of EAE
Since Vγ4 CD27− γδ T cells play a key pathogenic role in EAE by producing IL-17A early in the disease, we examined the impact of blocking PD-1 in mice with EAE. Treatment with anti-PD-1 before and during the development of EAE significantly increased the severity of EAE when compared with mice treated with an isotype control antibody (Fig. 2A). Furthermore, when compared with isotype control antibody-treated mice, the anti-PD-1-treated mice lost significantly more weight from day 11 of EAE (Fig. 2B).
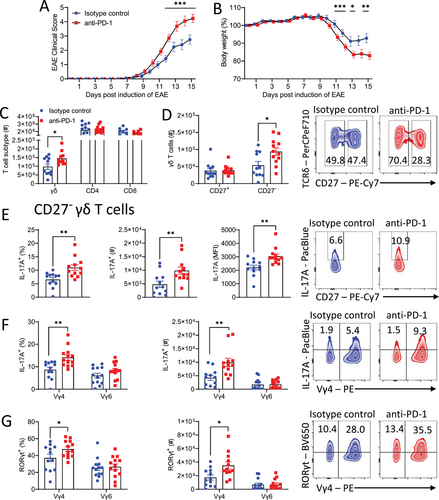
There was a significant increase in the total number of γδ T cells, but not of CD4 or CD8 T cells, in the LN following treatment with anti-PD-1 on day 6 of EAE (Fig. 2C). The expansion of γδ T cells was restricted to the CD27− γδ T cell population, with a significant increase in the absolute number of CD27− γδ T cells, but not of CD27+ γδ T cells on day 6 of EAE in mice treated with anti-PD-1 (Fig. 2D). An examination of cytokine production by γδ T cells showed a significant increase in the frequency, mean fluorescence intensity, and total number of IL-17A-producing CD27− γδ T cells in the LN of anti-PD-1-treated mice with EAE (Fig. 2E). The increase in IL-17A production was confined to the Vγ4 population of CD27− γδ T cells; there was a significant increase in the frequency and absolute number of IL-17A-producing CD27− Vγ4, but not Vγ6, CD27− γδ T cells in anti-PD-1-treated mice (Fig. 2F). Furthermore, there was a significant increase in frequency and absolute number of RORγt-expressing CD27− Vγ4, but not CD27− Vγ6 γδ T cells in anti-PD-1-treated mice (Fig. 2G).
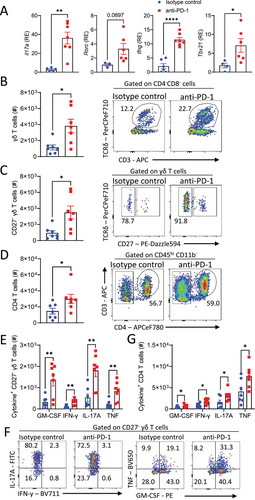
We next examined the effect of anti-PD-1 treatment on T cell infiltration and cytokine production in the CNS of mice with EAE. Expression of Il17a, Ifng, and Tbx21 mRNA were significantly increased in the spinal cord of anti-PD-1-treated mice at the peak of EAE (Fig. 3A). Expression of Rorc was also increased although not significantly (Fig. 3A). An examination of CNS-infiltrating T cells revealed that there was a significant increase in the number of γδ T cells in the brain (Fig. 3B). Furthermore, there was a significant increase in the number of CD27− γδ T cells (Fig. 3C) and CD4 T cells (Fig. 3D) in the brains of anti-PD-1-treated mice at the peak of EAE. The absolute number of GM-CSF, IFN-γ, IL-17A, and TNF-producing CD27− γδ T cells was significantly augmented in the brain of anti-PD-1-treated mice at the peak of EAE (Fig. 3E and F). There was also significant enhancement in the absolute number of GM-CSF, IFN-γ, IL-17A, and TNF-producing CD4 T cells in the brains of anti-PD-1-treated mice during EAE (Fig. 3G). These findings demonstrate that treatment with anti-PD-1 exacerbates EAE by enhancing the production of IL-17A and other inflammatory cytokines by peripheral CD27− γδ T cells and augmenting CNS infiltration of CD27− γδ T cells and CD4 T cells into the CNS where they promote disease pathology.
Anti-PD-1 exacerbates EAE by regulating γδ T-cell function
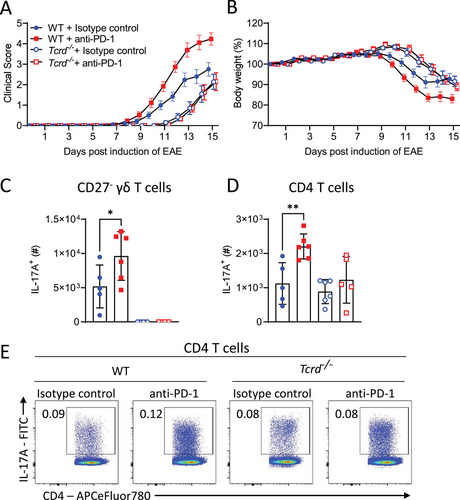
We have previously reported that IL-17A-producing γδ T cells are pathogenic in EAE, where they amplify MOG-specific CD4 T-cell responses [20]. Here we used Tcrd−/− mice to examine the role of γδ T cells in the exacerbation of EAE induced by anti-PD-1 treatment. Consistent with previous reports, EAE was less severe in Tcrd−/− mice when compared with WT mice. Although anti-PD-1 enhanced the severity of EAE in WT mice, there was no difference in clinical score between isotype control and anti-PD-1-treated Tcrd−/− mice (Fig. 4A). Furthermore, anti-PD-1 treatment exacerbated the weight loss in WT but not in Tcrd−/− mice (Fig. 4B). There was a significant increase in the number of IL-17A-producing CD27− γδ T cells in the LN of anti-PD-1-treated mice on day 9 of EAE (Fig. 4C). There was an increase in the absolute number of IL-17A-producing CD4 T cells in anti-PD-1-treated WT mice, but not Tcrd−/− mice, with EAE (Fig. 4D and E). These findings suggest that anti-PD-1 treatment during EAE increases the severity of disease by modulating the function of γδ T cells that further impacts CD4 T cell function.
PD-1 regulates TCR- but not cytokine-induced IL-17A-production by Vγ4 γδ T cells
Vγ4 γδ T cells are a key early source of IL-17A during the induction of EAE [19] and the present study has shown that they express high levels of PD-1 in the LN at the onset of EAE.
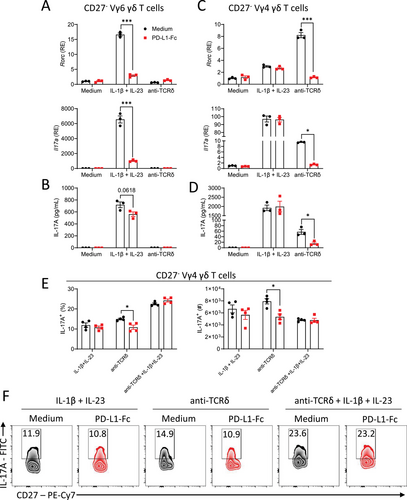
We examined the ability of PD-1 to regulate γδ T cells stimulated with the cytokines IL-1β and IL-23 or via the TCR. Since it had previously been reported that Vγ4+ and Vγ6+ cells are differentially regulated by PD-1 and other immune checkpoints [12], we compared Vγ4 and Vγ6 CD27− γδ T cells. Purified Vγ4 or Vγ6 γδ T cells were stimulated with IL-1β and IL-23 or anti-TCRδ with or without plate-bound PD-L1-Fc (PD-1 agonist), which activates PD-1 signaling. IL-1β and IL-23 activation induced Il17a and Rorc expression and IL-17A production by Vγ4+ and Vγ6+ cells. Engagement of PD-1 significantly suppressed IL-1β and IL-23-induced expression of Il17a and Rorc and reduced though not significantly IL-17A production by purified Vγ6+ cells (Fig. 5A and B). TCRδ activation did not induce Il17a and Rorc expression or IL-17A production by purified Vγ6+ cells (Fig. 5A and B). In contrast, activation of PD-1 on Vγ4+ cells with PD-L1-Fc did not affect IL-1β and IL-23-induced expression of Il17a and Rorc or IL-17A production (Fig. 5A and B). Conversely, anti-TCRδ-induced expression of Il17a and Rorc and IL-17A production by Vγ4 γδ T cells was significantly suppressed upon PD-1 engagement with PD-L1-Fc (Fig. 5C and D). Flow cytometry analysis confirmed that activation of PD-1 with PD-L1-Fc significantly suppressed the IL-17A-producing Vγ4 γδ T cells stimulated through the TCR but not with IL-1β and IL-23 or with IL-1β, IL-23, and anti-TCRδ (Fig. 5E and F). Although it has been reported that IL-7 is required for survival and expansion of TCR-activated IL-17 producing CD27− γδ T cells [24], we found that survival of Vγ4 γδ T cells was higher in cells stimulated with anti-TCRδ when compared with IL-1β and IL-23 (Supporting information Fig. S2). These findings suggest that PD-1 regulates TCR-induced activation of Vγ4+ cells and cytokine activation of Vγ6+ cells, but also reveal that innate Vγ4 γδ T cells escape regulation by this immune checkpoint.
Discussion
The significant new finding of our study is that PD-1 regulates activation of γδ T cells during the development of CNS autoimmunity. We found that PD-1 was expressed on a very high frequency of CD27− γδ T cells in the LN and CNS of mice with EAE. Furthermore, treatment of mice with anti-PD-1 at the onset of EAE enhanced IL-17A production by CD27− Vγ4 γδ T cells, promoting their infiltration into the CNS, and enhanced disease severity. PD-1 controlled TCR-induced activation of IL-17A-producing CD27− Vγ4 γδ T cells. However, cytokine-activated IL-17A-producing CD27− Vγ4 γδ T cells were resistant to regulation by PD-1 and this may in part explain the pathogenicity of these innate γδ T cells in EAE and other autoimmune diseases.
γδ T cells are key mediators of pathology in EAE and other murine models of autoimmunity including psoriasis, where they produce early IL-17A and promote the development of Th17 cells [20, 25]. In EAE, Vγ4 γδ T cells can produce IL-17A without TCR engagement, responding to the cytokine IL-1β/IL-18 and IL-23 in an innate-like fashion [20, 26]. We have also reported that lung Vγ6 γδ T cells provide an early source of innate IL-17A during Bordetella pertussis infection, but that antigen-specific Vγ4 γδ T cells function in adaptive immunological memory against this bacterial pathogen [27]. In a tumor model, it has been reported that PD-1 regulates lung Vγ6 γδ T cells through induction of FOXO1, which inhibits RORγt, whereas Vγ4 γδ T cells were regulated by the checkpoint molecule TIM-3 [12]. In the present study, we found that a PD-1 agonist did not suppress IL-17A production by CD27− Vγ4 γδ T cells stimulated with IL-1β and IL-23. This may explain how EAE progresses in the presence of high PD-1 expression and is consistent with the demonstration that CD27− Vγ4 γδ T cells play a key pathogenic role in EAE.
It has been reported that depletion of PD-1 expressing cells with either diphtheria toxin-derived anti-PD-1 immunotoxin [28] or radioisotope-labelled anti-PD-1 [29] reduced the progression of EAE. Although γδ T cells were not examined, these depleting approaches would have removed Vγ4 γδ T cells, which express high levels of PD-1 and are highly pathogenic in EAE. Consistent with this, our study demonstrated that blocking the function of PD-1 with anti-PD-1 enhanced proliferation and IL-17A production by CD27− Vγ4 γδ T cells and consequently exacerbated EAE. Furthermore, the exacerbating effect of anti-PD-1 in EAE was lost in mice lacking γδ T cells. We found that PD-1 engagement with PD-L1 suppressed IL-17A production by TCR-activated Vγ4 γδ T cells, which is consistent with the demonstration that PD-1 constrains CD4 T cell responses via inhibition of TCR signaling [30] and with the recent demonstration that TCR-activated human skin-derived Vδ1+ γδ T cells are regulated by PD-1 [31]. Our findings demonstrate that PD-1 regulates TCR-activated murine Vγ4 γδ T cells and suggests that antigen-specific Vγ4 γδ cells must also contribute to pathology in EAE. The antigens recognized by the Vγ4 γδ cells during EAE are unclear but may arise from immunization with complete Freund's adjuvant (CFA) and MOG. We have previously reported the killed Mycobacterium tuberculosis in CFA activates IL-1β production by neutrophils and inflammatory monocytes and together with IL-23, activated innate γδT17 cells [19]. However, we also have preliminary evidence that MOG may activate IL-17A-secreting CD27− γδ T cells (as well as CD4 T cells) through the TCR (unpublished observations). IL-17 fate mapping studies have revealed that TCR stimulation synergized with IL-1β and IL-23 to enhance the acquisition of IL-17-expression by γδ T cells [32].
ICIs have revolutionized the treatment of cancer, but they are associated with significant immune-mediated adverse events that are similar to autoimmune diseases [1, 2]. Activation of autoreactive CD4 and CD8 T cells is considered to be the key mediator of these immune-mediated adverse events [10]. However, the present study together with studies in a psoriasis model [33], suggest that anti-PD-1 may also enhance autoimmune diseases in cancer patients by enhancing the activation of IL-17A-producing γδ T cells. The study in the psoriasis model suggested that anti-PD-1 enhanced IL-17A production by innate γδ T cells that have intermediate expression of the γδ receptor [33]. Furthermore, treatment with recombinant PD-L1-Fc suppressed IL-17 production and attenuated disease in imiquimod-treated mice [34]. Interestingly, PD-L1-Fc suppressed IL-17 production by CD27− Vγ6 γδ T cells activated via the TCR, but not by IL-1β and IL-23, which is consistent with our findings on CD27− Vγ4 γδ Tcells in the EAE model. Our study also demonstrated that anti-PD-1 treatment enhanced IL-17A production by TCR-activated CD27− Vγ4 γδ T cells. Collectively, these studies suggest that treatment with drugs or antibodies that block IL-17A signaling in γδ T cells may be useful in treating immune-related adverse events in cancer patients.
The impact of ICIs on γδ T cell function also has relevance to the treatment of cancer, as IL-17A can have a protumor role in certain cancers [35-37]. Anti-PD-1 treatment did not reduce tumor growth in certain murine cancer models where IL-17A is produced by γδ T cells [38]. In NSCLC patients treated with the anti-PD-1 monoclonal antibody pembrolizumab, the response to treatment was associated with decreased expression of Rorc in the lungs [39]. Furthermore, the blockade of IL-17A improved the response to anti-PD-1 treatment in a murine model of colorectal cancer by increasing infiltration of CD8 T cells into the tumor [40]. In the present study, anti-PD-1 treatment promoted expansion and enhanced IL-17A production by γδ T cells. This suggests that increased IL-17A production by γδ T cells in the tumor microenvironment could prevent ICIs from effectively reducing tumor growth. These data could explain why certain cancer types are resistant to treatment with ICIs.
Our findings also suggest that modulation of IL-17A production by PD-1 could be exploited for therapeutic benefit in autoimmune diseases. PD-1 agonistic monoclonal antibodies that induce PD-1 signaling can specifically suppress T cell activation via the TCR [41]. Peresolimab, a humanized monoclonal antibody, which stimulates PD-1 signaling in vivo, has shown efficacy in a phase II clinical trial in patients with rheumatoid arthritis [42]. The findings from the present study in EAE, together with the demonstration that MS disease pathogenesis is associated with Rorc expression [43], suggest that a PD-1 agonistic monoclonal antibody may be effective in the treatment of MS and other autoimmune diseases. However, Vδ1 γδ T cells in MS patients primarily secrete IFN-γ [44] and in melanoma patients do not secrete IL-17 [31]. Nevertheless, IL-17-secreting CD161+CCR6+ γδ T cells have been shown to accumulate in the cerebrospinal fluid of patients with MS [45]. Although the expression of PD-1 by γδ T cell subsets has not been examined in MS patients, the role of immune checkpoints in controlling MS and other autoimmune diseases disease in humans is worthy of further investigation.
Materials and methods
Mice
C57BL/6J mice and Tcrd−/− mice on a C57BL/6J background were bred by the Comparative Medicine Unit, Trinity College Dublin, and were housed under specific-pathogen-free conditions. Mice were maintained under guidelines and regulations of the Health Products Regulatory Authority and experiments were carried out under license with the approval of Trinity College Dublin Animal Research Ethics Committee. Experiments were conducted with female 6–12-week-old mice. All mice within the experiments were age-matched.
Induction and assessment of active EAE
EAE was induced in C57BL/6J mice by subcutaneous immunization with 100 μg MOG35-55 (Genscript, referred to as MOG throughout) peptide emulsified 1 in 2 in CFA (Chondrex) which contains 4 mg/mL of H37 Ra M. tuberculosis. Mice were injected intraperitoneally with 250 ng of pertussis toxin (Native Antigen Company) on day 0 of EAE. Disease severity was assessed according to percentage weight change and EAE clinical scores were 0: no clinical signs, 1: limp tail, 2: ataxic gait, 3: hind limb weakness, 4: hind limb paralysis, 5: tetra paralysis. In certain experiments mice were injected with 200 μg anti-PD-1 antibody (RMP1-14; AssayGenie) or rat IgG2a isotype control antibody (AssayGenie) on the day prior to induction and on days 2 and 5 post-induction of EAE. The dose of 200 μg of anti-PD-1 per mouse was based on a dose that we found to be effective as an immune checkpoint inhibitor in a study with a tumor vaccine [46].
Isolation of mononuclear cells from the spleen and lymph nodes
Spleen and LN cell suspensions were prepared by manual disruption of the organs through a 70 μm filter, washing through with complete RPMI (cRPMI) medium continuously. Cells were centrifuged (342 × g, 5 min), lysed with RBC lysis buffer (0.87% NH4Cl; Sigma-Aldrich), and re-suspended in cRPMI, that included 10% heat-inactivated FCS (Biosera), 100 mM L-glutamine (Sigma-Aldrich), 100 μg/mL penicillin/streptomycin (Gibco) supplemented with β-mercaptoethanol (50 μM; Thermo Fisher Scientific).
Isolation of mononuclear cells from the CNS
Mice were sacrificed by CO2 anesthesia and perfused through the left ventricle with 20 mL of ice-cold PBS before removal of the brain and spinal cord. Organs were lysed in 1 mL cRPMI using a tissue lyser (Qiagen) at 27.5 rpm/s for 5 min. The brain and spinal cord cells were re-suspended in 5 mL of 40% isotonic Percoll (GE Healthcare) in cRPMI and layered over 5 mL of 70% Percoll in PBS. The Percoll gradients were centrifuged at 342 × g for 20 min at room temperature with the centrifuge brake off. Mononuclear cells were removed from the interface of the Percoll gradients and passed through a 70 μm filter. Cells were washed in cRPMI.
Flow cytometry analysis
Mononuclear cells from the brain, spinal cord, or LN were processed into an RBC-lysed, single cell suspension prior to staining. Cells were incubated with live/dead stain (LIVE/DEAD Fixable Aqua Dead Cell Stain Kit; Invitrogen, 1 in 1000) for 30 min. Cells were washed and surface stained in the presence of Fcγ block (BD Biosciences) with antibodies specific for B220 (RA3-6B2), CD3ε (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD27 (LG.7F9), CD45 (30-F11), PD-1 (29F.1A12), TCRβ (H57-597), TCRδ (GL3), TCR Vγ1.1 (2.11), or TCR Vγ4 (UC3-10A6). For intracellular cytokine staining, cells were stimulated for 4 h with PMA (50 ng/mL; Sigma-Aldrich), ionomycin (500 ng/mL; Sigma-Aldrich), and brefeldin A (5 μg/mL; Sigma-Aldrich) or brefeldin A alone. For intracellular and intranuclear staining, cells were fixed and permeabilized using the FoxP3/Transcription factor staining buffer kit (Thermo Fisher Scientific) according to the manufacturer's protocol. Cells were stained intracellularly with antibodies specific for GM-CSF (MP1-22E9), IFN-γ (XMG1.2), IL-17A (TC11-18H10.1), RORγt (Q31-378), or TNF (MP6-XT22). The details of antibodies used are shown in Table S1. Flow cytometric data were acquired using the LSRFortessa flow cytometer (BD Biosciences) with FACS Diva software or Cytek Aurora full spectrum analyzer with SpectroFlo software, and data were analyzed with FlowJo software (TreeStar Inc.) Percentage and absolute number of cells were calculated based on single, live cells. Flow cytometry gating strategies are shown in Supporting information Figs. S3 and S4.
Purification and stimulation of CD27− Vγ4 and Vγ6 γδ T cells
Mononuclear cells were prepared from the LN of mice with EAE. CD27− Vγ4 γδ T cells were isolated using a FACSAria Fusion Cell Sorter (BD Biosciences). Propidium iodide Ready Flow reagent (Invitrogen) was added to the cells prior to cell sorting to allow for discrimination of live from dead cells. FACS-purified CD27− Vγ4 γδ T cells were either stimulated with recombinant murine IL-1β (2.5 ng/mL; Biolegend) and IL-23 (10 ng/mL; Biolegend) or anti-TCRδ (UC7-13D5; 1 μg/mL, Biolegend) in the presence or absence of plate-bound recombinant PD-L1-Fc (30 μg/mL; R&D Systems). For qPCR analysis, cells were lysed after 2 h and RNA was extracted using an RNeasy mini kit (Qiagen) and reverse transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's protocols. qRT-PCR was performed using commercially available Il17a (Mm00439618), Ifng (Mm01168134), Rorc (Mm01261022), and Tbx21 (Mm00450960) primers (Applied Biosystems). Eukaryotic 18S ribosomal RNA was used as an endogenous control. Samples were assayed on an Applied Biosystems 7500 Fast Real-Time PCR machine. Alternatively, cells were stimulated for 48 h and IL-17A concentrations in the supernatants were determined by ELISA using a DuoSet ELISA kit (R&D Systems) according to the manufacturer's protocol.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. Differences were analyzed by unpaired Student's t-test (two conditions) or one-way ANOVA with Tukey's post-test (three or more conditions). Differences between groups for clinical scores and percentage weight change in EAE were analyzed by two-way ANOVA with repeated measures followed by Sidak's multiple comparisons test. Error bars indicate the mean ± SEM or SD as shown in individual figure legends. p values of 0.05 or less were considered to be statistically significant.
Acknowledgements
This work was supported by research grants from Science Foundation Ireland (16/Ia/4468 and 22/FFP-A/10297) to K.H.G.M. and by an Irish Research Council Government of Ireland Postgraduate award (IRCLA/2023/1664) to C.M.L.
Open access funding provided by IReL.
Conflict of interest
Kingston Mills is an inventor on a number of patent applications, co-founder of a start-up company, and has collaborative research funding from and acts as a consultant to pharmaceutical and biotech companies.
Author contributions
Kingston H.G. Mills conceived and supervised the study. Charlotte M. Leane designed and performed the experiments and analyzed and interpreted the data. Caroline E. Sutton provided critical input and feedback on the study design and the manuscript. Barry Moran carried out FACS sorting and provided critical feedback on the manuscript. Charlotte M. Leane and Kingston H.G. Mill wrote and revised the manuscript.
Open Research
Data Availability Statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Abbreviations
-
- BTN
-
- butyrophilin
-
- EAE
-
- experimental autoimmune encephalomyelitis
-
- ICIs
-
- immune checkpoint inhibitors
-
- LN
-
- lymph node
-
- MS
-
- multiple sclerosis
-
- NSCLC
-
- non-small cell lung cancer
-
- TCR
-
- T-cell receptor
-
- WT
-
- wild type




